Hip fracture in the population aged 75 years and older is one of the most disabling pathologies. Likewise, disease related malnutrition (DRM) and sarcopenia are two frequent diagnoses in this age group, whose prevalence may be increased in patients with hip fracture.
AimsTo determine the prevalence of malnutrition and/or sarcopenia in patients admitted for hip fracture and evaluate the existence of malnutrition related to disease and sarcopenia, and the differences between the sarcopenic and non-sarcopenic group.
Methods186 patients aged 75 years or over, hospitalised for hip fracture from March 2018 to June 2019 were included. Demographic, nutritional and biochemical variables were collected. Nutritional screening was carried out with the Mini-Nutritional Assessment (MNA), the presence of DRM was established with The Global Leadership Initiative on Malnutrition (GLIM) criteria. For sarcopenia screening, the Strength, Assistance with walking, Rising from a chair, Climbing stairs and Falls (SARC-F) was used and the diagnosis of sarcopenia was made using the criteria from the European Working Group on Sarcopenia in Older People (EWGSOP) reviewed in 2019 (EWGSOP2). Muscle strength was determined by hand-grip strength, body composition by measurement of bioelectrical impedance.
ResultsThe mean age was 86.2 years, most of the patients were women (81.7%). 37.1% of patients were at nutritional risk (MNA 17−23.5) and 16.7% were malnourished (MNA < 17). 72.4% of women and 79.4% of men, were diagnosed with DRM. 77.6% of the women and 73.5% of the men had low muscle strength. The appendicular muscle mass index was below the cut-off points for sarcopenia in 72.4% of the women and 79.4% of the men. Patients with sarcopenia had a lower body mass index, older age, poorer previous functional status and higher disease burden. The relationship between weight loss and hand grip strength (HGS) was significant (p = 0.007).
Conclusions53.8% of patients admitted for hip fracture present malnutrition or are at risk after screening with MNA. Sarcopenia and DRM affects at least three out of four patients older than 75 years admitted for hip fracture. Older age, worse functional status, lower body mass index and high number of comorbidities, are associated with these two entities. There is a relationship between DRM and sarcopenia.
La fractura de cadera en la población de edad igual o superior a 75 años es una de las patologías más incapacitantes. Así mismo, la desnutrición relacionada con la enfermedad (DRE) y la sarcopenia son dos diagnósticos frecuentes en este grupo de edad, cuya prevalencia puede estar aumentada en los pacientes con fractura de cadera.
ObjetivoConocer la prevalencia de desnutrición y/o sarcopenia en pacientes ingresados por fractura de cadera y evaluar la coexistencia de DRE y sarcopenia, y las diferencias entre el grupo de pacientes con sarcopenia y aquellos que no.
MétodosSe incluyeron 186 pacientes de 75 años o más, hospitalizados por fractura de cadera desde marzo de 2018 a junio de 2019. Se recogieron variables demográficas, nutricionales y bioquímicas. El cribado nutricional se realizó con Mini-Nutritional Assessment (MNA). La presencia de DRE se estableció con los criterios The Global Leadership Initiative on Malnutrition (GLIM). Para el cribado de sarcopenia se utilizó el Strength, Assistance Walking, Rise from a chair, Climb stairs, and Falls (SARC-F) y el diagnóstico de sarcopenia se realizó mediante los criterios European Working Group on Sarcopenia in Older People (EWGSOP) revisados en 2019 (EWGSOP2). Se determinó la fuerza muscular mediante dinamometría de mano y la composición corporal mediante bioimpedanciometría.
ResultadosLa edad media fue de 86,2 años, la mayor parte de los pacientes fueron mujeres (81,7%). Un 37,1% de los pacientes estaban en riesgo nutricional (MNA 17-23.5) y un 16,7% desnutridos (MNA < 17). Un 72,4% de las mujeres y un 79,4% de los varones, se diagnosticaron de DRE. Presentaron baja fuerza muscular un 77,6% de las mujeres y el 73,5% de los varones. El índice de masa muscular apendicular se encontraba por debajo de los puntos de corte para sarcopenia en el 72,4% de las mujeres y el 79,4% de los varones. Los pacientes con sarcopenia presentaban un índice de masa corporal más bajo, mayor edad, peor situación funcional previa y mayor carga de enfermedad. Resultó significativa (p = 0,007), la relación entre pérdida de peso y baja fuerza de presión manual.
ConclusionesEl 53,8% de los pacientes que ingresan por fractura de cadera presentan desnutrición o están en riesgo tras cribado con MNA. La sarcopenia y la DRE afectan al menos a tres de cada cuatro pacientes mayores de 75 años que ingresan por fractura de cadera. Una mayor edad, peor situación funcional, menor índice de masa corporal y mayor número de comorbilidades se asocia con estas dos entidades. Existe una relación entre DRE y sarcopenia.
Hip fracture is the fragility fracture with the greatest negative consequences in geriatric age. Hip fractures involve a functional deterioration that is not always reversible, due to the process itself and the immobilisation that they entail in the preoperative and immediate postoperative periods. They also contribute to institutionalisation and increased mortality, more markedly during the first year following the fracture.1
The processes related to fractures and falls are being increasingly studied in relation to the possibility of preventing their occurrence; and, should they occur, reducing the functional deterioration that they entail. The factors that have the greatest impact at this level are malnutrition2 and sarcopenia.3
According to the latest criteria established by The Global Leadership Initiative on Malnutrition (GLIM),4 disease-related malnutrition (DRM) is commonly diagnosed in these patients.
The first to use the term sarcopenia (from the Greek sarx: flesh and penia: loss) was Rosenberg in 1995.5 This process was defined as an age-related abnormal loss of muscle mass, which predicts functional decline and is associated with loss of mobility and nutritional deterioration. Subsequently, Manini and Clark6 reviewed the concept of “sarcopenia”, as loss of muscle mass, versus “dynapenia”, a term that was coined as early as 2008 to describe a loss of muscle strength.7 Although initially, loss of muscle strength and function was directly related to loss of muscle quantity, these authors conducted an extensive review in 2012 of what had been published so far, concluding that loss of strength does not depend solely on a decrease in muscle mass, but that there were other factors involved, some of neurological origin, and that, in general, muscle strength deteriorated faster than mass. These researchers reviewed studies that linked loss of strength to disability, but found no relationship between loss of muscle mass and functional decline. In the diagnostic algorithms of “sarcopenia” published to date,8,9 functional tests (gait speed) and hand grip strength measurements are included. It is for this reason that at the present time it is postulated that we are talking about two different entities; dynapenia versus sarcopenia.6
One of the most widely-used definitions of sarcopenia is that published in 2010 by the European Working Group on Sarcopenia in Older People (EWGSOP). This definition is based on muscle mass, strength and function.8 In 2019, a review of the criteria was published by this same group, with better defined cut-off points.10 In addition, in its diagnostic algorithm, the study of sarcopenia is initiated with tests that measure muscle strength (hand grip strength, chair stand test), the quantity of muscle being a confirmatory measurement (by DEXA [Dual-energy X-Ray Absorptiometry], BIA [Bioimpedancemetry], NMRI [Nuclear Magnetic Resonance Imaging], CT [computed tomography]), and once again basing the severity of sarcopenia, or perhaps it would be better to say "dynapenia", on functional tests (Short Physical Performance Battery [SPPB], gait speed, Timed Up and Go [TUG]).6,7 In this regard, in 2016 sarcopenia was recognised with its own code in the International Classification of Diseases (ICD-10).11
A relationship been observed between loss of bone mass and decrease in muscle mass (sarcopenia), leading to the coining of the term osteosarcopenia.12,13 In fragility fractures there is a correlation between the two entities as a risk factor.14
Muscle and bone are highly interrelated due to a common embryonic origin; both come from the same mesenchymal stem cells that differentiate into one or the other cell lineage.15 From these stem cells also come the adipocytes found in both tissue types, which would explain the greater muscle and bone fat infiltration in elderly patients, since pluripotent cells tend to differentiate towards this lineage with ageing.15,16 In addition, the two tissues are integrated and have shared functions. Thus, there is a sophisticated regulation between muscle and bone of the balance between processes of synthesis and degradation/resorption of both tissues. Genetic and endocrine factors, the nervous system, proinflammatory cytokines, the patient's physical activity and drugs are involved in this regulation.16 There is a paraendocrine mechanism, by which the muscle secretes myokines that affect the bone, and this, in turn, secretes osteokines with which it communicates with the muscle.15,16 Changes in bone mass affect muscle mass and vice versa.17
DRM leads to a loss of muscle mass, and therefore, although they are different entities, there is a relationship between DRM and sarcopenia.18 In addition, it would be expected that its prevalence would be higher in patients with hip fracture due to osteoporosis.
For this reason, a study was carried out to determine the prevalence of DRM and/or sarcopenia in patients admitted to an Orthogeriatric Unit of a tertiary care hospital due to fragility hip fracture.
Material and methodsStudy designA cross-sectional study of routine clinical practice was designed for all patients aged 75 years or over with a diagnosis of fragility fracture of the proximal end of the femur (hip fracture).
Patients who were admitted to the Orthogeriatric Unit of the Hospital Clínico Universitario de Valladolid from March 2018 to June 2019, and who were able to undergo the initial assessment prior to surgery and in less than 48 h from admission, were included. All patients or their representatives agreed to participate after signing an informed consent.
As this was a study of routine clinical practice, in all cases of DRM diagnosis there was nutritional intervention during admission and on discharge: diet enrichment or nutritional supplements to meet each patient's individualised energy and protein requirements. In addition to progressive exercise recommendations adapted to the patient's clinical course, muscle strength and power exercises, and balance exercises when the patient was standing (multicomponent exercise) were also prescribed.
The exclusion criteria were: emergency surgery; underlying disease determining a life expectancy below six months: we considered that an advanced organ disease or neoplasm alone would explain the DRM and/or sarcopenia and would be confounding factors; persistent oedema that did not respond to treatment of its cause (heart failure in most cases) in 24 h; prior treatment with nutritional supplements: this could influence the results, although laboratory values are not included in the diagnostic criteria for DRM or sarcopenia; cognitive impairment preventing the patient's collaboration; having a pacemaker or bilateral metallic prostheses that would alter the measurements; patients who could not previously walk; and non-acceptance by the patient.
The study was approved by the Independent Ethics Committee (IEC) of the Hospital Clínico Universitario de Valladolid (PI 17-653). The investigation and processes were carried out in accordance with the good clinical practices stipulated in the Declaration of Helsinki.
VariablesAfter signing the informed consent, epidemiological, medical history and physical examination data were collected, and a comprehensive geriatric assessment was carried out. Prior functional status was measured with the Barthel index,19 burden of illness with the CIRS-G (Cumulative Illness Rating Scale-Geriatric)20 and nutritional screening with the MNA (Mini Nutritional Assessment),21 with those patients who scored less than 17 being considered malnourished, and those who scored between 17 and 23.5 points being at nutritional risk. For the initial screening for sarcopenia, we used the SARC-F (Strength, assistance with walking, rising from a chair, climbing stairs and falls),22 with those subjects who scored four or more in this screening test considered to have probable sarcopenia.
Hand grip strength was measured with a Jamar® Hydraulic Hand Dynamometer, model J00105, and was considered decreased, according to EWGSOP criteria, when it was less than 16 kg in women and less than 27 kg in men. The body composition study was carried out, always with the patient not having had fluid replacement therapy for at least six hours, by Bioelectrical Impedance Analysis using the BIA 101 Anniversary model (Akern, Italy).
In the first 24 h following admission, a blood test was performed to assess protein, albumin and prealbumin levels, as well as calcium, phosphorus, vitamin D and PTH, and complete blood count and kidney function.
Patients were considered to have DRM, according to GLIM criteria, when they met a phenotypic criterion: weight loss (>5% in the last six months or >10% beyond six months), or BMI < 22 kg/m2, or low muscle mass (ALMI < 7 kg/m2 in men and <5.5 kg/m2 in women); and an aetiological criterion (inflammation, reduced intake or assimilation of nutrients); in this case, all patients met the criteria for acute disease with inflammation.4
For the diagnosis of sarcopenia, the revised EWGSOP2 criteria were used,10 using hand grip strength. Physical performance could not be evaluated with gait speed, SPPB or other tests, since the patients were unable to walk at the time of evaluation.
Total muscle mass was estimated according to variables obtained with BIA with Sergi's equation23: TLM (total lean mass): −3.964 + (0.227 × RI) + (0.095 × weight in kg) + (1.384 × gender) + (0.064 × reactance), where resistance index (RI) = height2 in cm/resistance, and gender: men 1, women 0.
Conversion to appendicular lean mass was performed according to the model described by Kim et al.,24 and finally the appendicular lean mass index was calculated, correlating the above with the squared height: ALM (appendicular lean mass): TLM/1.19 + 1.65; ALMI (appendicular lean mass index): ALM/height2 in m.
Statistical analysisThe data were processed using the statistical package SPSS (SPSS for Windows version 22.0.00, 2013. SPSS INC., Chicago III, USA). Quantitative variables with normal distribution were described with mean and standard deviation (mean [SD]), and qualitative variables as total number and percentages (total number [%]). The analysis tests used were: Student's t-test to compare means of normal quantitative variables and Chi-square test to compare qualitative variables. For non-normal quantitative variables, the Mann–Whitney U test was used. The significance level was conventionally set at a p-value of less than 0.05 (<0.05).
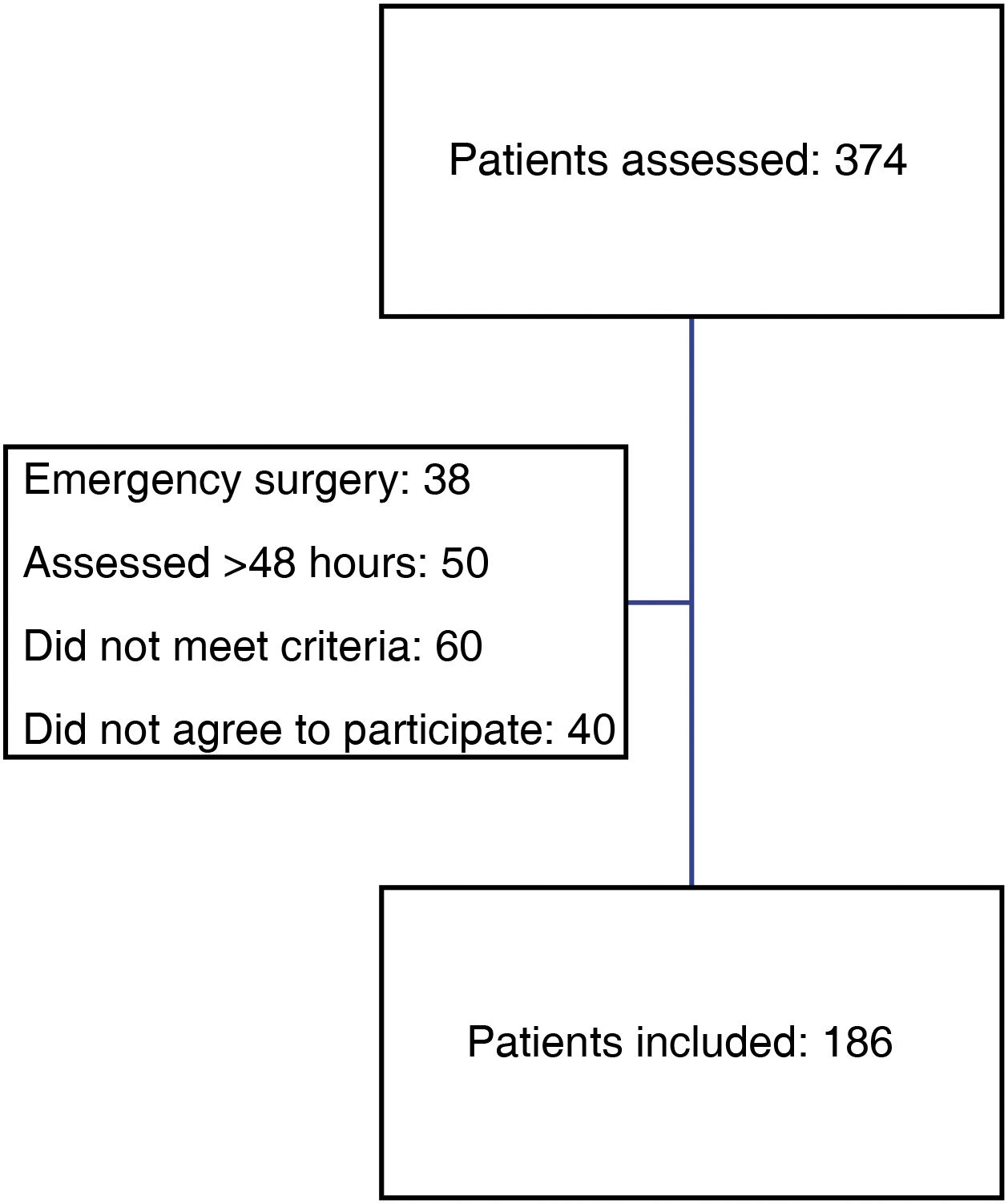
ResultsDuring the study period, 374 patients were diagnosed with proximal femoral fragility fracture and were admitted to the Orthogeriatric Unit of the Traumatology and Orthopaedic Surgery Department.
Overall, 186 patients were included in the study, which was 49.73% of the total (Fig. 1).
Sample descriptionThe baseline data are shown in tables 1 and 2. The majority of patients were women, with a mean age of 86.2 years. The most frequent type of fracture was extracapsular.
Baseline data of the patients included in the study.
| Variables | Total (%–range) |
|---|---|
| Gender | |
| Female | 152 (81.7%) |
| Male | 34 (18.3%) |
| Mean age (years) | 86.20 years (82−90) |
| Barthel (0−100 points) | 83.47 (25−100) |
| Pfeiffer (errors) | |
| 0−2 | 135 (72.6%) |
| 3−4 | 51 (27.4%) |
| Type of fracture | |
| Extracapsular | 101 (54.3%) |
| Pertrochanteric | 91 (48.9%) |
| Subtrochanteric | 10 (5.4%) |
| Intracapsular | 85 (45.7%) |
| HTN | 144 (77.4%) |
| DM | 48 (25.8%) |
| AFib | 40 (21.5%) |
| Ischaemic heart disease | 37 (19.9%) |
| Heart failure | 51 (27.4%) |
| COPD | 17 (9.1%) |
| Thyroid problems | 7 (3.8%) |
| Previous fractures | 36 (19.4%) |
| Antiplatelet therapy | 43 (23.2%) |
| ASA | 36 (19.4%) |
| Clopidogrel | 7 (3.8%) |
| Anticoagulants | 34 (18.3%) |
| Acenocoumarol | 22 (11.8%) |
| DOACs | 12 (6.5%) |
| BMI (kg/m2) | |
| ≥22 | 142 (76.3%) |
| 18.5–21.5 | 31 (16.7%) |
| <18.5 | 13 (7%) |
| % weight loss | |
| <5% | 67 (36%) |
| 5−10% | 42 (22.6%) |
| >10% | 77 (41.4%) |
| MNA | |
| <17 | 31 (16.7%) |
| 17−23.5 | 69 (37.1%) |
| 24−30 | 86 (46.3%) |
| SARC-F | |
| <4 | 92 (49.5%) |
| ≥4 | 94 (50.5%) |
AFib: atrial fibrillation; ASA: acetylsalicylic acid; BMI: body mass index; COPD: chronic obstructive pulmonary disease; DM: diabetes mellitus; DOACs: direct-acting oral anticoagulants; HTN: arterial hypertension; MNA: Mini Nutritional Assessment; SARC-F: Strength, assistance with walking, rising from a chair, climbing stairs and falls.
Strength and muscle mass at admission.
| Variables | Mean (SD) |
|---|---|
| Hand grip strength (kg) | 12.2 (7.3) |
| Women | 11.1 (5.2) |
| Men | 19.8 (10.1) |
| ALM (kg) | 14.84 (5.2) |
| Women | 14.00 (5.0) |
| Men | 18.60 (3.2) |
| ALMI (kg/m2) | 5.7 (0.8) |
| Women | 5.5 (0.6) |
| Men | 6.5 (1.1) |
ALM: appendicular lean mass; ALMI: appendicular lean mass index; SD: standard deviation.
Weight was 59.8 (12.3) kg; 57.9 (11) kg in women and 68.5 (14.1) kg in men. Calf circumference was 29.72 (3.09) cm; 29.64 (3.13) cm in women and 30.06 (2.93) cm in men. BMI was 23.82 (4.2) kg/m2; 23.81 (4.2) kg/m2 in women and 23.84 (4.1) kg/m2 in men.
After screening with the MNA, we suspected malnutrition in 16.7% of the patients and 37.1% were at nutritional risk.
Some 76% of the total sample (72.4% of women and 79.6% of men) met the phenotypic criterion for low muscle mass (<7 kg/m2 in men and <5.5 kg/m2 in women). In total, 21.1% of women and 29.4% of men had lost 5−10% of their weight (22.6% overall) and 43.4% of women and 32.4% of men more than 10% (41.4% overall). BMI was <18.5 kg/m2 in 7% of cases, and 16.7% had a BMI 18.5−21.5 kg/m2. The common aetiological criterion was inflammation due to acute disease.
Some 50.5% of our patients scored four or more on the SARC-F.
Sarcopenia prevalence and disease-related malnutritionAccording to hand grip strength, 76.9% met the criteria for probable sarcopenia (63.4% women and 13.4% men). Grouped by gender, 77.6% of women and 73.5% of men had low hand grip strength. Finally, and measuring quantity of muscle, 76.9% of the sample had low muscle mass: 72.4% of women and 79.6% of men were below the cut-off points defined by the EWGSOP2. Some 76.9% of the total sample met both criteria: low hand grip strength and quantity of muscle.
DRM was diagnosed according to GLIM criteria in 72.4% of women and 79.6% of men using the phenotypic criterion of low muscle mass (ALMI in this case), not differentiating between a severe or moderate form as the literature does not define clear cut-off points. Selecting percentage of weight loss as the phenotypic criterion, 43.3% of women and 32.4% of men would meet the criteria for severe DRM (41.4% overall) and 21.1% of women and 29.4% of men would meet the criteria for moderate DRM (22.6% overall). However, with the phenotypic criterion of BMI below the cut-off points, only 7% of the total sample showed severe DRM, and 16.7% moderate.
Inferential statisticsBy comparing the MNA screening scores for DRM with the SARC-F score for sarcopenia screening, we found statistical significance both in the total sample (p < 0.001) and by gender (p = 0.008 in women and p = 0.005 in men) (Table 3).
Comparison of sarcopenia and DRM screening tests.
| Overall data | ||||
|---|---|---|---|---|
| SARC-F | Total | |||
| <4 | ≥4 | |||
| MNA | ||||
| <17 | ||||
| n | 3 | 20 | 23 | |
| % | 13.0% | 87.0% | 100.0% | |
| 17−23.5 | ||||
| n | 46 | 48 | 94 | |
| % | 48.9% | 51.1% | 100.0% | p < 0.000 |
| ≥24 | ||||
| n | 43 | 26 | 69 | |
| % | 62.3% | 37.7% | 100.0% | |
| Total | ||||
| n | 92 | 94 | 186 | |
| % | 49.5% | 50.5% | 100.0% | |
| Data by gender | ||||
|---|---|---|---|---|
| Gender | SARC-F | Total | ||
| <4 | ≥4 | |||
| Female | ||||
| MNA | ||||
| <17 | ||||
| n | 3 | 15 | 18 | |
| % | 16.7% | 83.3% | 100.0% | |
| 17−23.5 | ||||
| n | 37 | 41 | 78 | |
| % | 47.4% | 52.6% | 100.0% | p = 0.008 |
| ≥24 | ||||
| n | 33 | 23 | 56 | |
| % | 58.9% | 41.1% | 100.0% | |
| Total | ||||
| n | 73 | 79 | 152 | |
| % | 48.0% | 52.0% | 100.0% | |
| Male | ||||
| MNA | ||||
| <17 | ||||
| n | 0 | 5 | 5 | |
| % | 0.0% | 100.0% | 100.0% | |
| 17−23.5 | ||||
| n | 9 | 7 | 16 | |
| % | 56.3% | 43.8% | 100.0% | |
| ≥24 | ||||
| n | 10 | 3 | 13 | p = 0.005 |
| % | 76.9% | 23.1% | 100.0% | |
| Total | ||||
| n | 19 | 15 | 34 | |
| % | 55.9% | 44.1% | 100.0% | |
DRM: disease-related malnutrition; MNA: Mini Nutritional Assessment; SARC-F: Strength, assistance with walking, rising from a chair, climbing stairs and falls.
The SARC-F score was compared with the hand grip strength, and a statistically significant relationship was found (p < 0.001) (Table 4).
Differences between the groups according to hand grip strength upon admission.
| Low HGS (<16 kg in women//<20 kg in men) | Normal HGS (≥16 kg in women//≥20 kg in men) | p | |
|---|---|---|---|
| Age (years) | 87.1 (5.4) | 83.1 (4.8) | <0.01 |
| Gender | |||
| Female | 118 | 34 | 0.608 |
| Male | 25 | 9 | |
| % weight loss | |||
| <5% | 43 | 24 | |
| 5−10% | 26 | 16 | 0.007 |
| >10% | 65 | 12 | |
| MNA (women) | |||
| <17 | 23 | 2 | |
| 17−23.5 | 51 | 7 | 0.001 |
| ≥24 | 44 | 25 | |
| MNA (men) | 6 | 0 | |
| <17 | 8 | 3 | 0.115 |
| 17−23.5 | 11 | 6 | |
| ≥24 | |||
| BMI (kg/m2) | 22.5 (4.4) | 27.5 (3.4) | <0.01 |
| CIRS-G | 10.1 (4.2) | 7.9 (4.3) | <0.01 |
| Barthel Index | |||
| <60 | 13.3% | 2.3% | |
| 60−80 | 25.9% | 0% | <0.01 |
| >80 | 60.8% | 97.7% | |
| SARC-F | |||
| <4 | 54 | 38 | |
| ≥4 | 80 | 14 | <0.001 |
| ALM <15 kg in women//<20 kg in men | 106 | 34 | |
| ALM >15 kg in women//>20 kg in men | 28 | 18 | 0.05 |
| ALMI | |||
| <5.5 kg/m2 (women) | 77 | 50 | |
| <7 kg/m2 (men) | |||
| ALMI | 0.080 | ||
| ≥5.5 kg/m2 (women) | 20 | 24 | |
| ≥7 kg/m2 (men) | |||
| Proteins (g/dl) | 5.7 (0.6) | 6.0 (0.6) | 0.03 |
| Prealbumin (mg/dl) | 14.5 (4.4) | 16.8 (4.9) | <0.01 |
| Albumin (g/dl) | 3.4 (0.5) | 3.6 (0.4) | 0.06 |
| Vitamin D (ng/ml) | 11.7 (10.5) | 13.8 (7.8) | 0.22 |
ALM: appendicular lean mass; ALMI: appendicular lean mass index; BMI: body mass index; CIRS-G: Cumulative Illness Rating Scale-Geriatric; HGS: hand grip strength; MNA: Mini Nutritional Assessment; SARC-F: Strength, assistance with walking, rising from a chair, climbing stairs and falls.
Performing the correlation between nutritional screening by MNA and hand grip strength revealed statistical significance in women (p = 0.001), but not in men (p = 0.115) (Table 4). Similarly, the comparison between the MNA and ALMI scores was statistically significant in women (p < 0.00), but not in men (p = 0.393) (Table 5).
Relationship between nutritional screening and muscle mass.
| ALMI <7 kg/m2 in men or <5.5 kg/m2 in women | ALMI ≥7 kg/m2 in men or ≥5.5 kg/m2 in women | p | |
|---|---|---|---|
| MNA (women) | |||
| <17 | 21 | 4 | |
| 17−23.5 | 25 | 33 | <0.00 |
| ≥24 | 25 | 44 | |
| MNA (men) | |||
| <17 | 5 | 1 | |
| 17−23.5 | 10 | 1 | 0.393 |
| ≥24 | 12 | 5 | |
| BMI (kg/m2) (mean) | 22.5 | 27.5 | <0.000 |
ALMI: appendicular lean mass index; BMI body mass index; MNA: Mini Nutritional Assessment.
The differences between patients with or without probable sarcopenia according to hand grip strength that were significant were: age (87.13 [5.43] years vs 83.14 [4.82] years, p < 0.001), burden of illness measured by CIRS-G (10.06 [4.19] vs 7.91 [4.26], p = 0.004), functional dependence measured with the Barthel index (p < 0.001), mean total protein levels (5.73 [0.64] g/dl vs 5.98 [0.63] g/dl, p = 0.029) and mean prealbumin levels (14.55 [4.37] mg/dl vs 16.76 [4.89] mg/dl, p = 0.005) (Table 4).
Significant differences (p < 0.001) were found in BMI (22.5 [3.24] vs 27.5 [4.4]) between patients with and without sarcopenia according to the appendicular lean mass index (Table 5).
The relationship between weight loss and low hand grip strength was also statistically significant (p = 0.007) (Table 3).
Other dataSurgical delay was 58.93 (42) hours, with 49.9% of patients undergoing surgery in the first 48 h. The overall length of hospital stay was 7.05 (4.03) days.
Barthel index at admission was 83.47 (20.70) points (median 95). At discharge, the Barthel index was 53.06 (20.64) (median 60). This represented a loss of 30.41 points (functional loss of 36.43%).
Prior to the fracture, 74.4% of the patients walked without assistance or with technical assistance both outside and inside the home and 16.6% did so only at home.
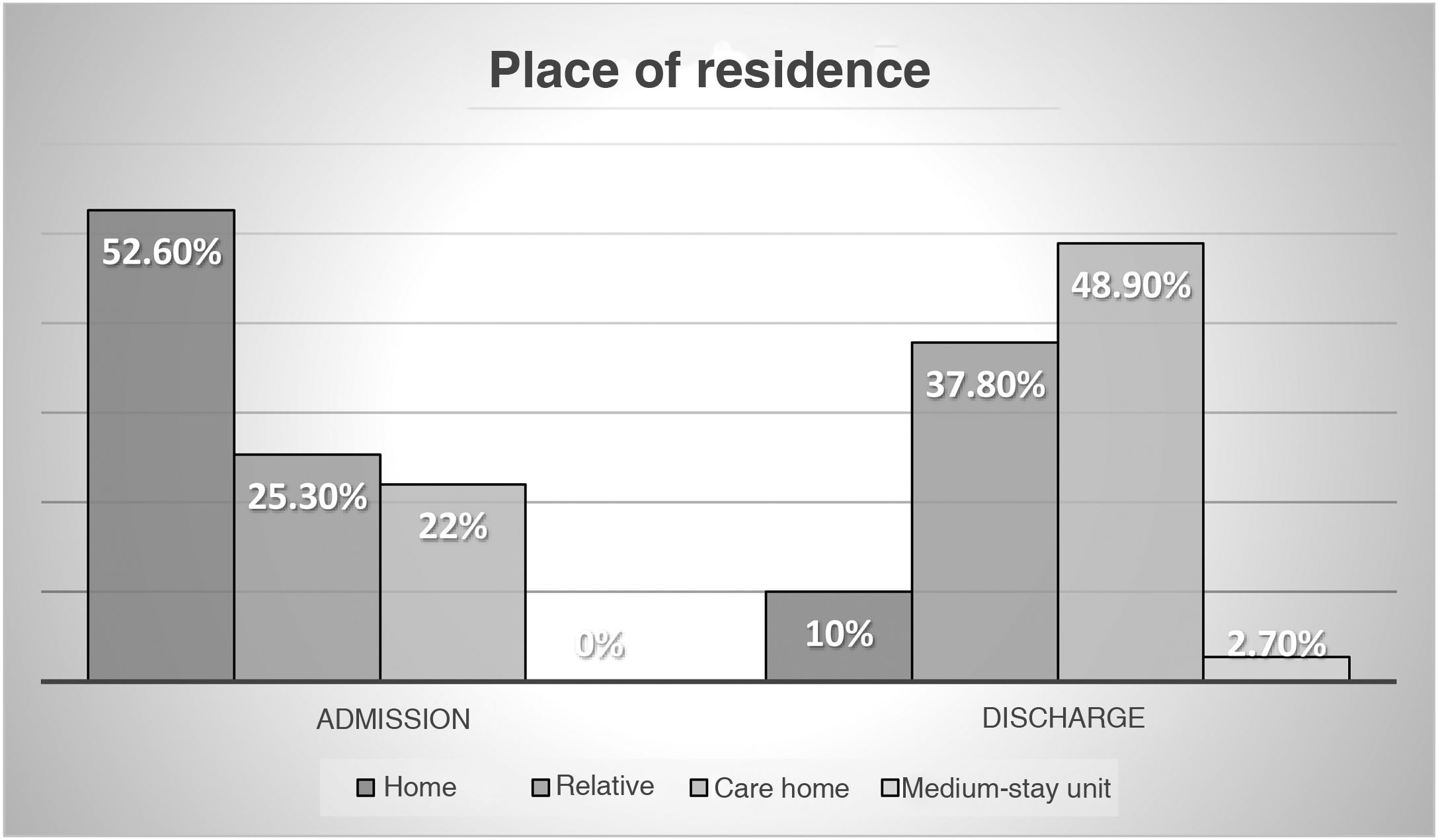
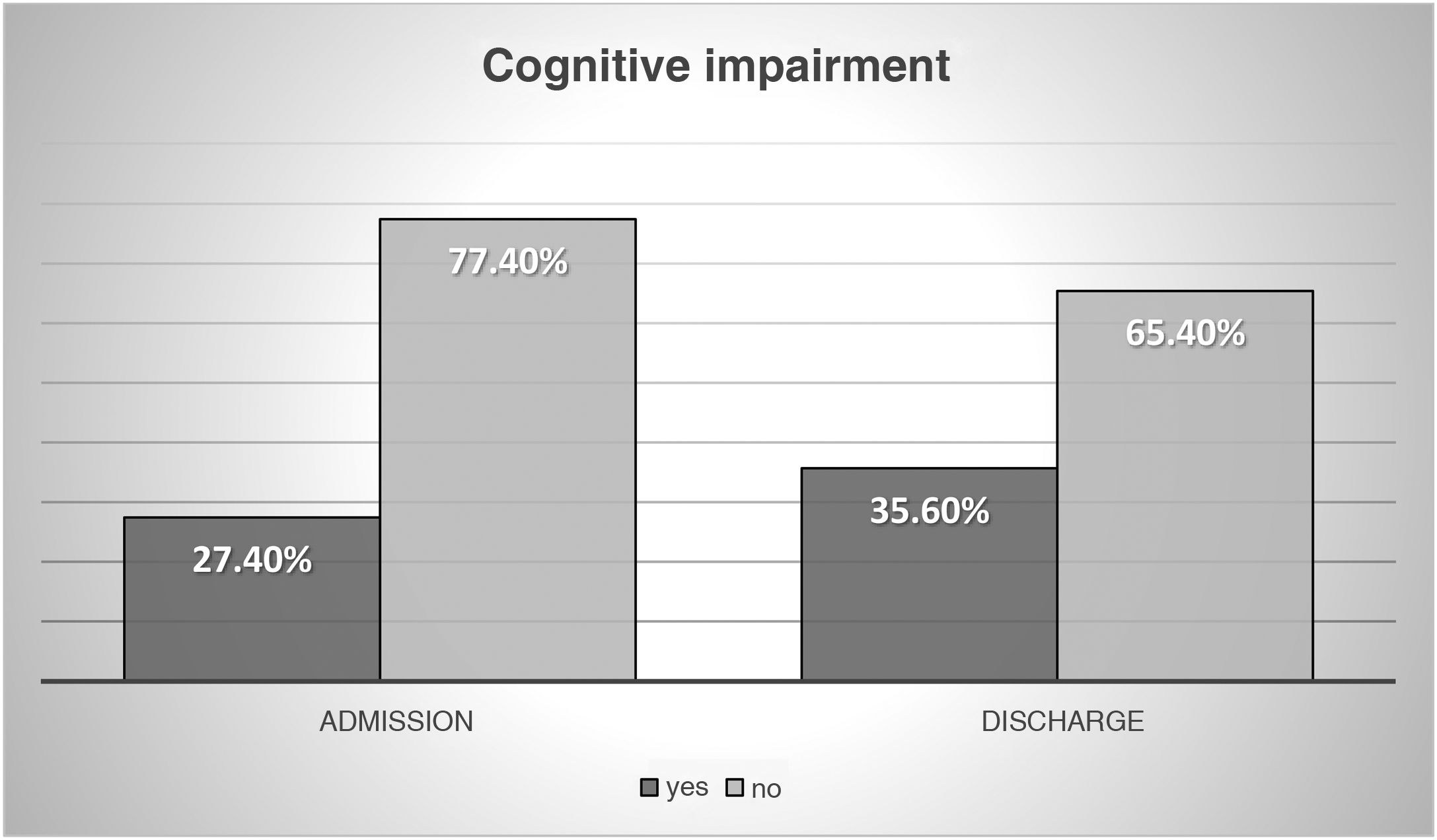
The socio-family and cognitive situation is shown in Figs. 2 and 3.
The most frequent comorbidity was arterial hypertension (HTN). In total, 19.4% of the patients had suffered a previous fracture, despite which none was receiving osteoprotective treatment with antiresorptive agents or bone-building drugs, nor were they taking treatment with vitamin D and/or calcium supplements.
The most common complications during admission were: acute confusional state (44.1%), urinary infection (9.7%) and acute urinary retention (8.6%).
The most frequent lab test abnormality was vitamin D deficiency with secondary hyperparathyroidism. Some 85.5% of the total sample had levels <20 ng/ml of vitamin D, and 57% had levels less than 10 ng/ml. In our centre, levels <10 ng/ml are considered a severe deficiency and between 10−20 ng/ml below the optimal range of vitamin D. The mean levels of PTH were 71.63 (42.93) pg/ml (normal range in our centre 15–65 pg/ml).
DiscussionThe prevalence of DRM in patients with hip fracture was 76.9% using the phenotypic criterion of low muscle mass, and somewhat lower using weight loss. However, in both cases, this prevalence was higher than that described in patients hospitalised for other causes. According to a study published in 2008 in patients admitted for other reasons, this prevalence was 20–50%,25 although Norman et al. based their conclusions on a review of studies between 1991 and 2006 with diverse criteria. The prevalence was not entirely comparable between the different studies since the GLIM criteria had not yet been published. Subsequently, and using the GLIM criteria, Bellanti et al.26 found a 46% prevalence of DRM, which was lower than that found in the study conducted in patients with hip fracture. This would support the hypothesis that malnutrition and sarcopenia are the cause of falls and fractures,27,28 and therefore their prevalence is higher in these patients than in those admitted for other reasons.
In our study, when using the phenotypic criterion of low muscle mass, all patients with sarcopenia had DRM and vice versa. Because the GLIM and EWGSOP2 criteria for the diagnosis of DRM and sarcopenia share the criterion of low muscle mass, in these patients, all with acute disease, diagnosing one of the geriatric syndromes automatically led to the diagnosis of the other. Some 64% of the sample had more than 5% weight loss. Perhaps the greater loss of lean mass, compared to fat, would explain why the diagnosis of DRM is more likely if we use the ALMI values.
The study conducted found a very high prevalence of sarcopenia, higher than that of previous studies in Spanish hospitals. In 2015, González-Montalvo et al. found a prevalence of 17.1% (12.4% in men and 18.3% in women) in a population of patients with comparable hip fracture (79.3% of the sample were women, mean age 85.3 years) from Hospital La Paz [La Paz Hospital] (Madrid).29 Sánchez-Castellano et al. also published a lower prevalence in 2019; 11.5% (10.3% in women and 16.1% in men) at Hospital Ramón y Cajal [Ramón y Cajal Hospital] (Madrid) (mean age 87.6 years, 78.7% women).30
In contrast, a study in an Asian population with hip fracture14 found a prevalence of sarcopenia of 73.6% in men and 67.7% in women, figures that are closer to our findings. They included patients over 60 years old, although the mean age was 81.85 years, and applied the criteria recommended by the Asian Working Group for Sarcopenia (AWGS), where the cut-off points for the appendicular lean mass index are 7 kg/m2 in men and 5.4 kg/m2 in women,31 similar to those defined in the 2019 revised EWGSOP2 criteria.10
An observational study published in 2019 at the University of Melbourne found a different prevalence of sarcopenia depending on the criteria applied8,31,32: older than 70 years, mean age 79.7 years, in men between 12–75.9%, and in women between 3.1–75.3%,33 but they used different cut-off points for the appendicular lean mass index than those defined in the 2019 EWGSOP criteria used in our study.10 The most similar criteria would be those of the 2011 IWGS (International Working Group on Sarcopenia) (ALMI < 7.23 kg/m2 in men and <5.67 kg/m2 in women),32 in which the prevalence of sarcopenia in men would be 44.3% and in women 24.5%, figures also lower than those obtained in our study.
The baseline characteristics of the patients with and without sarcopenia were similar except for BMI, a result comparable to that found in the studies by González-Montalvo et al. and Sánchez-Castellano et al.29,30
In this study, significant differences were only found between BMI, functional status measured by the Barthel index, burden of illness according to the CIRS-G, age and total protein and prealbumin levels in laboratory tests on admission, among patients with probable sarcopenia or not according to hand grip strength. Sánchez-Castellano et al.30 also found significant differences both in the functional status measured by Barthel and in the mean previous mobility by FAC (Functional Ambulation Categories); the latter was not analysed in our patients. Significant differences were also not found according to the type of fracture, unlike the study at Hospital Ramón y Cajal in Madrid30 where patients with pertrochanteric fracture had lower hand grip strength.
Given the importance that is being placed on the role of vitamin D in this area of knowledge,34,35 the fact that there were no differences in the levels of this vitamin between the groups is striking. Perhaps the absence of any difference is related to the existing high deficiency rate of this vitamin. More than 85% of the sample had levels below 20 ng/ml, and in 57%, levels were below 10 ng/ml.
Only in women was a relationship found between screening for malnutrition using the MNA with decreased hand grip strength and low appendicular lean mass index. This could be because the number of men included in the study was very low (34 patients). Given the existing evidence, differences between patients with and without sarcopenia would be expected to be greater. This could be explained by the fact that the risk factors that contribute to falls are more relevant than the sarcopenia itself.
This study contributes evidence on the incidence of DRM and of a syndrome such as sarcopenia in Spain, which is so important for the development and progression of fragility fractures. It also relates DRM with sarcopenia in these patients; the comparison between the screening tests used for the diagnosis of one process or the other was significant. There are few publications in the literature that relate DRM with sarcopenia. In one of them, Meyer and Valentini36 reviewed previous studies that demonstrated that the presence of both sarcopenia and DRM was associated with worse outcomes, more complications and increased mortality in different processes. In many patients, the two conditions occur simultaneously, and so a new clinical syndrome has been proposed: “malnutrition-sarcopenia syndrome” (MSS). Questions should be raised about whether it is sufficient to screen for one to automatically diagnose the other, or which is more important. If the differences and similarities in the screening tests (MNA/SARC-F) and their diagnostic criteria (GLIM and EWGSOP2) are compared, DRM would share the criterion of low muscle mass with sarcopenia. In this review, therefore, it was proposed that, although there are no official recommendations, it seems reasonable to perform nutritional screening and evaluate sarcopenia when there are signs of low strength (dynapenia) or low muscle mass. In the case of hip fracture, and after the results of this study, it could be argued that the two go hand-in-hand.
In 2021, Ballesteros-Pomar et al. published a study on DRM and sarcopenia in patients admitted to an Internal Medicine department in a Spanish hospital.37 The differences with this study on hip fracture lie in the fact that they used the MUST (Malnutrition Universal Screening Tool) for nutritional risk screening. For the functional assessment they chose the Katz index, which is less sensitive to changes and in the detection of slight dependency and need for support than the Barthel index, and for comorbidity, the Charlson index. In common with this study, sarcopenia screening was conducted with the SARC-F following EWGSOP2 recommendations, and DRM and sarcopenia were diagnosed with the same criteria (GLIM and EWGSOP). Their objectives were: to determine the prevalence and impact of hospitalisation on the quantity of muscle mass and strength and how it influenced patient outcomes (death, hospital stay, readmission at three months and quality of life); variables that have not been analysed in this study. These were subjects with a younger mean age (75.4 years in the overall sample) than the patients with fragility hip fracture, from a medical department, as opposed to a surgical one. A high prevalence of sarcopenia (33% on admission) and DRM (27.5% according to GLIM criteria) and of both combined (10.5%) was also found. As in the Meyer review, worse outcomes (higher mortality, readmission and poorer quality of life) were observed, which were associated with low hand grip strength. However, this relationship with the quantity of muscle was not found, which would support the importance of the concept of dynapenia.6 On the other hand, they demonstrated that sarcopenic patients are older and have greater comorbidity (burden of disease), as is the case in our sample. Although the prevalence is high, it is still lower than that found in patients with hip fracture, which would reinforce the idea that we can speak of osteosarcopenia.
It is striking that, despite 19% of patients with previous fragility fractures, none had been prescribed treatment with vitamin D, with or without associated calcium, or antiresorptive or bone-building treatment. As a result of this study, we believe that it is necessary to promote detection of osteoporosis in Primary Care and foster the establishment of Fracture Liaison Services (FLS), where all patients who suffer from a first osteoporotic fracture are assessed: mainly wrist, humerus and vertebral compression, but any low-energy trauma fractures would be assessed and treated, if appropriate.
The findings of this study emphasise the importance of including nutritional and sarcopenia screening as part of the comprehensive assessment of all patients admitted after a fragility hip fracture, as well as establishing treatment protocols for these two syndromes and osteoporosis.
As a consequence of these results, at our centre, all patients with hip fracture are screened using MNA and SARC-F and their hand grip strength is measured, with an individualised care plan established during admission and discharge. Moreover, we have begun to treat osteoporosis more and better, and to raise awareness of the importance of the first fracture and assessment of the bone health of these patients, in order to initiate treatments and prevent new fragility fractures from occurring.
The strengths of this study include the sample size and the variables collected in a group of patients who are not usually studied so thoroughly from a nutritional point of view during admission. Although it was not one of the objectives of the study, it is important to point out a lower mean length of hospital stay (7.05 days) and a shorter surgical delay (58.93 h) than that published in the RNFC (Registro Nacional de Fracturas de Cadera [Spanish National Registry of Hip Fractures]) (9.8 days/64.6 h, respectively), as well as a slightly higher number of patients (49.9% vs 48.1%) undergoing surgery in the first 48 h.38
The main limitations of the study were the fact that due to the special situation of our patients, physical function was not measured, but only hand grip strength. This variable (measured by SPPB, Timed Up and Go, etc.), in accordance with the EWGSOP 2019 criteria, indicates the degree of severity, and could also explain the absence of differences in any of the points. Therefore, unlike for DRM, we could not establish different degrees of severity for sarcopenia. After reviewing previous publications, we also believe that it would have been more correct to speak of dynapenia than sarcopenia, although when dealing with patients with acute mobility limitations, functional assessment on admission is very limited. Applying the Barthel scale at discharge allowed us to determine acute functional loss caused by fracturing the hip even after undergoing repair surgery. Knowing the Barthel scale score when the patient left the hospital also made it possible to assess functional recovery in this period at the one-month check-up in the Orthogeriatric clinic.
Further studies are needed to determine the relationship between DRM in this type of patient and sarcopenia, or perhaps even better, to talk about dynapenia in the future, as well as the influence that these two geriatric syndromes have on the functional recovery of patients who suffer a hip fracture.
The need to detect and treat osteoporosis is also evident, especially after a first fragility fracture.
ConclusionsThe prevalence of DRM and sarcopenia in the patients was very high; higher than previously published in other studies. DRM affected more than 75% of the sample when taking into account low muscle mass, and up to three quarters of the patients were diagnosed with sarcopenia, the two conditions occurring simultaneously. This corroborates the significance of these two syndromes in patients with a fragility fracture.
Older patients, with greater functional dependency, higher disease burden and lower body mass index have a greater tendency towards worse muscle function as measured by hand grip strength (dynapenia), while loss of quantity of muscle was only associated with body mass index. This would support the idea that the quality (dynapenia) is more important than the quantity of muscle in patients with hip fracture.
Ethical considerationsThe research was carried out in accordance with the World Medical Association International Code of Medical Ethics (Declaration of Helsinki) and was approved by the Independent Ethics Committee of the Hospital Clínico de Valladolid (PI 17-653).
FundingNo funding of any kind was received.
Conflicts of interestThe authors declare that they have no conflicts of interest.