Effect of aluminium salts on the synthesis of AlOOH nanostructures has been successfully investigated in detail using solvothermal method, when ethanol and NaOH are solvent and pH adjusting agent, respectively. Fourier transform infrared spectroscopy (FTIR), X-ray powder diffraction (XRD), and field emission scanning electron microscopy (FESEM), were used to characterize the synthesized samples. The specific surface area, pore size distribution and pore structure of the different AlOOH structures were also discussed by the N2 adsorption/desorption test. According to our experimental results, the structure characterization revealed that for synthesized AlOOH nanostructures no obvious XRD peaks arising from other phases of alumina are found indicating pure AlOOH phase of products. Furthermore, the nitrogen adsorption and desorption measurements indicated that the obtained AlOOH nanoparticles from aluminium chloride at basic condition possess a high BET surface area of approximately 90m2/g.
El efecto de las sales de aluminio sobre la síntesis de las nanoestructuras de AlOOH ha sido analizado en detalle satisfactoriamente usando el método solvotérmico, cuando el etanol y el NaOH son el disolvente y el agente de regulación del pH, respectivamente. Para clasificar las muestras sintetizadas se utilizaron la espectroscopia infrarroja por transformada de Fourier (FTIR), difracción de rayos X por polvo (XRD) y microscopia electrónica de barrido de emisión de campo (FESEM). El área superficial específica, la distribución del tamaño del poro y la estructura de poros de las diferentes estructuras de AlOOH también se debatieron mediante la prueba de adsorción/desorción de N2. De acuerdo con nuestros resultados experimentales, la clasificación de la estructura reveló que en las nanoestructuras de AlOOH sintetizadas no se observan picos XRD evidentes derivados de otras fases de la alúmina que indican la fase pura de AlOOH de los productos. Además, las mediciones de adsorción y desorción de nitrógeno indicaron que las nanopartículas de AlOOH obtenidas a partir de cloruro de aluminio en estado básico poseen un área superficial BET elevada, de aproximadamente 90m2/g.
The preparation and characterization of boehmite (aluminium oxyhydroxides) nanoparticles and their applications in different fields such as ceramic, catalysts and supports have received considerable attention during the past decades [1–24]. Moreover, different alumina oxides, such as transition aluminas (γ-Al2O3, θ-Al2O3, δ-Al2O3, etc.) and corundum (α-Al2O3) are obtained from boehmite (AlOOH). Transition aluminas include a series of metastable forms that exist on an extended temperature range, but all of them lead to α-Al2O3 by calcining at high temperatures. Transition aluminas are extensively used as ceramics [8,25,26], catalysts and catalyst supports [1–3,10,15,17,18,20,24], and membranes [27,28] because of their high surface area, mesoporosity, and surface acidity. Owing to these, several researches have broadly addressed to develop new routes to prepare boehmite structures [6,7,11,22,29–40]. Additionally, our group have reported some results on synthesis of boehmite nanostructures, and also as catalyst supports [1,3,6,7,9–11,17,18,20–24].
It is worth noting that for many of the current applications of boehmite nanoparticles not only is it needed to control the size and morphology of particles but it is also crucial to achieve long-time stability of their aqueous dispersion. Many routes have been generally used for the preparation of boehmite nanoparticles. Most of the functionalized boehmite nanoparticles used in several applications were synthesized by using hydrolysis and condensation of aluminium alkoxide under ambient conditions and its further peptization [41]. Furthermore, sol–gel [39], precipitation [36,42,43] and solvothermal or hydrothermal routes [6,11,22,33,35] can be achieved under a very wide range of synthesis conditions. Among them, solvothermal technique has been broadly employed as the effective method do to the mild synthesis conditions and flexible change of experimental parameters. Generally speaking, the experimental reaction parameters usually have great effects on the preparation and characterization of boehmite nanoparticles under solvothermal condition. In this method, the synthesis conditions, such as the temperature and time of the solvothermal processing, the initial pH, the pH adjusting agents, template and surfactant as well as the aluminium salts is also important [6,7,11,16,21,22,31,44–47]. Undoubtedly, the research and development of producing AlOOH with various structures are beneficial for many branches of modern science and technology. However, the reports concerning the synthesis of boehmite by a simple and environmentally benign method and their interesting properties are still limited. To our best knowledge the effect of different aluminium salts on the synthesis of AlOOH via solvothermal route have not been reported. This work introduces the synthesis of boehmite nanoparticles using aluminium chloride and nitrate as the precursor via solvothermal method. The structure and morphology were investigated by the XRD, FTIR, N2 adsorption–desorption, and FESEM.
Experimental detailsThe starting materials utilized are Al(NO3)3·9H2O, AlCl3, NaOH and ethanol 96%, were purchased from Scharlau, Spain, all chemical reagents were of analytical grade and were used as purchased without further purification. In order to investigate the effect of different anion in alkali solution on boehmite synthesis, two aluminium salts, nitrate and chloride were used as sources.
Typically, 40mmol either Al(NO3)3·9H2O or AlCl3 was dissolved in 120ml of ethanol (96%), and it was stirred for 10–15min at room temperature. NaOH solution (2M) were subsequently added drop by drop to the solution to give lacteous precipitates. At this point, the pH value of the reaction mixture was ∼5 or 11, then transferred into Teflon-lined stainless steel autoclave (250ml, volume), and then placing them in an oven with a temperature of 180°C for 24h. These samples were treated by centrifugation, rinsed with ethanol 96% and DI water several times, and then dried overnight at 65°C in an oven. The boehmite samples prepared by nitrate (N) and chloride (C) at different pH were labelled N-5, C-5, N-11 and C-11, respectively.
Fourier transform infrared (FTIR) spectra were obtained using a RAYLEIGH WQF-510 spectrometer in the range 400–4000cm−1 at room temperature. The product phases obtained under different experimental conditions were identified using a X-ray powder diffraction (XRD) patterns, using a D8 ADVANCE, BRUKER X-ray diffractometer, equipped with CuKα radiation (λ=1.54Å). Data were collected from 5 to 80° 2θ, counting for 10sec every 0.02° 2θ step. The surface morphology and particles sizes were analyzed by a field emission scanning electron microscope (FESEM, HITACHI S-4160 XL30). The specific surface area of synthesized samples was determined using BEL SORP, MINI II-310 analyser. In this technique the Brunauer–Emmett–Teller (BET) equation was employed to calculate the specific surface area and the mean sizes of pores were calculated using the original Barrett, Joyner, and Halenda (BJH) method. Before analyses, the samples were degassed under a vacuum at 120°C for at least 4h.
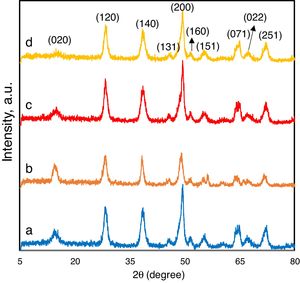
Experimental results and discussionThe phase structure and purity of the typical samples were examined by XRD. Fig. 1 shows the XRD patterns of the samples synthesized in this paper. All diffraction peaks of boehmite nanoparticles were in good agreement with AlOOH (JCPDS no. 001-1283) which is the orthorhombic cell with lattice parameters of a=3.78Å, b=11.8Å, and c=2.85Å. The distinguished peaks at the angles of 14.3, 28.1, 38.3, 45.7, 48.9, 51.6, 54.9, 64.6, 66.7, and 72.1° corresponded to the (020), (120), (140), (131), (200), (160), (151), (071), (022) and (251) plans of the orthorhombic boehmite, respectively. The orthorhombic structure of AlOOH is proven by comparing the XRD pattern with others reported in literature [6,11,45]. No obvious XRD peaks arising from other phases of alumina are found indicating pure AlOOH phase of the solvothermal product. The intensities of all the diffraction peaks were gradually increased when the nitrate salt is used, indicating that higher crystallinity or larger crystal size of boehmite can be obtained when the aluminium source was nitrate. Generally, the crystallinity of boehmite depended on the experimental parameters such as type of aluminium salt and pH. Very often, additional crystalline phases such as gibbsite and or bayerite are formed beside boehmite. However, this work showed that the precipitation of boehmite from aluminium salt (nitrate or chloride) solution with NaOH via solvothermal route was able to produce boehmite as a single phase. Generally, it is known that the chemical and physical properties can be significantly different for low- and well-crystallized boehmites, even though their crystal structure is just the same. Furthermore, there is no evident shift of the diffraction lines, suggesting that the layers are not rotated or displayed due to the change of pH or aluminium salt.
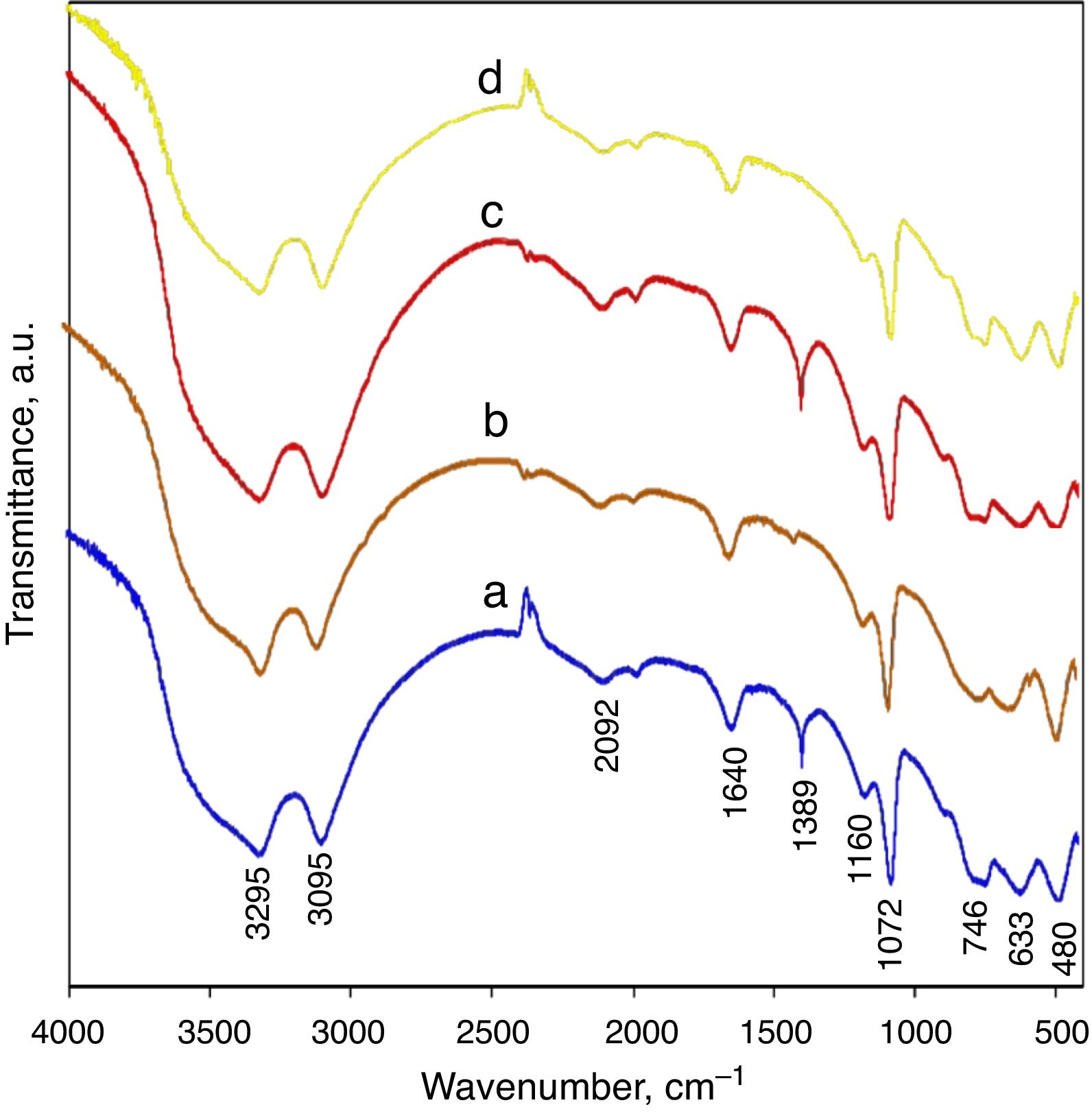
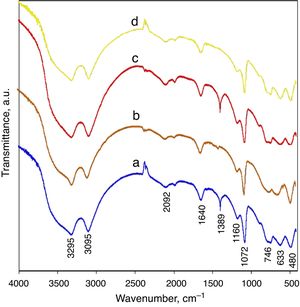
The FTIR analysis of AlOOH has been well studied [6,11,16,22,23] and is characterized by prominent OH stretching and bending modes associated with the interlayer hydrogen bonds of the structure. Fig. 2 shows the infrared spectrum of a AlOOH nanoparticle prepared as described earlier in this research. Generally, for all samples, the FTIR spectrums were similar regardless of the slight difference in intensity some of peaks. As shown in Fig. 2, for the boehmite samples, five strong bands at 480, 633, 746, 1073, and 1160cm−1 were observed. The band at 480cm−1 is assigned to the angle deformation of OA(OH), and the (OH)AlO angle bending results in the peak at 633cm−1, and 746cm−1 which is attributed to the stretching vibrations of AlOAl in the distorted AlO6. The sharp peak at 1072cm−1 and small shoulder at 1160cm−1 are assigned to the angle bending of the H bonds in the octahedral structure of boehmite (OHAlO) and Angle deformation (wagging) of the H bonds in the octahedral structure of boehmite (OHAlO), respectively. The acute peak in 1389cm−1 corresponds to the amounts of nitrate anion, which was not thoroughly removed by washing. As a comparison, the intensity of this band is stronger for the synthesized samples with nitrate anion as has not been washed well. The weak band at 1640cm−1 can be assigned to the stretching and bending modes of the adsorbed water molecule, and this absorbance in the spectra of AlOOH nanoarchitectures are very weak, indicating a very small amount of physically adsorbed water molecules. The asymmetric and symmetric stretches of the interlayer OH groups are seen at 3295 and 3095cm−1, respectively (Fig. 3).
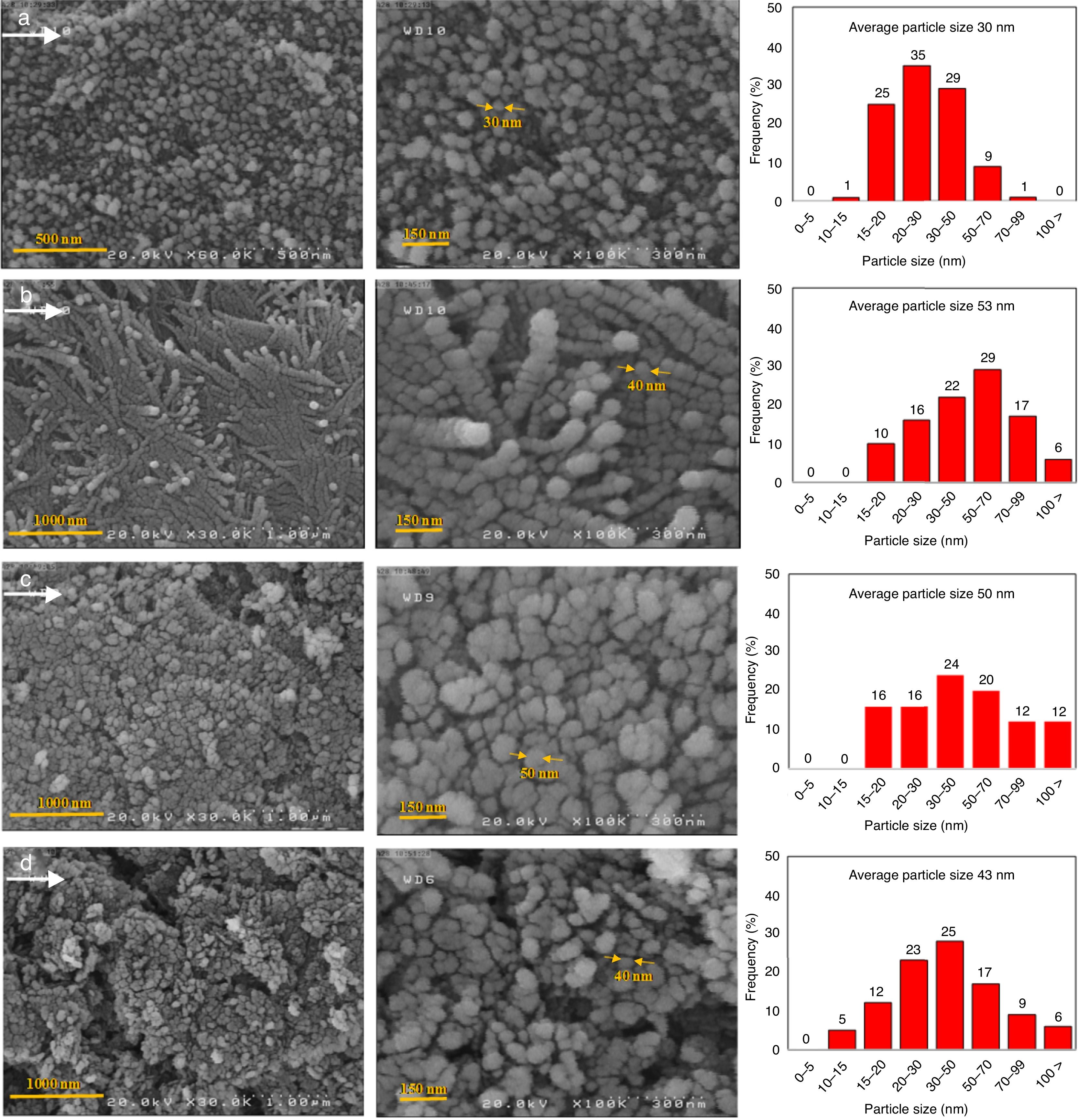
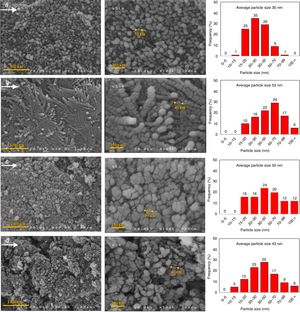
The microstructure of the synthesized AlOOH nanoparticles were studied using FE-SEM. Fig. 4 shows the representative FESEM images of N-5 (a), C-5 (b), N-11 (c) and C-11 (d) samples, and we carried out the higher and lower magnification analysis for all samples. According to FESEM images, different anion has a low effect on the morphologies of as-prepared AlOOH. The obtained images indicate that all samples are nearly spherical in shape. We can see that the formed particles only for N-5 sample possess uniform size distribution and are homogeneous without preferentially oriented shapes, for this sample more than 90 percent of particles were sized lower than 50nm in diameter. It is interesting that the particles in the C-5 sample were formed nano rods with size several ten nanometres to several micrometres in length. The particle size distribution histograms are shown in right hand of FESEM images in Fig. 4. As comparison, the nano-rods are produced when pH adjusting agent is NaOH and same pH (∼5), and nitrate salt applied with hydrothermal method [11], whereas, we have obtained nanoparticles with narrow particle size distribution. Therefore, the solvent in the solvothermal synthesis has more effect on the characteristics and structure of final products.
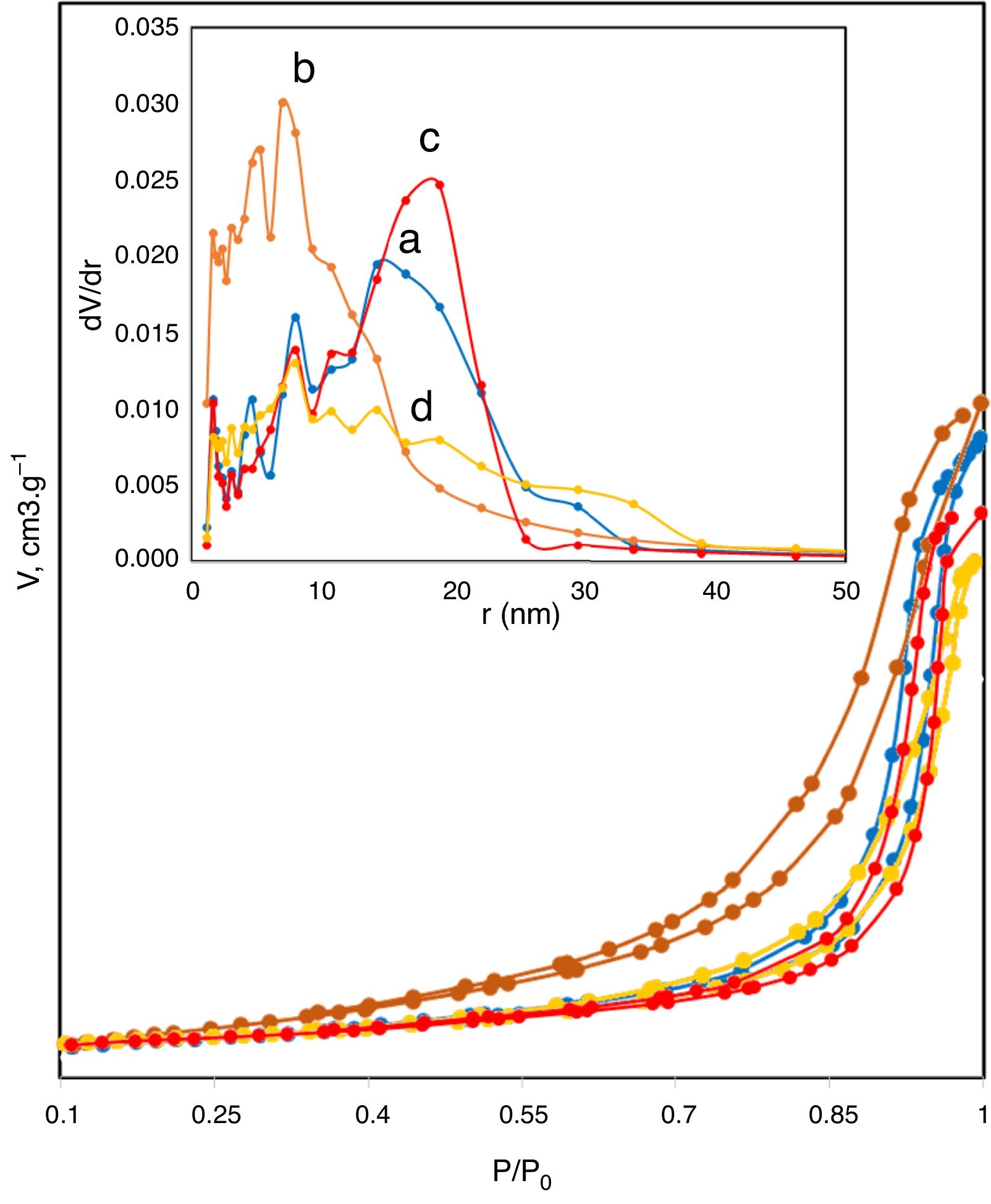
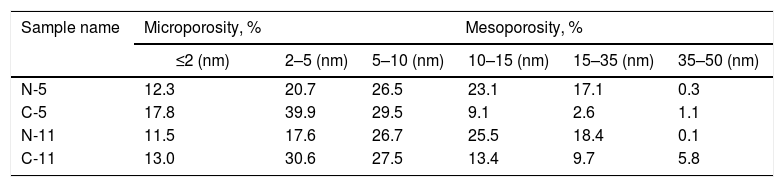
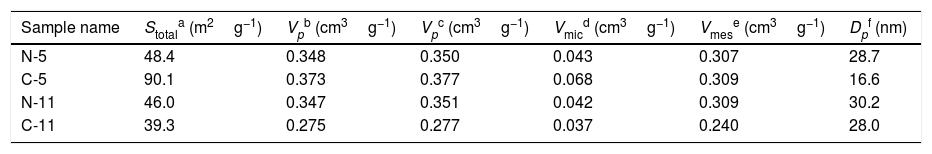
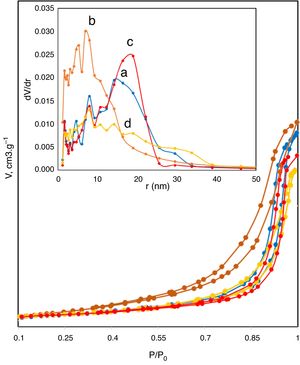
The N2 adsorption–desorption isotherms shown in Fig. 4 were used to determine the surface area and the type of porosity for boehmite samples synthesized under different conditions. Table 1 lists the results of textural properties, porosity structures and positions of synthesized AlOOH nanostructures. These results imply that onions in the solvothermal method is quite important to prepare AlOOH. Fig. 4 shows the N2 adsorption–desorption isotherms measured at 77K and the corresponding pore size distributions curves (inset) calculated via the Barret-Joyner-Halenda (BJH) [48] for all samples. The shape of these isotherms belonged to type IV, as indicated by convex curvature of the isotherms at the sub-monolayer range and by occurrence of a narrow hysteresis loop at high P/P0 range, which proved that this fibrous boehmite was a mesoporous material. The hysteresis loops of these samples seem to be type H2, indicating that they have good pore connectivity with ink-bottle or channel-like pores [49]. It could be seen that the pore sizes for samples synthesized by nitrate and chloride were mostly located between 2-35nm and 2–40, respectively. The pore structure parameters for all the samples including total surface area from BET, total pore volume from BET and BJH method, micro porosity, mesoporosity, average pore diameter are listed in Table 2. The C-5 and C-11 have highest and lowest surface area and pore volume, respectively. But samples synthesized by nitrates did not show more change on the surface area with increasing of solution pH. In other hand, the C-5 sample exhibited the high specific surface area of 90m2/g. Therefore, the obtained surface area for sample C-5 is comparable and higher than other boehmites, such as cantaloupe-like AlOOH (55.5m2/g) [50], γ-AlOOH hollow microspheres (93.6m2/g) [51], lamellar γ-AlOOH architectures (75.02m2/g) [52], micro-mesoporous flower-like γ-AlOOH (69m2/g) [6], boehmite (87.5m2/g) [53]. In general, larger specific surface area and pore volume are favourable for many applications such as catalysis and adsorbents.
Porosity structures and positions of synthesized boehmite samples.
Boehmite nanoparticles were successfully prepared via a chemical solvothermal treatment without using any surfactant or hard templates. The synthetic parameters such as different anions and two pH value of 5 and 11 were systematically studied to achieve a good porous property. The boehmite sample with middle crystallinity, nano particle morphology and its surface area of 90m2/g was prepared when used aluminium chloride as precursor and pH value was controlled at 5. The advantages of these nanoparticles include its simplicity and applications in ceramics, adsorption, catalyst and catalyst supports, and this method could be applied in the preparation of other oxyhydroxide powders.
The authors are grateful for the supports from Islamic Azad University, Kermanshah Branch.