Intermediate Inborn Errors of Metabolism (IEM) are a group of inherited diseases that include phenylketonuria (PKU), tyrosinemia II (TSII), organic acidaemias and ornithine transcarbamylase deficiency (OTCD), among others. They are increasingly more common in adults due to improved management. This has allowed more affected women to consider having children with good prospects. However, pregnancy may worsen metabolic control and/or increase maternal-fetal complications. The objective is to analyse the characteristics and outcomes of pregnancies of our patients with IEM.
MethodsRetrospective descriptive study. Pregnancies of women with IEM attended to at the adult IEM referral unit of the Hospital Universitario Virgen del Rocío were included. The qualitative variables were described as n(%) and the quantitative as P50 (P25–P75).
Results24 pregnancies were recorded: 12 newborns were healthy, 1 inherited their mother’s disease, 2 had maternal phenylketonuria syndrome, 1 was stillborn (gestational week 31 + 5), 5 were spontaneous abortions and 3 were voluntarily terminated. The gestations were divided into metabolically controlled and uncontrolled.
ConclusionsPregnancy planning and multidisciplinary management through to postpartum is essential to ensure maternal and fetal health. The basis of treatment in PKU and TSII is a strict protein-limited diet. Events that increase protein catabolism in organic acidaemias and DOTC should be avoided. Further investigation of pregnancy outcomes in women with IEM is needed.
Los Errores Innatos del Metabolismo (EIM) intermediario son un conjunto de enfermedades hereditarias que incluye la fenilcetonuria (PKU), la tirosinemia II (TSII), las acidemias orgánicas y el déficit de ornitina transcarbamilasa (DOTC) entre otras. Son cada vez más frecuentes en adultos debido a las mejoras en su abordaje. Esto ha permitido que más mujeres afectas puedan plantearse deseos gestacionales con buenas perspectivas. Sin embargo, el embarazo puede empeorar el control metabólico y/o aumentar las complicaciones materno-fetales. El objetivo es analizar las características y resultados de los embarazos de nuestras pacientes con EIM.
MétodosEstudio descriptivo retrospectivo. Se incluyeron los embarazos de mujeres con EIM atendidas en la unidad de referencia de EIM de adultos del Hospital Universitario Virgen del Rocío. Las variables cualitativas se describieron como n(%); las cuantitativas como P50 (P25–P75).
ResultadosSe registraron 24 embarazos: 12 recién nacidos fueron sanos, 1 heredó la enfermedad materna, 2 presentaron el síndrome de fenilcetonuria materna, 1 nació muerto (semana de gestación 31 + 5), 5 fueron abortos espontáneos y 3 se interrumpieron voluntariamente. Se dividieron las gestaciones en metabólicamente controladas y no controladas.
ConclusionesEs fundamental la planificación del embarazo y su manejo multidisciplinar hasta el postparto para garantizar la salud materno-fetal. La base del tratamiento en la PKU y TSII es la dieta estricta limitada en proteínas. Deben evitarse eventos que aumenten el catabolismo proteico en las acidemias orgánicas y el DOTC. Se necesita una mayor investigación de los resultados de embarazos de mujeres con EIM.
Inborn errors of metabolism (IEM) are genetic diseases characterised by the defect of a protein (enzyme, transporter or cofactor) in which blockage of the metabolic pathway occurs, generating different consequences: substrate accumulation, deficiency of the product or activation of alternative pathways.1 There are currently more than 1050 known disorders, with an estimated global prevalence of 50.9/100,000 births.2,3 The development of neonatal screening programmes (in Spain since 1968 for phenylketonuria [PKU] and since 2000 for the rest) and the subsequent application of tandem mass spectrometry with which up to seven studies of different IEM can be performed, have led to enormous advances in their early detection4 and a significant improvement in the prognosis and quality of life of these patients, enabling their transition to adulthood in good health.5
This has made it easier for more and more women with these diseases to consider pregnancy. However, this can be associated with worsening metabolic control and an increase in complications and maternal risk.6 In addition, there may be difficulty conceiving due to the effect of the disease on the gonads and some disorders are associated with significant neurological or physical disability, limiting the ability of these patients to socialise.6 There may also be fetal complications due to malnutrition or the transplacental passage of teratogenic toxic substances5 such as phenylalanine, which can cause maternal phenylketonuria (mPKU) syndrome,7 characterised by intellectual disability, microcephaly, congenital heart disease and intrauterine growth retardation (IUGR), among other complications; or tyrosine, which may have adverse effects on fetal neurodevelopment. The safety of IEM treatments in pregnancy is also not known.6 In general, given the low frequency of these disorders, there is little evidence and experience regarding the management and monitoring of pregnancy in these patients.
Currently, nutritional support is the most useful tool in the management of these diseases. Therein lies the importance of adult inborn errors of metabolism units, in cooperation with endocrinologists, nutritionists, geneticists, gynaecologists, obstetricians and paediatricians.1,8 This multidisciplinary approach is key to optimising the management of these women in family planning and the pregnancy itself. However, there are still very few IEM specialist centres in Spain. In 2012, the referral centre for all IEM in western Andalusia was created and accredited as a Reference Centre, Service or Unit (CSUR).
The objectives of this study were: to analyse the maternal-fetal complications and neonatal sequelae in relation to the treatment and degree of IEM control; to draw conclusions about metabolic monitoring and control during pregnancy, the postpartum phase and breast-feeding, and if possible, before conception; and to collect information that facilitates the counselling of future pregnant women with IEM.
MethodsA retrospective observational study was conducted that included pregnant women with IEM who were monitored in adult inborn errors of metabolism consultations by the Endocrinology and Nutrition Unit of the Hospital Universitario Virgen del Rocío. All included patients were informed about the characteristics and purpose of the study and gave informed consent. The study was approved by the centre's Ethics Committee.
The inclusion criteria were: (1) women older than 18 years of age capable of giving informed consent; (2) diagnosed with an IEM; (3) who were pregnant, even if it did not reach term. The exclusion criteria were the refusal of informed consent and any condition that, in the investigator's opinion, could pose an unacceptable risk for their participation in the study.
The variables analysed were those related to the pregnant woman (the IEM; age at diagnosis—neonatal, childhood or adult screening—; year of birth; anthropometric variables—weight, height and body mass index [BMI]—; cardiovascular risk factors and concomitant diseases; number of pregnancies; the woman's age at conception; elective termination of pregnancy (ETP) or otherwise; miscarriage or otherwise; week of gestation at delivery; mode of delivery—caesarean section, spontaneous vaginal delivery (SVD) or induced vaginal delivery (IVD)—; diseases during pregnancy and obstetric complications; admissions for metabolic decompensation; treatment compliance; control of Phe levels in PKU; levels in routine blood tests in other IEM; breastfeeding; and those related to the newborn (birth weight; outcome of the disease—healthy or sick—; existence and type of anomaly at birth; fetal complications during pregnancy). To assess the degree of compliance with the diet, a 24-h dietary record was performed at each visit. In addition, in the case of PKU, phenylalanine levels were checked weekly by dried blood spot testing. Adherence to the rest of the treatments was verified in each consultation as part of the clinical interview. The maintenance of Phe levels <6 mg/dl on average was considered good treatment compliance.
Statistical analysis was performed using the software SPSS® for Windows, version 26.0 (IBM Corp., Armonk, NY, United States of America). The descriptive analysis was performed by obtaining the median and quartiles of the quantitative variables (expressed as P50 [P25–P75]) and the frequency for the qualitative variables (expressed as n [%]).
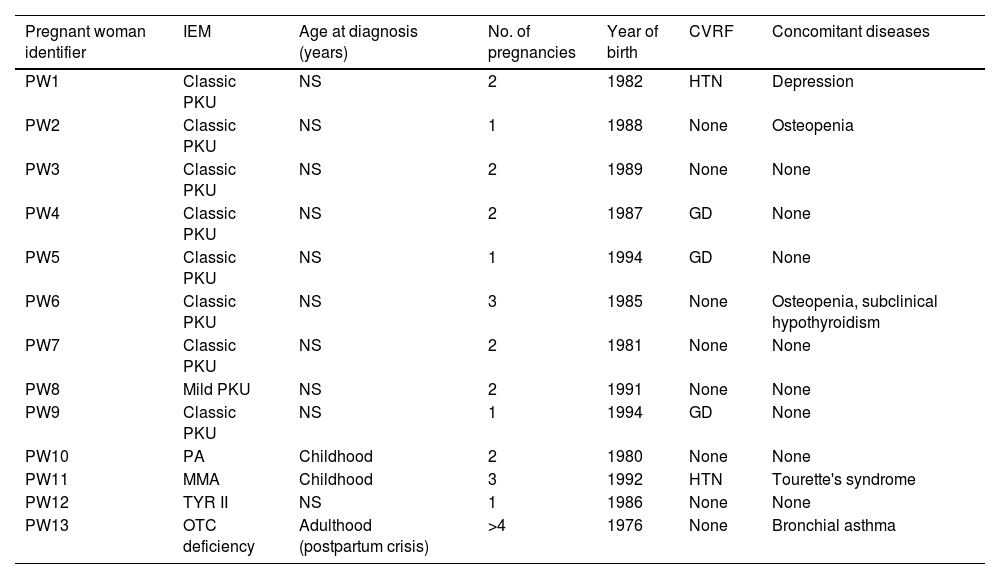
ResultsA total of 24 pregnancies in 13 patients were included (Table 1): 16 (66.6%) in mothers with PKU, two (8.3%) in mothers with ornithine transcarbamylase (OTC) deficiency, three (12.5%) in mothers with methylmalonic aciduria (MMA), two (8.3%) in mothers with propionic acidaemia (PA) and one (4.1%) in which the mother had tyrosinaemia type II (TYR II). Age at pregnancy was 26.5 (24.7–33) years; 12 (92.3%) patients had an IQ within the normal average and one (PKU, PW [pregnant woman] 4, P [pregnancy] 6 and P7) had an IQ of 80. Only one (7.6%) (OTC deficiency, PW13, P23 and P24) was diagnosed in adulthood after having an affected child, since this disease is not included in neonatal screening.
Main characteristics of the patients included in the study.
| Pregnant woman identifier | IEM | Age at diagnosis (years) | No. of pregnancies | Year of birth | CVRF | Concomitant diseases |
|---|---|---|---|---|---|---|
| PW1 | Classic PKU | NS | 2 | 1982 | HTN | Depression |
| PW2 | Classic PKU | NS | 1 | 1988 | None | Osteopenia |
| PW3 | Classic PKU | NS | 2 | 1989 | None | None |
| PW4 | Classic PKU | NS | 2 | 1987 | GD | None |
| PW5 | Classic PKU | NS | 1 | 1994 | GD | None |
| PW6 | Classic PKU | NS | 3 | 1985 | None | Osteopenia, subclinical hypothyroidism |
| PW7 | Classic PKU | NS | 2 | 1981 | None | None |
| PW8 | Mild PKU | NS | 2 | 1991 | None | None |
| PW9 | Classic PKU | NS | 1 | 1994 | GD | None |
| PW10 | PA | Childhood | 2 | 1980 | None | None |
| PW11 | MMA | Childhood | 3 | 1992 | HTN | Tourette's syndrome |
| PW12 | TYR II | NS | 1 | 1986 | None | None |
| PW13 | OTC deficiency | Adulthood (postpartum crisis) | >4 | 1976 | None | Bronchial asthma |
CVRF: cardiovascular risk factors; GD: gestational diabetes; HTN: arterial hypertension; NS: neonatal screening.
Of the 24 pregnancies (Table 2), 12 (50%) resulted in healthy newborns, one (4%) was born with the maternal disease (OTC deficiency, PW13, P23), two (8%) were born with mPKU syndrome (PKU, PW4, P6 and P7), five (20%) resulted in spontaneous abortions and three (12%) underwent ETP.
General characteristics of the patients and their pregnancies.
| Pregnant woman identifier | Disease | Pregnancy identifier | Maternal BMI | Planned pregnancy | Maternal age at conception (years) | Good treatment control during pregnancy | Fetal outcome |
|---|---|---|---|---|---|---|---|
| PW1 | Classic PKU | P1 | 27.4 | No | 19 | * | Healthy |
| P2 | 32.8 | No | 33 | * | ETP | ||
| PW2 | Classic PKU | P3 | 20 | No | 26 | No | ETP |
| PW3 | Classic PKU | P4 | * | No | * | * | Miscarriage |
| P5 | 16.6 | Yes | 30 | Yes | Healthy | ||
| PW4 | Classic PKU | P6 | 37 | No | 21 | No | mPKU syndrome |
| P7 | 37.5 | No | 27 | No | mPKU syndrome | ||
| PW5 | Classic PKU | P8 | 35.3 | No | 25 | Yes | Stillborn |
| PW6 | Classic PKU | P9 | 23.4 | Yes | 33 | Yes | Miscarriage |
| P10 | 24.6 | Yes | 34 | * | Miscarriage | ||
| P11 | 27.1 | No | 35 | Yes | Healthy | ||
| PW7 | Classic PKU | P12 | 21.4 | No | 32 | Yes | Healthy |
| P13 | 23.7 | Yes | 37 | Yes | Healthy | ||
| PW8 | Benign PKU | P14 | 27.4 | No | 26 | * | Miscarriage |
| P15 | 29 | No | 26 | Yes | Healthy | ||
| PW9 | Classic PKU | P16 | 27.5 | No | 24 | Yes | Healthy |
| PW10 | PA | P17 | 35 | Yes | 26 | Yes | Healthy |
| P18 | 35 | Yes | 29 | Yes | Healthy | ||
| PW11 | MMA | P19 | 29.2 | No | 17 | No | Healthy |
| P20 | 23.3 | No | 19 | No | ETP | ||
| P21 | 28.3 | Yes | 25 | Yes | Healthy | ||
| PW12 | TYR II | P22 | 25 | Yes | 35 | Yes | Healthy |
| PW13 | OTC deficiency | P23 | * | * | 31 | * | OTC deficiency |
| P24 | * | * | * | * | Miscarriage |
This group included a total of 16 pregnancies in nine women. The median age was 27 (25–33) years. Only four (25%) were planned and two of them resulted in spontaneous abortion (PKU, PW6, P9 and P10). Median BMI was 27.4 (23.4–32.8) kg/m2. Phe and Tyr measurements during pregnancy were collected with dried blood spots on filter paper (Table 3); eight (50%) maintained Phe levels within the established target range (mean <6 mg/dl). Data for these levels were not found in the medical records of two pregnancies (12.5%) (PKU, PW1, P1 and P2).
Phenylalanine (mg/dl) (Phe) and tyrosine(μmol/l) (Tyr) levels collected monthly during pregnancy from patients with PKU.
| PI | 1στ M | 2νδ M | 3ρδ M | 4τη M | 5τη M | 6τη M | 7τη M | 8τη M | GTC | FO |
|---|---|---|---|---|---|---|---|---|---|---|
| P1 | * | * | * | * | * | * | * | * | * | H |
| P2 | * | * | * | * | * | * | * | * | * | ETP |
| P3 | Phe: 17.3 | Phe: 17.4 | Phe: 27.4 | Phe: 16.4 | Phe: 17.5 | * | * | * | No | ETP |
| Tyr: 33 | Tyr: 42 | Tyr: 47 | Tyr: 36.6 | Tyr: 26 | ||||||
| P4 | * | * | * | MIS | ||||||
| P5 | Phe: 5.1 | Phe: 5.2 | Phe: 1.5 | Phe: 1.8 | Phe: 1.2 | Phe: 0.7 | Phe: 0.7 | Phe: 7.3 | Yes | H |
| Tyr: 63 | Tyr: 43 | Tyr: 89 | Tyr: 88 | Tyr: 96 | Tyr: 35 | Tyr: 27.4 | Tyr: 57.6 | |||
| P6 | Phe: 16.5 | * | * | * | Phe: 7.9 | Phe: 13.5 | Phe: 12.7 | Phe: 10.9 | No | SIC |
| Tyr: * | Tyr: * | Tyr: * | Tyr: * | Tyr: * | ||||||
| P7 | Phe: 16.3 | Phe: 11.2 | Phe: 11.1 | Phe: 12.3 | Phe: 18.7 | Phe: 13.5 | Phe: 14.1 | Phe: 19.1 | No | SIC |
| Tyr: 97 | Tyr: 22 | Tyr: * | Tyr: * | Tyr: 69 | Tyr: * | Tyr: 42 | Tyr: 58 | |||
| P8 | * | Phe: 8.0 | Phe: 5.5 | Phe: 4.4 | Phe: 3.2 | * | Phe: 2.6 | * | Yes | SB |
| Tyr: 88.8 | Tyr: 33.2 | Tyr: 28.1 | Tyr: 36 | Tyr: 28.7 | ||||||
| P9 | Phe: 5.1 | Phe: 4.8 | Yes | MIS | ||||||
| Tyr: 80 | Tyr: 30 | |||||||||
| P10 | Phe: 6.4 | * | * | MIS | ||||||
| Tyr: * | ||||||||||
| P11 | Phe: 5.9 | Phe: 6.9 | Phe: 3.2 | Phe: 1.8 | Phe: 2.4 | Phe: 1.4 | Phe: 2.1 | Phe: * | Yes | H |
| Tyr: 63.8 | Tyr: 33.3 | Tyr: 71 | Tyr: 70.8 | Tyr: 65 | Tyr: 46 | Tyr: 55.6 | Tyr: 55 | |||
| P12 | Phe: 3.2 | Phe: 3.2 | Phe: 3.9 | Phe: 3.04 | Phe: 4.7 | Phe: 3.2 | Phe: 1.9 | Phe: 3.8 | Yes | H |
| Tyr: 40 | Tyr: 36 | Tyr: * | Tyr: 46 | Tyr: 37 | Tyr: 32 | Tyr: 25 | Tyr: 30 | |||
| P13 | Phe: 3.3 | Phe: 2.2 | Phe: 3.1 | Phe: 3.2 | * | * | Phe: 1.6 | Phe: 1.8 | Yes | H |
| Tyr: 51 | Tyr: 27.5 | Tyr: 45 | Tyr: 36 | Tyr: 32 | Tyr: * | |||||
| P14 | Phe: 2.4 | * | MIS | |||||||
| Tyr: 35 | ||||||||||
| P15 | Phe: 3.3 | Phe: 3.4 | Phe: 2.16 | * | Phe: 1.8 | Phe: 1.83 | Phe: 1.4 | Phe: 1.7 | Yes | H |
| Tyr: * | Tyr: * | Tyr: * | Tyr: * | Tyr: * | Tyr: * | Tyr: * | ||||
| P16 | Phe: 8.2 | Phe: 6.9 | Phe: 4.2 | Phe: 6.09 | Phe: 5.6 | Phe: 4.06 | Phe: 4.5 | Phe: 5.2 | Yes | H |
| Tyr: 139 | Tyr: 31 | Tyr: 40 | Tyr: 81 | Tyr: 34 | Tyr: 44 | Tyr: 27 | Tyr: 43 |
FO: fetal outcome; GTC: good treatment control during pregnancy; H: healthy; M: month; MIS: miscarriage; P: phenylalanine (Phe); PI: pregnancy identifier; SB: stillborn; SIC: sick; T: tyrosine (Tyr).
Regarding treatment during pregnancy, diets and oral nutritional supplements without phenylalanine were prescribed in all pregnancies. Sapropterin was prescribed in three pregnancies (23.1%). Other prescribed treatments were iron in three pregnancies (18.8%), folic acid supplements in 11 (68.7%), vitamin D and vitamin B12 in nine (56.2%), insulin in four (25%), levothyroxine in three (18.8%) and antiemetics in one pregnancy (6.2%).
Regarding pregnancy planning, the differences between planned and unplanned pregnancies are shown in Table 4.
Comparison of neonatal complications and sequelae between planned and unplanned pregnancies.
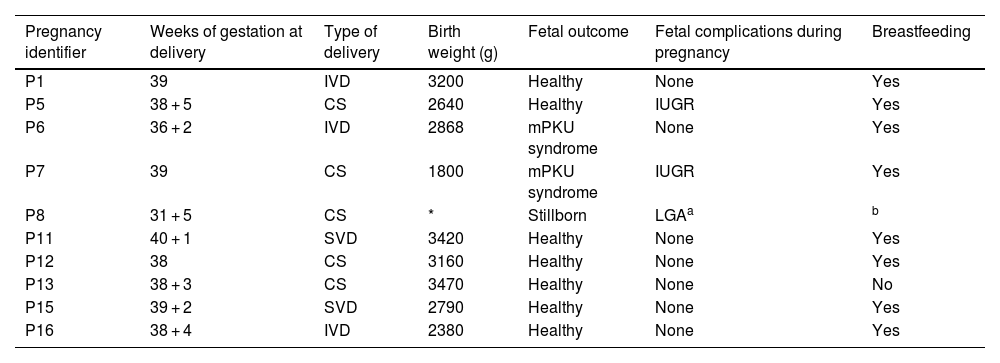
Of the 16 pregnancies, nine (56.3%) resulted in live births; one foetus (6.3%) was stillborn by immediate caesarean section due to a ruptured splenic artery aneurysm in the mother causing internal bleeding at 31 + 5 weeks of gestation (PKU, PW5, P8). Of the remaining six pregnancies, four (25%) resulted in first-trimester miscarriages and two pregnancies (12.5%) were terminated voluntarily due to the high probability of mPKU syndrome (PKU, PW1, P3; and PKU, PW2, P3). The median number of weeks of gestation at delivery was 38 (37.5–39) (Table 5).
Characteristics of babies born to mothers with PKU.
| Pregnancy identifier | Weeks of gestation at delivery | Type of delivery | Birth weight (g) | Fetal outcome | Fetal complications during pregnancy | Breastfeeding |
|---|---|---|---|---|---|---|
| P1 | 39 | IVD | 3200 | Healthy | None | Yes |
| P5 | 38 + 5 | CS | 2640 | Healthy | IUGR | Yes |
| P6 | 36 + 2 | IVD | 2868 | mPKU syndrome | None | Yes |
| P7 | 39 | CS | 1800 | mPKU syndrome | IUGR | Yes |
| P8 | 31 + 5 | CS | * | Stillborn | LGAa | b |
| P11 | 40 + 1 | SVD | 3420 | Healthy | None | Yes |
| P12 | 38 | CS | 3160 | Healthy | None | Yes |
| P13 | 38 + 3 | CS | 3470 | Healthy | None | No |
| P15 | 39 + 2 | SVD | 2790 | Healthy | None | Yes |
| P16 | 38 + 4 | IVD | 2380 | Healthy | None | Yes |
LGA: foetus large for gestational age.
Two live births (22.2%) (PKU, PW4, P6 and P7), both from the same pregnant woman, with poor control during both pregnancies, manifested mPKU syndrome and one of them was diagnosed with PKU by neonatal screening. The newborn resulting from pregnancy P6 required admission due to presenting with obstetric brachial plexus palsy at birth, anomalies in the head (microcephaly and cephalohaematoma), nose (broad nasal bridge), central nervous system (hyperreflexia and clonus after minimal stimulation and asymmetrical Moro reflex), extremities (scarce spontaneous movements of the left upper limb and hypertonia of the lower limbs), eyes (epicanthal folds) and heart (patent foramen ovale-type atrial septal defect); and psychomotor retardation with intellectual disability. The newborn from pregnancy P7 presented with an Apgar score of 5/8 at birth. The baby also manifested growth failure, anomalies in the head (microcephaly and cephalohaematoma), facies (broad nasal bridge, low-set ears and thin upper lip), central nervous system (asymmetrical Moro reflex, focal seizures, intellectual disability) and eye (hyperopia and myopia).
Maternal complications during pregnancy arose in eight women (50%) (one case of pregnancy pruritus, three cases of emetic syndrome, one polymorphic eruption of pregnancy, one case of normochromic normocytic anaemia, one case of threatened preterm labour and one case of mild polyhydramnios and splenic artery rupture). Three (18.8%) manifested obstetric complications (all of them perineal laceration). In one of the pregnancies (PKU, PW4, P7), the pregnant woman (who had poor control during pregnancy) required hospital admission due to high phenylalanine levels and low tyrosine levels, requiring nutrition through a nasogastric tube.
Eight live newborns (88.9%) were breastfed. In one of them (PKU, PW7, P13) the mother did not breastfeed, since she was receiving treatment with sapropterin and she preferred not to do so after being informed that its safety during breastfeeding had not been proven. This pregnant woman did breastfeed her first newborn (PKU, PW7, P12), deciding after delivery to suspend this treatment to do so.
Pregnancies with other IEMThis group included eight pregnancies in four women (Table 6). Information could only be obtained from six pregnancies, since those of PW13 (P23 and P24) with OTC deficiency were followed up at another centre. The only data obtained was that the pregnant woman had never had metabolic decompensation until the delivery of her first child, in which she suffered a hyperammonaemic coma, with OTC deficiency diagnosed in mother and child.
Characteristics of the outcomes of pregnancies of mothers with IEM other than PKU.
| Pregnancy identifier | IEM | WG at end of pregnancy | Mode of delivery or termination | Birth weight (g) | Fetal outcome | Fetal complications during pregnancy | Breastfeeding |
|---|---|---|---|---|---|---|---|
| P17 | PA | 39 | IVD | 2944 | Healthy | None | No |
| P18 | PA | 38 | SVD | 3470 | Healthy | None | Yes |
| P19 | MMA | 35 + 4 | CS | 2160 | Healthy | IUGR | Yes |
| P20 | MMA | a | ETP | a | a | a | a |
| P21 | MMA | 36 + 4 | CS | 1795 | Healthy | None | Yes |
| P22 | TYR II | 39 + 6 | IVD | 3690 | Healthy | LGA | Yes |
| P23 | OTC deficiency | * | * | * | OTC deficiency (male) | Brain damage | No |
| P24 | OTC deficiency | a | MIS | a | a | a | a |
CS: caesarean section; ETP: elective termination of pregnancy; IVD: induced vaginal delivery; LGA: foetus large for gestational age; MIS: miscarriage; SVD: spontaneous vaginal delivery; WG: weeks of gestation.
Four pregnancies (66.6%) were planned, and of the total eight pregnancies, six (75%) resulted in live births. One pregnancy (12.5%) (MMA, PW11, P20) was voluntarily terminated and the remaining pregnancy (OTC deficiency, PW13, P24) resulted in spontaneous abortion. Only one newborn (OTC deficiency, PW13, P23) manifested IEM at birth (OTC deficiency). A liver transplant was proposed, but it was denied due to the development of irreversible brain damage. The baby finally died at eight months of age.
Regarding maternal complications, in P23 (OTC deficiency, PW13) the pregnant woman suffered hyperammonaemic decompensation triggered by the delivery that required hospital admission, with no further information about this available. This pregnant woman also had an emetic syndrome. The pregnant woman with MMA (PW11) during her second pregnancy (P20) underwent ETP due to poor metabolic control secondary to hyperemesis together with the appearance of choreoathetosis secondary to lesions at the basal ganglia (suspicion of metabolic stroke), which has now evolved into Tourette's syndrome. In her first and third pregnancy (PW11, P19 and P21), this patient maintained adequate renal function and normal ammonia values during pregnancy and the postpartum period.
The pregnancy of the patient with TYR II (PW12, P22) was planned and she exhibited tyrosine and phenylalanine levels within normal range (40−72 μmol/l and 40−64 μmol/l, respectively) during almost the entire pregnancy.
The patient with PA (PW10) had two pregnancies (P17 and P18), in which very high levels of propionylcarnitine (C3) and glycine were detected, with normal values of free carnitine (C0) or values at the lower limit of normal, for which high doses of carnitine were required. The rest of the amino acids in blood remained within normal ranges (NR). In the first pregnancy, ammonia levels were collected during the prepartum period, in the immediate postpartum period and in subsequent days, and were 36.9 µm/l, 101.6 μm/l and 38.6 μm/l, respectively (NR = 11–51). In the second pregnancy, the ammonia level in the immediate postpartum period was 28.4 μ m/l and during this period the pregnant woman presented with elevated levels of glycine, alanine, glutamine, arginine and lysine, and undetectable levels of isoleucine and methionine. During the second pregnancy, she exhibited decreases in methionine, tyrosine, leucine, isoleucine and valine.
DiscussionAlthough pregnancy can worsen the health of a woman with IEM, many women are capable of having pregnancies with excellent outcomes if planned well and if the disease is well managed throughout the pregnancy. Our study is a good example of this.
Pregnancy and PKUThe high number of pregnancies in women with PKU compared to other IEM is due to the fact that it is the most prevalent abnormality in the group of diseases related to amino acid degradation.9
In these women, in addition to genetic counselling, early education on pregnancy planning is essential in all cases, and when they occur they should be considered high-risk pregnancies. In our study, pregnancy planning was achieved in 25% of cases (four cases), with two spontaneous abortions and two pregnancies with excellent maternal-fetal outcomes. Following the current scientific evidence, we recommend that our patients maintain Phe levels <4 mg/dl from pregnancy planning onwards, since good management before and during pregnancy is essential to avoid mPKU syndrome.7,10 Although the risk of a foetopathy developing is considerably reduced when the mother maintains blood phenylalanine levels below 360 mmol/l (6 mg/dl) throughout the pregnancy,10 levels below 4 mg/dl are recommended to avoid Phe peaks.9 In accordance with the recommendations of the European guidelines for the management of phenylketonuria,11 we recommend that contraceptive strategies should only be discontinued after stable Phe levels within the target range have been achieved for a minimum of two weeks. This can become complicated, since adherence to treatment and follow-up in PKU tends to decrease after ten years.12 Our patients were adults with a median age at conception of 27 years and only 50% maintained Phe levels in the target range. Another pre-pregnancy factor associated with a lower risk of mPKU syndrome is normal maternal intelligence.12 All the patients included in this study were diagnosed through neonatal screening, which ensured they received appropriate early treatment and therefore a normal IQ, except in one case (PKU, PW4, P6 and P7), whose IQ was 80 and who had poor phenylalanine control during pregnancy.
During pregnancy, the mainstay of treatment is a low-phenylalanine diet. A daily intake >70 g of protein equivalents must be ensured12 by supplementing with phenylalanine-free formulas enriched with tyrosine and other vitamins and minerals. We recommend our pregnant women consume >2000 kcal/day, with a gain of 11−16 kg, although these aspects should be personalised. Excess weight is increasingly common in patients with PKU, mainly as a result of a sedentary lifestyle and poor nutrition, with a diet rich in carbohydrates and low in fruit and vegetables.13,14 This can lead to gestational diabetes, making dietary treatment even more difficult. It is therefore essential to emphasise the importance of early identification of nutritional disorders in these patients.
Depending on maternal Phe levels, protein restriction should be strict at the beginning, increasing its intake during the second and third trimesters, since, from week 26, if the foetus does not suffer from PKU it is capable of metabolising maternal phenylalanine.15,16 The guidelines recommend Phe monitoring once or twice a week.10 We carry out weekly Phe and Tyr dried blood spot testing on filter paper and at least monthly visits, more often if necessary, and we combine them with tele-consultations to facilitate follow-up and nutritional management. Nevertheless, the frequency was lower in our study. In addition to the weekly phenylalanine measurement, a 24-h dietary record was taken at each visit. Regarding adherence to the rest of the treatment, this was checked at each consultation as part of the clinical interview with the patient, although other indirect methods could be used, such as questionnaires. Hospital admission should also be considered when necessary, like our PW4 (PKU, P6 and P7), who required admission due to poor metabolic control at week 10 + 4 of pregnancy, her newborn developing mPKU syndrome. In the postpartum period, patients should be closely monitored for at least four weeks and every six to 12 months thereafter, with a low-protein diet due to its multiple benefits on mood and cognitive abilities,12 improving parenting ability. Breastfeeding is safe,8 since the healthy child can metabolise the phenylalanine in breast milk.
Regarding neonatal sequelae, following the reported pattern, the appearance of mPKU syndrome occurred in unplanned and uncontrolled pregnancies (PKU, PW4, P6 and P7). In contrast to other research studies, we identified eye abnormalities in P7. In P6, congenital heart disease was diagnosed, which could be due to the poor metabolic control of the pregnant woman, especially during weeks eight to 10, when the fetal heart develops. This pregnant woman also did not take vitamin B12, the deficiency of which is associated with an increased risk of congenital heart disease.17
On the other hand, it is likely that protein restriction influences the growth patterns of offspring. A low protein intake and/or with phenylalanine levels <120 μmol/l (1.98 mg/dl) has been associated with IUGR.18 Only two newborns suffered from IUGR: one in the context of mPKU syndrome, while the other (PKU, PW3, P5) weighed 2640 g at birth and its mother was underweight and manifested Phe values <1.98 mg/dl between weeks 10–32.
Sapropterin (pregnancy category C) was used in three pregnancies. Two had a normal fetal outcome and in the other pregnancy (PKU, PW5, P8), the foetus was stillborn due to a maternal complication at the end of pregnancy (splenic artery aneurysm rupture).
Pregnancy and other IEMThere are currently few published cases of pregnancies in the other IEM due to their lower frequency. As in PKU, pregnancy planning is important. In addition to the woman who was diagnosed with IEM after delivery (OTC deficiency, PW13, P23 and P24), and therefore could not plan her pregnancy, only two pregnancies were not planned, both in the same woman. In the first, the foetus (P23) developed IUGR and in the second (P24) it was voluntarily terminated. Our recommendations in planning have been to optimise the prior maternal condition, as well as dietary management that ensures adequate nutrition. In addition, it was recommended to measure blood pH, vitamin B12 and ammonia on a monthly basis.8
In these diseases, genetic counselling and prenatal genetic tests are also essential to facilitate the control of possible hyperammonaemia and its postpartum complications or to allow ETP in high-risk cases, as occurred in pregnancy P20 (MMA, PW11, P20).
Regarding organic acidaemia and OTC deficiency, there are three scenarios in which there is a high risk of metabolic decompensation: when the pregnant woman has not been diagnosed with the disease, when the pregnant woman has hyperemesis gravidarum and in the postpartum period.9,16
Regarding situations in which the pregnant woman had not been diagnosed, the example in our series is the mother of the newborn with OTC deficiency (PW13, P23), who suffered a hyperammonaemic coma in the immediate postpartum period, which led to the diagnosis of her disease. The diagnosis of late forms could be facilitated by a greater awareness of the non-specific symptoms of this disease. During pregnancy, the complications of hyperammonaemia can be masked by more common problems such as vomiting, nausea, headaches, mood swings and seizures, which are often attributed to hormonal changes.19 Hence the importance of preparing a detailed clinical history of the pregnant woman, preferably before conception. Similarly, ammonia concentration should be measured in encephalopathic patients or neuropsychiatric patients not diagnosed with OTC deficiency in the immediate postpartum period.
For its part, hyperemesis gravidarum can be both a cause and a consequence of hyperammonaemia.6,19 In our study, three pregnant women suffered from emetic syndrome during their pregnancies, although none of them was administered antiemetics to stop possible decompensation, which ultimately did not occur. Hyperemesis gravidarum was diagnosed in one of the women (MMA, PW11, P21), although she did not suffer from any acute hyperammonaemic state during or after pregnancy. It is also important to monitor the digestive tolerance of pregnant women with organic acidaemia to supplementation with carnitine and cofactors such as hydroxocobalamin and biotin, which in our experience has been excellent.
Regarding the increased risk of metabolic decompensation in the immediate postpartum period, this is a result of the catabolic state due to the involution of the uterus between days three to eight postpartum, as well as events that can increase catabolic stress (caesarean section, birth trauma, infection and/or a blood transfusion).20 Therefore, in the postpartum period, supplementary calories should be provided.20
This should be discussed on an individual basis depending on how the pregnancy ended. In our study there were no differences in terms of maternal-fetal complications in relation to this. There are studies that defend elective caesarean delivery with 10% dextrose infusion in patients with MMA15 and, in our study, the patient with MMA (PW11, P19 and P21) had two caesarean sections without complications, which could support this idea. In our experience, it is recommended to assess blood ammonia during labour and the immediate postpartum period every six to 12 h and up to 72 h postpartum, as well as to maintain intravenous fluids with dextrose to compensate for the catabolism produced.
ConclusionsMultidisciplinary management is important in women with IEM. Pregnancy planning should always be recommended and prior genetic counselling performed. Despite its importance, planning, management and follow-up during pregnancy and in the postpartum period of patients with IEM currently poses a significant challenge. Our study confirms that pregnancy planning in PKU patients improves maternal-fetal outcomes.
Ultrasound fetal monitoring should be used for anatomical study in conjunction with growth assessments to detect fetal complications in pregnant women with IEM. Strict dietary adherence is required for women with PKU and TYR II, ensuring good nutrition so that their levels remain within the safe range to prevent fetal abnormalities. In pregnant women with OTC deficiency and organic acidaemia, events that increase catabolism should be avoided due to the risk of developing hyperammonaemic states during pregnancy or delivery.
The forming of networks between centres with experience in IEM is required in order to provide the data and results of a significant number of patients and thereby improve the protocols and follow-up of these diseases.
FundingThis study did not receive any type of funding.
Conflicts of interestThe authors declare that they have no conflicts of interest.