Sarcoidosis is a chronic granulomatous inflammatory process of unknown etiology that can develop in any organ of the body1. Gastrointestinal involvement occurs in less than 1% of patients diagnosed with sarcoidosis, and pancreatic involvement is unusual2. The symptoms of pancreatic invasion can be confused with episodes of pancreatitis or pancreatic carcinoma, and its preoperative diagnosis is complicated1,2. We present the case of a pancreatic mass with no clear preoperative diagnosis and a final result of pancreatic sarcoidosis.
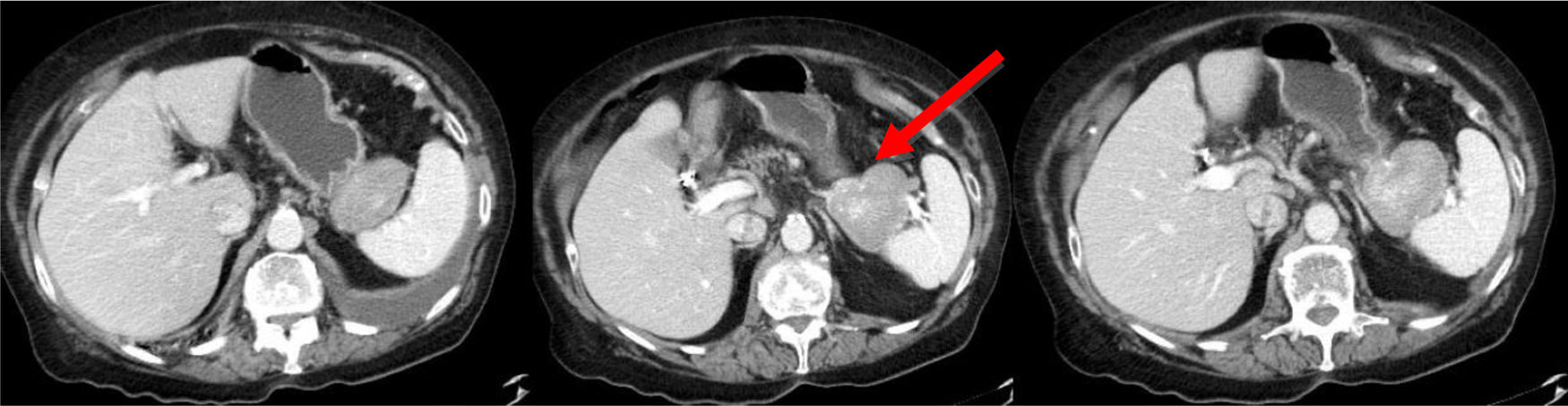
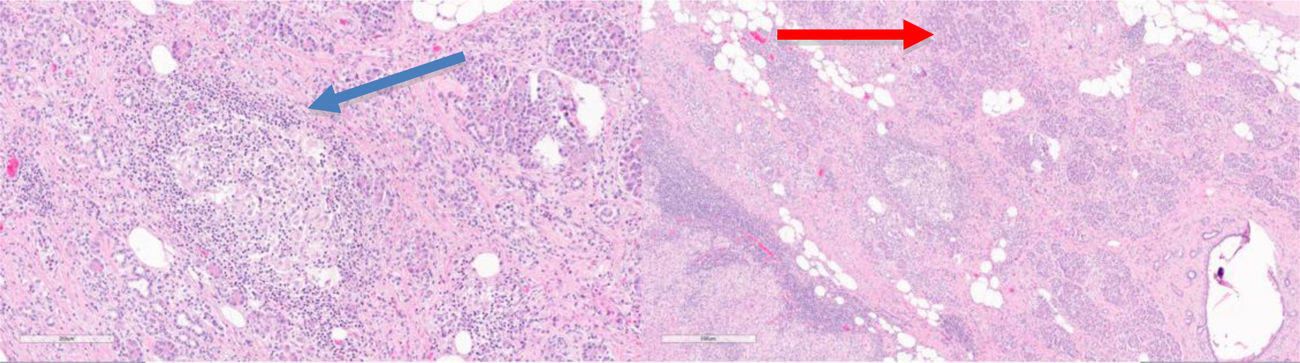
The patient is a 78-year-old woman with an allergy to non-steroidal anti-inflammatory drugs and a history of hypertension, type 2 diabetes mellitus and atrial fibrillation. She reported increasing abdominal pain over the previous 3 months located in the epigastrium and left hypochondrium, which did not subside with habitual analgesia and was associated with anorexia, nausea, vomiting and a weight loss of 4 kg. Lab work-up showed no relevant alterations. Thoracoabdominal CT scan revealed a 60 mm solid vascularized lesion in the portal phase between the greater curvature of the stomach and the splenic hilum, which it displaced without infiltrating. The lesion presented small satellite and periceliac lymphadenopathies. The findings indicated a possible stromal tumor (GIST) without ruling out association with the pancreas (Fig. 1). Endoscopic ultrasound-guided fine-needle aspiration provided evidence of lymphoid tissue with aggregates of epithelioid histiocytes, CD68+ and negative GIST markers (chronic inflammatory reaction with non-caseating histiocytic granulomas). Given the uncertain diagnosis, the patient’s symptoms and the impossibility to rule out malignancy, we decided to operate after presenting the case to a multidisciplinary committee. The initial laparoscopic approach was converted to a bilateral subcostal laparotomy due to hemodynamic instability and episodes of sustained ventricular tachycardia. We observed diffuse retroperitoneal granulomatous infiltrate and 3.5 L of ascites. Intraoperative peritoneal biopsies were negative for malignancy and reported as fibroconnective tissue with chronic inflammation, lymphoid and presence of granulomas with multinucleated giant cells. Distal pancreatectomy and splenectomy were performed. During postoperative ICU care, the patient required vasoactive drugs and amiodarone infusion due to an episode of atrial fibrillation. After favorable evolution, the patient was moved to the ward on the 3rd postoperative day and discharged on the 7th day without further incidents (Clavien-Dindo IVa). The histology study showed a smooth tumor measuring 60 × 50 mm dependent on the pancreas, with a focus of intratumoral calcification. Microscopically, the peripancreatic lymph nodes were observed to have granulomas rich in epithelioid cells, multinucleated giant cells, and chronic inflammation without necrosis. The morphology of the lesion indicated sarcoidosis (Fig. 2). The extending diagnostic tests of the histochemical study for acid-fast bacilli (Ziehl-Neelsen and Fite-Faraco) were both negative, which confirmed the final diagnosis of sarcoidosis. Subsequent follow-up visits were favorable, with no surgical or endocrine/metabolic complications.
Sarcoidosis is a chronic granulomatous disease of unknown cause characterized by epithelioid non-caseous granulomas with accumulation of mononuclear phagocytes and T-lymphocytes. It can appear systemically or with involvement of specific organs: lungs, lymph nodes, eyes, skin, nervous system and heart. In 1937, Nickerson published the first case of pancreatic involvement after the autopsy of a patient with systemic sarcoidosis3. Pancreatic localization of sarcoidosis is uncommon and estimated at 1%–4% of patients with systemic sarcoidosis and autopsy after death, with slightly higher figures in black women aged 50–70 years. Less than 50 cases with exclusively pancreatic involvement have been described in living patients4,5. Symptomatic pancreatic involvement is unusual and is usually due to either infiltration of the parenchyma or ductal involvement, causing clinical symptoms of acute pancreatitis, obstructive jaundice and hepatic biochemical alterations1,2,5. The presentation is varied, ranging from simple inflammatory processes or a nodular lesion to a pancreatic mass, which could sometimes mask a pancreatic carcinoma6. In more than 50%, they are located in the head of the pancreas in association with abdominal lymphadenopathies7.
Despite not having a definite preoperative histopathological diagnosis, abdominal CT scan, PET/CT or MRI, combined with ultrasound-guided FNA, help direct the diagnosis1,2,8. Possible analytical alterations include hyperbilirubinemia with a direct component, elevated amylase, lipase and hypercalcemia due to increased calcitriol levels in the macrophages of sarcoid granulomas and due to increased PTH-related proteins, which favors the conversion of vitamin D to active calcitriol9. Because of the differential diagnosis with pancreatic neoplasms, many patients are definitively diagnosed after surgery1,2. Noncaseating granulomas can be found in patients with malignant neoplasms like lymphomas and carcinomas, representing an immune reaction to a tumor antigen9.
Although there is controversy regarding treatment, long-term corticosteroid therapy is the treatment of choice as it results in biochemical improvement and reduced size of the tumor mass without improving fibrosis. The use of corticosteroids combined with ursodeoxycholic acid is recommended in symptomatic cases with marked cholestasis and/or high risk of developing liver complications10. Surgery is reserved for patients who present a significant intra-abdominal mass and involvement of critical organs, with associated symptoms and systemic repercussions1,2. Isolated histological involvement does not require the start of treatment, and asymptomatic patients can be observed.
Thus, although rare and infrequent, pancreatic sarcoidosis must be included in the differential diagnosis of a pancreatic mass. The evaluation of this type of exceptional cases in referral centers and by multidisciplinary committees can avoid errors in diagnosis and unnecessary treatments.
Please cite this article as: Cantalejo Díaz M, Palomares Cano A, Hörndler Algarate C, Ligorred Padilla LA, Serradilla Martín M. Presentación de sarcoidosis pancreática como masa infiltrativa retroperitoneal. Cir Esp. 2022;100:106–108.