Most evidence, including recent randomized controlled trials, analysing anal squamous cell carcinoma (SCC) do not consider immunocompromise patient population. The aim of this study was to compare clinical and oncological outcomes among immunocompetent and immunocompromised patients with anal squamous cell carcinoma.
MethodMulticentric retrospective comparative study including 2 cohorts of consecutive patients, immunocompetent and immunocompromised, diagnosed with anal SCC. This study evaluated clinical characteristics, clinical response to radical chemoradiotherapy (CRT) and long-term oncological results including both local and distant recurrence, overall survival (OS) and disease-free survival (DFS).
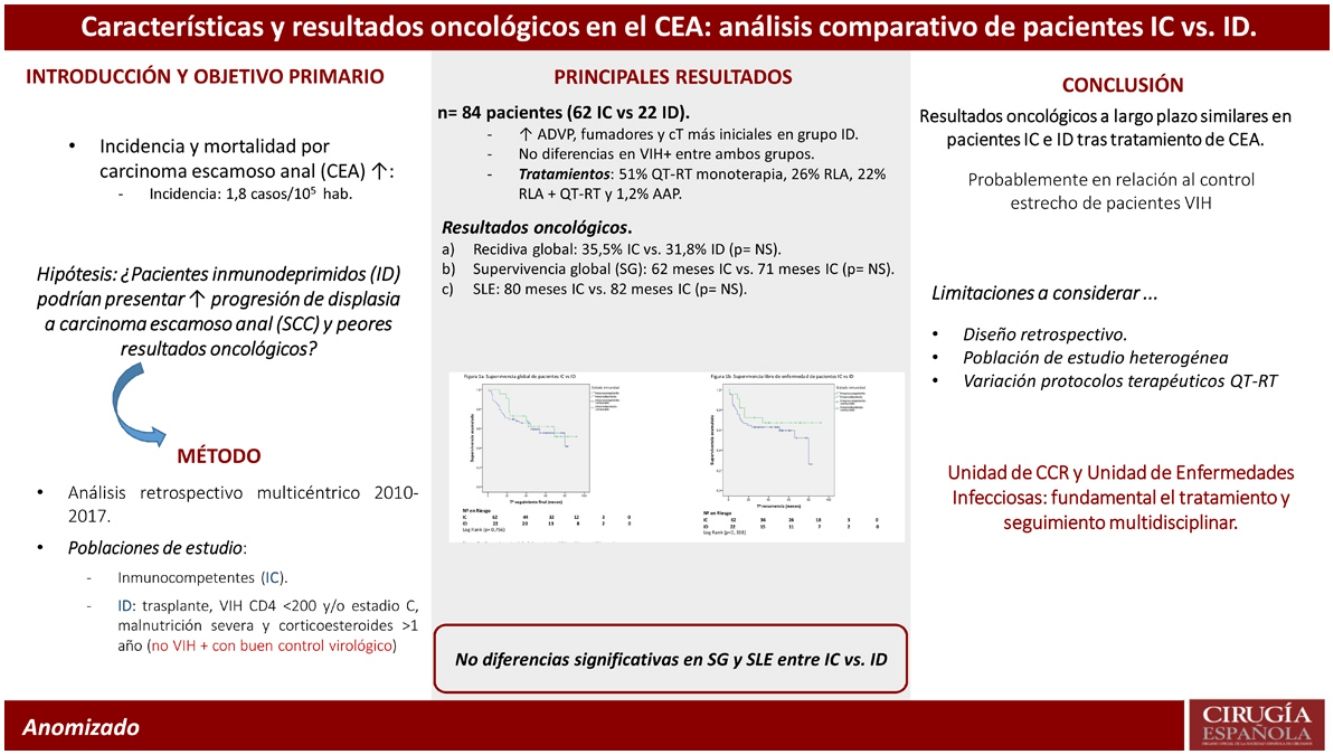
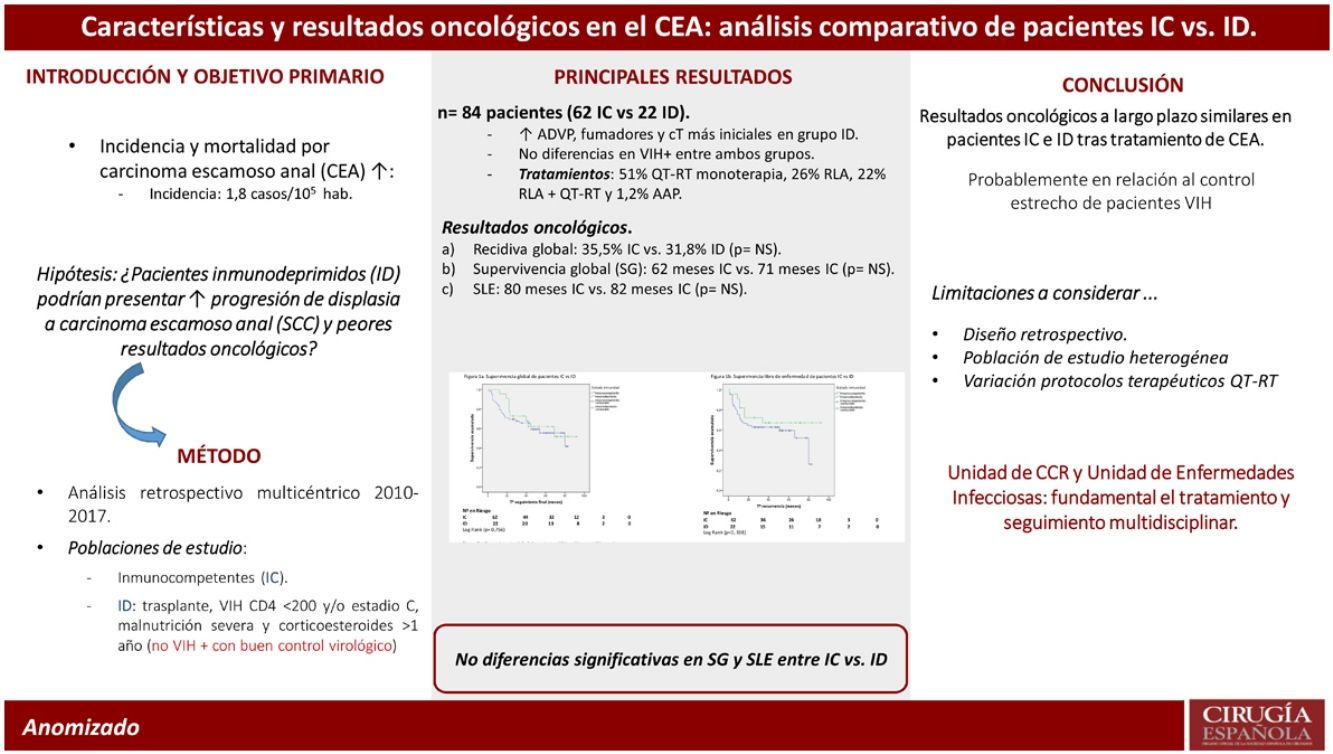
ResultsA total of 84 patients, 47 (55.6%) female, diagnosed with anal SCC from January 2012 to December 2017 were included, 22 (26%) and 62 (74%) patients in immunocompromised and immunocompetent groups respectively. Patients in immunocompromised group were significantly younger (53 vs. 61 years; P = 0.001), with smaller tumoral size (P = 0.044) and reported higher rates of substance abuse including tobacco use (P = 0.034) and parenteral drug consumption (P = 0.001). No differences were found in administered therapies (P = 301) neither in clinical response to chemoradiotherapy (83 vs. 100%). Moreover, similar 5-year OS (60 vs. 64%; P = 0.756) and DFS (65 vs. 68%; P = 0.338) were observed.
ConclusionThe present study shows no significant differences in long-term oncological results among immunocompetent and immunocompromised patients diagnosed with anal SCC, with a similar oncologic treatment. This evidence might be explained due to the close monitoring and adequate therapeutic control of HIV positive patients.
La mayoría de los ensayos clínicos realizados sobre pacientes con cáncer escamoso anal (CEA) excluyen pacientes inmunodeprimidos. El objetivo del presente estudio es comparar las características y los resultados oncológicos entre pacientes con CEA inmunocomprometidos e inmunocompetentes.
MétodosEstudio multicéntrico comparativo retrospectivo que incluye 2 cohortes consecutivas de pacientes, inmunocomprometidos e inmunocompetentes, diagnosticados de carcinoma escamoso anal. Se han investigado las características de los pacientes, los tratamientos realizados, la respuesta clínica al tratamiento con quimiorradioterapia (QRT), la recidiva local o a distancia, la supervivencia global (SG) y la supervivencia libre de enfermedad (SLE).
ResultadosDe enero 2012 a diciembre 2017 hemos estudiado a 84 pacientes, 47 (55,6%) mujeres, afectos de CEA, de los cuales 22 (26%) han sido pacientes inmunocomprometidos y 62 (74%) inmunocompetentes. Los pacientes inmunocomprometidos fueron más jóvenes (53 vs. 61 años; p = 0,001), con un menor tamaño tumoral (p = 0,044), y presentaban un mayor consumo de tabaco (p = 0,034) y de drogas de uso parenteral (p = 0,001). No se objetivaron diferencias significativas en los tratamientos administrados (p = 0,301), tampoco difirió la respuesta clínica a la QRT (83 vs. 100%). Tampoco se observaron diferencias significativas en la supervivencia global (60 vs. 64%; p = 0,756) o en la supervivencia libre de enfermedad a 5 años (SLE) (65 vs. 68%; p = 0,338).
ConclusionesEn el presente estudio no se observaron diferencias significativas en relación con los resultados oncológicos a largo plazo entre pacientes inmunocompetentes e inmunocomprometidos diagnosticados de CEA, con un grado de cumplimiento del tratamiento similar. Esta evidencia podría deberse al estrecho seguimiento y buen control terapéutico de pacientes infectados por HIV.
The epidemiology of anal squamous cell carcinoma (ASCC) has changed substantially over the second half of the 20th century. Although a rare disease, its incidence and mortality have increased over recent decades. In 2016, ASCC had an incidence of 1.8 per 100,000 US population, making it the 26th most common malignant disease in the US.1 ASCC cases have increased by 2%–3% per year over the past 10 years, as has the number of deaths due to this tumour (3% per year).2
Several factors are known to be associated with the risk of developing ASCC, including older age, tobacco use, increase in number of sexual partners, receptive anal sex, history of cervical, vulvar, or vaginal cancer, anogenital human papillomavirus (HPV) infection and immune system suppression, either due to conditions such as human immunodeficiency virus (HIV) infection or induced by immunosuppressive drugs (IMS), such as those used in organ transplantation.3
HPV is necessary, although not sufficient, to cause anal cancer. More than 150 different types of HPV have been identified; some have an affinity for the skin, causing skin lesions, and others for the mucous membranes, with the ability to infect the anogenital tract. Of these, some are high-risk or oncogenic and others are low risk, causing anogenital condylomas.
The average European prevalence of HPV infection in women (with normal Pap smears) is 8%–13%, and is higher among young women. It is 25%–30% and 40%–60% among heterosexual men and men who have sex with men (MSM), respectively.4,5 HPV serotype 16 is the most common viral type (85%) associated with ASCC, followed to a lesser extent by serotype 18 (7%). The cytological alterations caused by this virus in the anal margin and canal are well known; however, the mechanisms of progression towards squamous cancer or, conversely, those of regression of the lesions are less so.6
In situations where the immune system is impaired, due to HIV infection or IMS treatment, the likelihood of virus clearance decreases markedly, which may favour the progression of dysplastic cytological lesions to infiltrative neoplasia. Therefore, it could be assumed that immunosuppressed patients would present with more aggressive or advanced anal neoplasms and worse oncological outcomes than immunocompetent anal cancer patients. In this respect, HIV-infected patients would constitute a high-risk group. However, in these patients with better clinical control and with the implementation of more aggressive antiretroviral therapies, results have been obtained in the treatment of HPV-associated anal lesions similar to the non-HIV-infected population,7 suggesting that well-controlled HIV patients with normal CD4 counts may behave as immunocompetent patients.7–10
The aim of this study was to characterise the course of this disease in both immunocompetent and immunosuppressed patients, comparing data on patient characteristics and treatment outcomes in terms of recurrence, progression, and survival.
MethodPatient selectionRetrospective study including a consecutive series of patients treated for squamous cell carcinoma of the anal margin and anal canal in the Autonomous Community of the Basque Country from January 2012 to December 2017. Authorisation for the study was obtained from the Basque Research Ethics Committee (CEIm-E) on 10 November 2017.
Data were extracted from the clinical-administrative database of the Spanish national health system's minimum basic data set (MBDS) and from Osabide Global data (integrated socio-health clinical history of the Basque Health Service, Osakidetza), collected from the Oracle Business Intelligence Enterprise Edition (OBIEE) tool. A pre-selection was made using international codes (ICD) for topographical data (anus and perianal region) and morphological data (squamous carcinoma, squamous cell carcinoma, basal cell carcinoma, cloacogenic carcinoma, and verrucous carcinoma), including all confirmed and treated patients with an anatomopathological report of anal squamous cell carcinoma.
TNM 2009 (AJCC, 7th edition) was used for clinical tumour staging.
Study variablesData were collected on age, sex, ASA, history of alcoholism, smoking, intravenous drug addiction, presence of diabetes mellitus, presence, or history of cervical or vaginal disease associated with HPV virus (CIN or VIN), HIV infection and its stage (CDC classification 1993), treatments given, tumour size and staging, time, type, and treatment of tumour persistence and/or recurrence.
Patients with a history of solid organ transplantation, malignant bone marrow disease, HIV infection with CD4 below 200 and/or AIDS stage C (C1, C2, C3, A3 and B3), severe malnutrition (rapid weight loss greater than 10%, albumin <2 and cholesterol <100) and on steroid treatment (more than 30 mg) for more than one year at the time of ASCC diagnosis were considered immunosuppressed (IS) patients. Diabetes and alcoholism were considered comorbidities.
For HIV-infected patients, the year of diagnosis of infection, stage of HIV infection, CD4 nadir (lowest CD4 count), antiretroviral treatment (ART), 3-drug treatment started (HAART), previous suboptimal treatment (starting ART with mono- or dual therapy) were also collected, proportion of time on ART (time on ART divided by total known time of HIV), the length of time (in months) from HIV diagnosis to ASSC diagnosis, CD4 at time of ART initiation, CD4 at time of anal cancer diagnosis, viral load (VL) at time of anal cancer diagnosis (undetectable vs. detectable), proportion of time with undetectable VL (time with undetectable VL divided by time since known HIV infection), and proportion of time with undetectable VL on ART (time with undetectable VL divided by time on triple adherence ART).
In the chemo-radiotherapy treatment regimen, suboptimal treatment was considered to be when the radiotherapy dose was less than 50 Gy or if there were reduced doses of chemotherapy. Patients were examined 6 months after completion of treatment with curative intent following chemoradiotherapy (CRT) by physical examination and imaging tests.
Follow-up ended on 31 December 2019.
Statistical studyQuantitative variables are presented as absolute numbers and their distribution using appropriate measures of central tendency: mean or median and their interquartile range. The χ2 test or Fisher's exact test were used for qualitative univariate analysis when necessary. A normality study was performed with the Kolmogorov-Smirnov test, and according to the results, quantitative variables were analysed with the Student's t-test or Mann-Whitney test. Overall survival (OS) and disease-free survival (DFS) and recurrences were analysed using the Kaplan-Meier method, expressed as medians and their 95% CI. The log-rank test was used for univariate analysis of survival curves. The Mann-Whitney U test was used to test for sample heterogeneity. All statistical tests were performed with SPSS v. 26.0 software (SPSS®, Chicago, USA).
STROBE guidelines were followed.
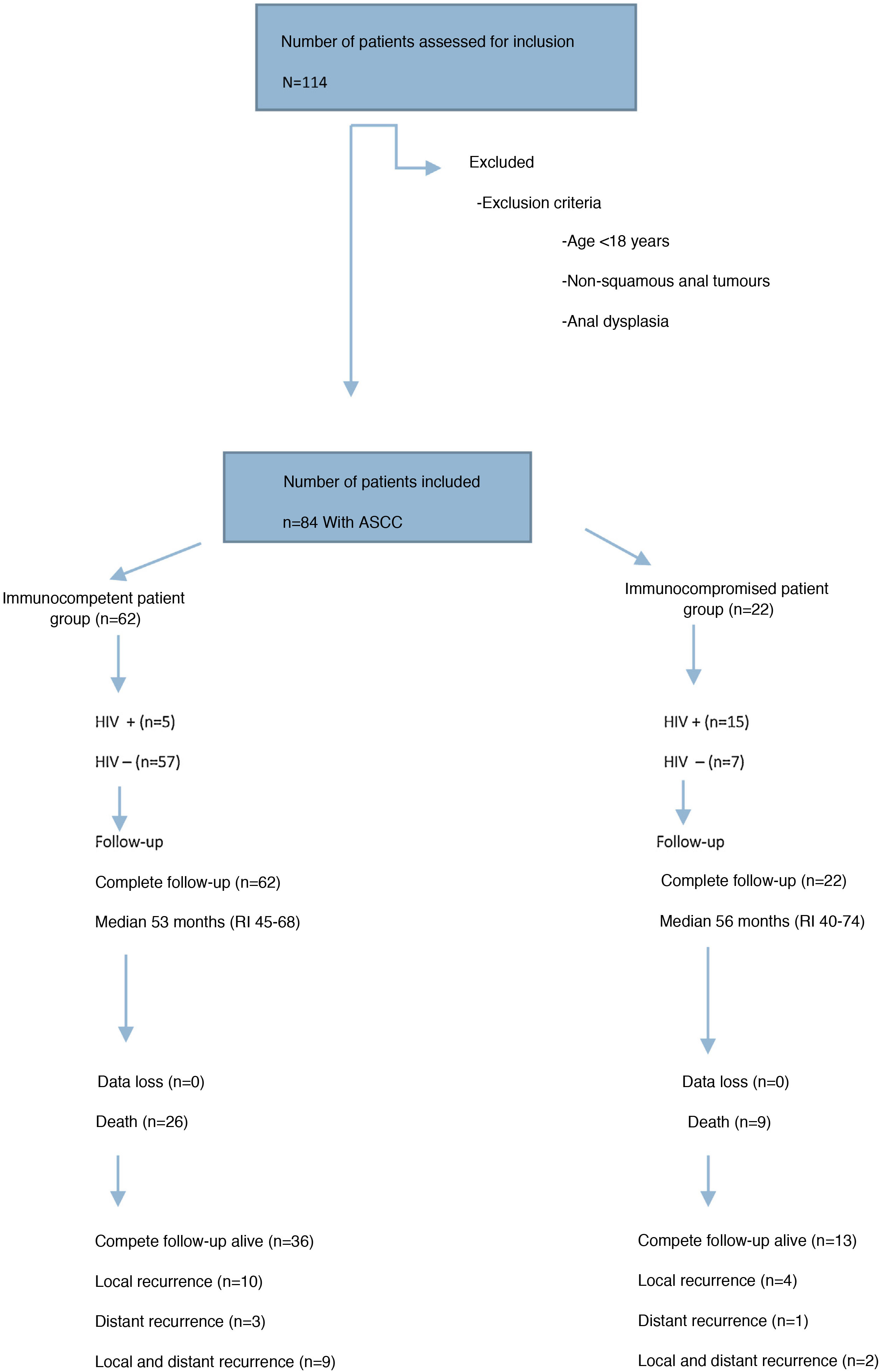
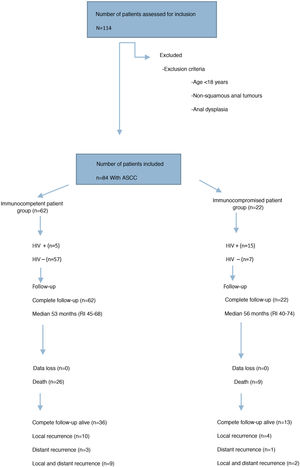
ResultsPatient characteristics and tumour stagingFrom January 2012 to December 2017, 84 patients consecutively treated for ASCC were registered, of which 22 (26%) were IS patients and 62 (74%) IC patients (Fig. 1).
Clinical-epidemiological and tumour staging data for both groups are presented in Table 1. The IS patient group included 15 patients with AIDS disease (13 stage C and 2 stage B3), one patient with B lymphoma, one patient with multiple myeloma, one patient with renal transplantation, two patients with anorexia and severe malnutrition and two patients with prolonged corticosteroid treatment. In the IC patient group, 5 patients were HIV-infected (3 stage A2 and 2 stage B1).
Characteristics of immunocompetent patients and immunocompromised patients.
| Patients 84 | IC | IS | p-value |
|---|---|---|---|
| n = 62 | n = 22 | ||
| Female | 36 (58.1%) | 11(50%) | 0.513* |
| Age (median) | 61 (IR: 52-76) years | 53 (IR: 47-55) years | 0.001** |
| ASA I/II | 36 (58%) | 7 (31.8%) | 0.054* |
| ASA III/IV | 26 (41.9%) | 15 (68.2%) | |
| Smokers | 35 (56.5%) | 18 (81.8%) | 0.034* |
| Diabetes mellitus | 9 (14.5%) | 2 (9.1%) | 0.517* |
| Intravenous drugs | 5 (8.1%) | 10 (45.5%) | 0.001* |
| CIN disease | 8 (22%) | 6 (54%) | 0.147* |
| HIV | 5 (5.9%) | 15 (17.8%) | 0 |
| cTNM stage | |||
| T1 | 7 (11.3%) | 8 (36.4%) | 0.044* |
| T2 | 30 (48.4%) | 7 (31.8%)) | |
| T3 | 16 (25.8%) | 6 (27.3%) | |
| T4 | 9 (14.5%) | 1 (4.5%) | |
| N− | 39 (62.9%) | 18 (81.8%) | 0.103* |
| N+ | 23 (37.2%) | 4 (18.1%) | 0.956* |
| M1 | 3 (4.8%) | 1 (4.5%) | 0.144* |
| Stage | |||
| I | 6 (9.7%) | 7 (1.8%) | |
| II | 31 (50%) | 10 (45.4%) | |
| IIIa | 4 (6.5%) | 1 (4.5%) | |
| IIIb | 18 (29%) | 3 (13.6%) | |
| IV | 3(4.8%) | 1 (4.5%) | |
| Mean tumour size (cm) | 6 | 3 | 0.133** (SD: 2.87–3.56) |
ASA: American Society of Anesthesiologists Physical Status Classification; SD: Standard Deviation; HIV: Human Immunodeficiency Virus: IC: Immunocompetent; IS: Immunosuppressed; CIN: Cervical Intra-epithelial Neoplasia.
When comparing both groups there were no significant differences in terms of sex, preoperative ASA, HPV-associated cervical disease, or presence of diabetes. However, we did observe differences between the two groups in terms of mean age at onset of ASCC, smoking rate and intravenous drug addiction.
Regarding clinical tumour staging, there were significant differences in T classification, more patients in the IS group were in early stages; however, the number of patients with metastases, lymph node or distant, was similar in both groups. The mean tumour size was 4.6 (0.7–14; SD: 2.87) cm and 3.5 (0.5-15; SD: 3.56) cm in the IC and IS group, respectively, with no significant difference.
Table 2 shows the clinical and analytical data of the HIV-infected patients in both groups and their corresponding antiretroviral treatments; as can be seen at the time of ASCC diagnosis, there were no differences between the two groups.
HIV-infected patients.
| IC (n = 5) | IS (n = 15) | p-value | |
|---|---|---|---|
| CD4 nadir | 181 | 114 | 0.142* |
| CD4 lymphocytes at time of tumour diagnosis | 450 | 491 | 0.735* |
| Stage | |||
| A | 3 (60%) | 0 | 0.001** |
| B | 2 | 2 | |
| C | 0 | 13 (86%) | |
| HAART | 4 (80%) | 10 (66,6%) | 0.517*** |
| Suboptimal previous treatment | 3 (60%) | 7 (46.5%) | 0.500*** |
| Proportion of time on ART from HIV diagnosis | 66.6% | 64.7% | 0.933* |
| Time from HIV diagnosis to anal cancer | 121.8 | 160 | 0.395* |
| VL detectable on diagnosis of anal cancer | 1 (20%) | 4 (26.6%) | 0.634*** |
| Proportion of time with undetectable VL | 40.2% | 41.40% | 0.735* |
| Proportion of time on ART with undetectable VL | 47.3% | 38.6% | 0.553* |
VL: viral load; IC: immunocompetent; IS: immunosuppressed; CD4 nadir: the lowest point of CD4; ART: antiretroviral treatment; HAART: highly active antiretroviral therapy.
Seventy-five patients (89%) were treated with curative intent at full doses, and 9 with suboptimal treatment (8 IC). Suboptimal doses of RT were given to 6 patients with haemostatic intent or local clinical control, all older than 80 years, 4 ASA IV patients and 2 alcoholic patients (one treated for prostate adenocarcinoma). CT was administered at suboptimal doses to 2 patients with a history of alcoholism (one ASA IV and the second treated with RT for prostate adenocarcinoma). The IS patient was an HIV+ male, T1N0M1 with suboptimal dose CT due to toxicity (CD4 131 at diagnosis). The treatments performed can be seen in Table 3. In the total series, 51% received either CRT or RT/CT alone (due to previous pelvic radiation or contraindication for CT), 26% underwent isolated local resection and 22% received CRT or RT after local resection due to positive or insufficient borders. There was no difference between the different treatments used in the two groups. Only one patient underwent abdominoperineal amputation as primary intention (IS patient, ASA IV, stage C for AIDS disease with previous radiotherapy for vulvar cancer).
Initial treatment.
| Patients 84 | IC (n = 62) | IS (n = 22) | p-value |
|---|---|---|---|
| CTRT | 24 (38.7%) | 7 (31.9%) | 0.301* |
| RTaor CTb | 9 (14.5%) | 3 (13.6%) | |
| Surgical | |||
| LR | 14 (22%) | 8 (36%) | |
| LR + CRT/RT | 14 (22%) | 3 (13.5%) | |
| APA | 1 (4.5%) | ||
| Symptomatic treatment | 1 (1.6%) |
APA: Abdominoperineal Amputation; IC: Immunocompetent; IS: Immunosuppressed; CT: Chemotherapy; CTRT: Chemoradiotherapy; LR: Local Resection; RT: Radiotherapy.
In the 43 patients who underwent chemotherapy, the most used regimen was mitomycin C with 5FU (34 patients, 80%). Radiotherapy doses were optimal in 46 patients (92%) with a mean of 55.5 Gy (50–63). Of the 57 patients who received radiotherapy, 4 received suboptimal doses (3 IC and 1 IS). Of these 4 patients, 2 had locoregional recurrence and 1 patient had distant recurrence.
Of the patients treated with CRT with radical intent, 4 (13%), all in the immunocompetent group, showed tumour persistence and/or progression, 3 requiring abdominoperineal amputation and palliative colostomy. There was complete clinical response to treatment in 100% of the IS patients.
Recurrence and treatmentThe median time to recurrence among the IC patients was 28 months (IR: 7–53) versus 40 months (IR: 15–68) in the IS group (p = 0.133, Mann-Whitney U test). As can be seen in Table 4, the overall recurrence rate was similar in both groups, 35.5% and 31.8% in the IC and IS, respectively. The most frequent form of recurrence was locoregional (30% IC vs. 27% IS); and the most frequent sites of tumour spread were lung, liver, and bone.
Recurrence in immunocompetent and immunosuppressed patients.
| Recurrence | Locoregional | Locoregional + distance | Distance | p = 0.861* |
|---|---|---|---|---|
| IC | 10 (16.1%) | 9 (14.5%) | 3 (4.8%) | |
| IS | 4 (18.2%) | 2 (9.1%) | 1 (4.5%) |
IC: Immunocompetent; IS: Immunosuppressed.
Regarding the treatment of recurrences, we found no differences between the two groups, as shown in Table 5. Of the 16 patients who required surgery, 11 underwent abdominoperineal amputation, 3 underwent local resection, and 2 underwent colostomy.
Treatment of recurrence.
| Patients 29 | IC (n = 22) | IS (n = 7) | p-value |
|---|---|---|---|
| CTRT | 1 (4.5%) | 1 (14.2%) | 0.123* |
| RT or CT | 5 (22.7%) | 4 (57.4%) | |
| Surg. ± (CRT/RT/CT) | 14 (63.6%) | 2 (28.4%) | |
| Palliative | 2 (9%) |
IC: Immunocompetent; IS: Immunosuppressed; Surg.: Surgical; CT: Chemotherapy; CTRT: Chemoradiotherapy; RT: Radiotherapy.
At the end of the study, we found that the overall colostomy rate was higher in the IC group (30% vs. 2%; p = 0.045).
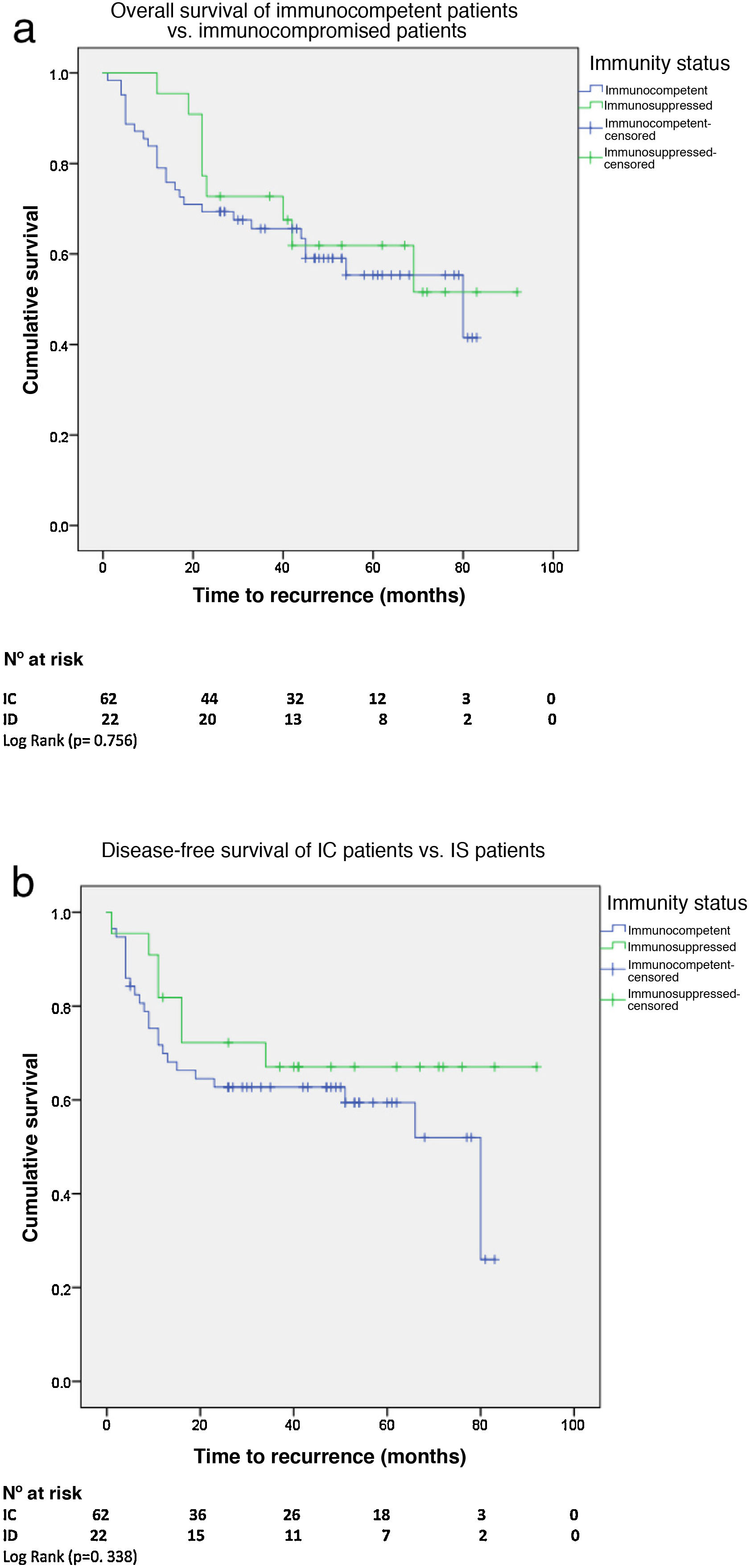
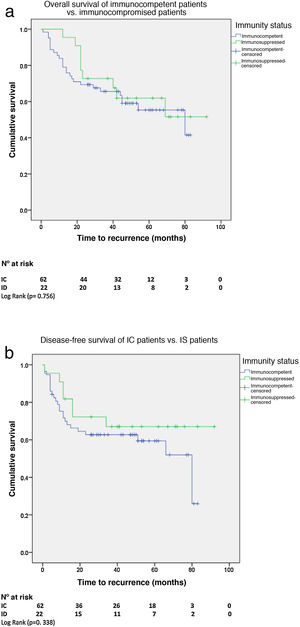
Overall and disease-free survivalWith a median survival of 62 months (95% CI 53–72) for the IC patients and 71 months (95% CI 59–83) for the IS patients, there was no significant difference in 5-year overall survival (OS). There was also no difference in 5-year disease-free survival (DFS) between the two groups with a median DFS for the IC patients of 80 months (95% CI 38–121) vs. 82 months for the IS group (95% CI 59–103) (Fig. 2A and B).
There was no difference in OS and DFS between HIV+ and HIV−patients. With a median OS of 76 months (95% CI 58–94) for HIV+ patients and 62 months (95% CI 50–74) for HIV−, and a median DFS for HIV+ patients of 80 months (95% CI 14-146) vs. 82 months (95% CI 58–105) for HIV−.
DiscussionThe main objective in this comparative study was to determine differences in clinical characteristics and oncological outcomes between IC and IS patients with ASCC. In our study we found no significant differences in terms of OS, DFS, type of treatment, tolerance to treatment, tumour regression after CRT, and tumour recurrence between either group. We did find differences in some patient characteristics and forms of tumour presentation.
Patients in the IC group of our study were younger and had smaller tumours at the time of ASCC diagnosis. Smaller tumours had a higher local resection rate among the IS patients. These findings are similar to those observed by authors such as Oehler-Jänne et al.11 where in a multicentre study of a large cohort of HIV positive and negative patients they found that HIV-infected patients had earlier clinical and pathological T stages and similar survival outcomes. In our series we also found no differences in OS or DFS between the IC and IS patients, and we believe, like these authors, that both earlier diagnosis and treatment management of HIV-infected patients in infectious disease units could explain why the oncological outcomes were similar to the non-HIV group of patients.
Immunodeficiency increases HPV activity, and therefore HIV-infected patients would be expected to have more advanced HPV-associated neoplastic lesions with worse prognosis. However, among HIV-infected patients, intensified antiretroviral therapy has changed the natural history of the disease, with a decreased incidence of Kaposi's sarcoma, non-Hodgkin's lymphoma. and other tumours associated with HIV immunodeficiency.9 Nevertheless, the changes that these more active treatments may have on the development and progression of ASCC, as well as the tolerance and response to treatment in this group of patients, are less well known.10 In our study, HIV-positive patients with more than 200 CD4/mm3 and/or HIV-associated disease CDC categories A1-2 and B1-2 were included as IS patients, on the understanding that, with a controlled immune status, their immunocompetence could be similar to that of non-HIV-infected patients.
In the era before intensified antiretroviral therapy, HIV-positive patients not only had a worse prognosis, but also experienced greater toxicity to CRT compared to IC patients.8,12 With the introduction of HAART, survival of HIV patients has increased substantially and outcomes in this group of ASCC patients treated with CRT have also improved.13–17 This is probably because HIV-positive patients with optimal immune status can tolerate treatment with full-dose RT regimens and more active drugs.18
CRT is the standard treatment for ASCC in HIV-negative patients, and achieves excellent results, with local disease control of more than 80%.19,20 A recent study published by Camandaroba et al.21 observed that HIV-positive patients on intensified antiretroviral therapy treated with CTRT need more time to achieve complete responses, and therefore they recommend waiting longer for a therapeutic decision to be made in order to reduce APA. One of the key factors for improvement in the local relapse rate of ASCC is tolerability and adherence to planned and intensified dose of CTRT.11,13,22 In our study, the degree of toxicity was not an objective in itself, however the degree of compliance with treatment, the need to change the regimen, the need to decrease the dose or discontinue treatment was. The IS patients who required curative CRT treatment were able to complete treatment without discontinuation, with optimal radiotherapy doses in all of them and a complete clinical response in 100% and 84% of the IS and IC patients, respectively. None of the 10 HIV-positive patients treated with CTRT required an APA. We believe that the fact that optimal treatment doses were achieved in most patients with RT, with more effective CT regimens such as mitomycin C, and together with good control and management of HIV-infected patients, resulted in good locoregional oncological response.
The mechanisms by which retrovirals promote tolerance and response to CRT in ASSC are still debated. HIV protease inhibitors have been shown to cause radiosensitization in infected cells in vitro,23 although their effect in vivo is unknown. In our series of HIV patients, the mean CD4 count at the time of CEA diagnosis was well above 200/mm3 and 70% of them were on intensified antiretroviral therapy. This may explain why the relapse rate is similar between the IC and IS patients.
The immunosuppression produced by the HIV virus in infected patients, but with intensified antiviral treatment, is probably different and lower than that produced by the immunosuppressive treatment of transplanted patients or by other causes of immunosuppression. There are few studies published in the literature where IC versus IS patients are studied regardless of HIV stage or cause other than HIV infection. The recent study by Bingmer et al.,24 which mainly includes patients with solid organ transplantation, does show worse tolerance to CT and worse response to CRT, with an increased recurrence rate. In contrast, with results similar to those found in our study, a retrospective study by Fraunholz et al.22 observed similar results between HIV-positive and negative patients.
The present study has limitations inherent to its retrospective design: heterogeneous samples, with incomplete data such as the absence of serotyping of all lesions, slight variations in CT protocols or different sources of RT emission, as it brings together cases from 4 different centres. Nevertheless, it comprises a large series of patients with ASCC with and without HIV, with no patient losses, with access to data on management, treatment, follow-up, and close monitoring of patients with HIV by infectious disease units, with common institutional protocols and a computerised clinical history shared with primary and specialist care, which facilitates the complete follow-up of patients.
ConclusionIn our study, oncological outcomes for ASCC between IC and IS patients were similar, although the IS patients were younger and with smaller tumours than the IC patients. Tolerance and compliance with treatment was similar in both groups, which we think may be due to the close follow-up and good control of HIV-infected patients treated with modern medication by the infectious disease units, in collaboration with the coloproctology unit.
Further studies are needed to confirm these conclusions.
FundingNo specific support from public sector agencies, commercial sector or non-profit organisations was received for this research.
Conflict of interestsThe authors have no conflict of interests to declare.
We would like to thank Dr Iribarren, Head of the Infectious Diseases Unit of the Hospital Universitario Donostia, Dr Montejo, Head of the Infectious Diseases Unit of the Hospital de Cruces and Dr Portu, head of the Infectious Diseases Unit of the Hospital Universitario de Araba for their help.