Although implant abutments made of yttria-stabilized tetragonal zirconia polycrystal (3Y-TZP) show great predictability, some issues related to its aging require further study. The objective of this in vitro study is to assess the stability of 3Y-TZP zirconia implant abutments with two different implant connections when subjected to aging simulation through thermocycling and mechanical loading (TCML).
MethodsTen 3Y-TZP zirconia abutments were selected and equally divided into two groups (n=5): CEZr, abutments for externally hexed implants; and CIZr, abutments for implants with an internal conical connection. The samples were subjected to thermocycling (5000 cycles; 5–55°C) and mechanical loading (1.2×106 cycles; 88.8N; 4Hz). Before and after the aging procedures, X-ray diffraction (XRD) analysis was conducted to observe tetragonal-monoclinic (t-m) phase transformation, and topographic surface analysis was performed by 3D profilometry, and data were analyzed using Mann-Witney test (p<0.05).
ResultsXRD measurements revealed no monoclinic phase in any of the abutments after aging. The comparative analysis regarding roughness (using the Sa parameter) at the abutments’ seating platforms (using 3D profilometry) revealed a slight increase in roughness in both connections after TCML. Statistically significant differences (test U=57.0, p=0.161>0.05), before and after TCML, and between implants connections (test U=57.0, p=0.053>0.05) were not detected.
ConclusionsAfter a 5-year simulation of its clinical use, the analyzed 3Y-TZP zirconia abutments did not show signs of aging. The connection's geometry does not interfere in aging.
Os pilares de zircónia tetragonal policristalina (Y-TZP) apresentam grande previsibilidade na sua utilização clínica. No entanto, existem ainda alguns aspetos relacionados com o envelhecimento que necessitam de ser estudados. O presente trabalho pretendeu avaliar a estabilidade de pilares de zircónia 3Y-TZP de 2 conexões implantares diferentes, quando submetidos a simulação de envelhecimento (TCML).
MétodosForam selecionados 10 pilares em zircónia 3Y-TZP, que foram divididos em 2 grupos (n=5): CEZr, pilares para implantes de conexão de hexágono externo; e CIZr, pilares para implantes de conexão interna cónica-lobular. As amostras foram submetidas a termociclagem (5000 ciclos; 5-55°C) e carga cíclica (1,2×106 ciclos; 88,8N; 4Hz). Antes e após TCML, os pilares foram sujeitos a análise por difração de raios X para determinação de alteração de fase cristalográfica de tetragonal para monoclínica (t-m) e a interferometria ótica sobre a superfície de assentamento das 2 conexões para a medição da sua topografia por perfilometria 3D. Análise estatística: Mann-Whitney test (p<0,05).
ResultadosApós TCML não foi detetada fase monoclínica em nenhum dos pilares. A análise comparativa da rugosidade (com utilização do parâmetro Sa) sobre as plataformas protéticas dos pilares revelou ligeiro aumento da Sa em ambas as conexões após TCML. Não foram observadas diferenças estatisticamente significativas nos valores de Sa (test U=57,0, p=0,161>0,05), antes e após TCML, e entre conexões dos implantes (test U=57,0, p=0,053>0,05).
ConclusõesApós uma simulação de 5 anos de utilização clínica, os pilares de zircónia 3Y-TZP analisados não apresentaram sinais de envelhecimento. A geometria das conexões não interferiu no envelhecimento.
Due to its high predictability, titanium has become the favorite material for implants.1–4 Recent systematic reviews have reported success rates higher than 95%, five and ten years after the implantation.5–8
Currently, the esthetic result is one of the success criteria for oral rehabilitation with dental implants, and thus it is the greatest limitation of using titanium abutments. In fact, the main failure of titanium abutments has been causing a grayish staining in the peri-implant mucosa.8–10 Junge et al.8 compared the use of metal versus ceramic components and observed significant color changes in the peri-implant mucosa of patients who had peri-implant tissues with thicknesses of up to 2mm and no significant effects on the ones with 3mm thickness.
The combination of ceramic crowns and abutments has revealed to provide a better esthetic performance, hence eradicating the risk of reduced illumination and grayish staining in the mucosa.11,12
Although different types of zirconia-based ceramic exist, their use in Dentistry is mostly limited to the 3Y-TZP structure (yttria-stabilized tetragonal zirconia polycrystal),13,14 and should meet the requirements described by International Organization for Standardization (ISO) 1356 and 6872.15
Clinical studies of up to 5 years that evaluated the performance of zirconia abutments in supporting single crowns on implants in the posterior, anterior, and premolar regions revealed success rates higher than 94%.10,16–20
Systematic reviews analyzing studies of at least 5-years follow-up about the performance of ceramic versus metal abutments21,22 concluded that zirconia abutments performed similar to metal abutments and, thus, were a valid alternative. Also, the implant/zirconia abutment set has similar strength to that of the implant/titanium abutment set.23 More recently, prospective clinical studies with more than 10-years follow-up focused on the performance of zirconia abutments in the anterior-premolar regions obtained success rates of approximately 96%.24,25 Despite these results, case reports with catastrophic failures have been reported in the literature.26
Zirconia is a polymorphic ceramic that takes three different crystallographic forms at ambient pressure, with distinct spatial arrangements according to temperature.27,28
During cooling after sinterization, the tetragonal phase becomes monoclinic below 970°C. To maintain that phase at room temperature, stabilizing oxides (dopants, such as Y3O2) are added, allowing the stabilized tetragonal zirconia to control that transformation and acquire excellent properties29–31 such as “transformation toughening”, the ability of zirconia to pass from the tetragonal to the monoclinic phase when submitted to tensile forces generated at crack tips. That transformation is accompanied by an increase of 3 to 5% in volume, resulting in compressive stress in the area near the microcrack in opposition to propagation.32
The greatest problem regarding 3Y-TZP lies in its sensitivity to low-temperature degradation and aging in the presence of water, steam, and body fluids, as well as during sterilization procedures. Aging occurs through a slow progressive transformation of the grains from the tetragonal to the monoclinic phase. This transformation originates from defects pre-existent in the surface of isolated granules (nucleation) and extends deeply throughout the remaining body, accompanied by the formation of micro-and/or macrocracks. It results in lower mechanical properties33–37 and changed surface properties, including increased roughness. This process has been reported to be accelerated by mechanical stresses and exposure to humidity.38 In vitro studies use many different methods to simulate and assess the aging of zirconia.39–43 Most of them use polished samples (bars) instead of more-complex geometric forms.44,45
Although zirconia abutments show great predictability in its use, there are still some issues related to its aging that must be further studied. This study aimed to assess the stability of 3Y-TZP zirconia implant abutments with two different implant connections — externally hexed and internal conical — when subjected to TCML, according to the null hypothesis: TCML had not influence on stability of 3YTZP abutments.
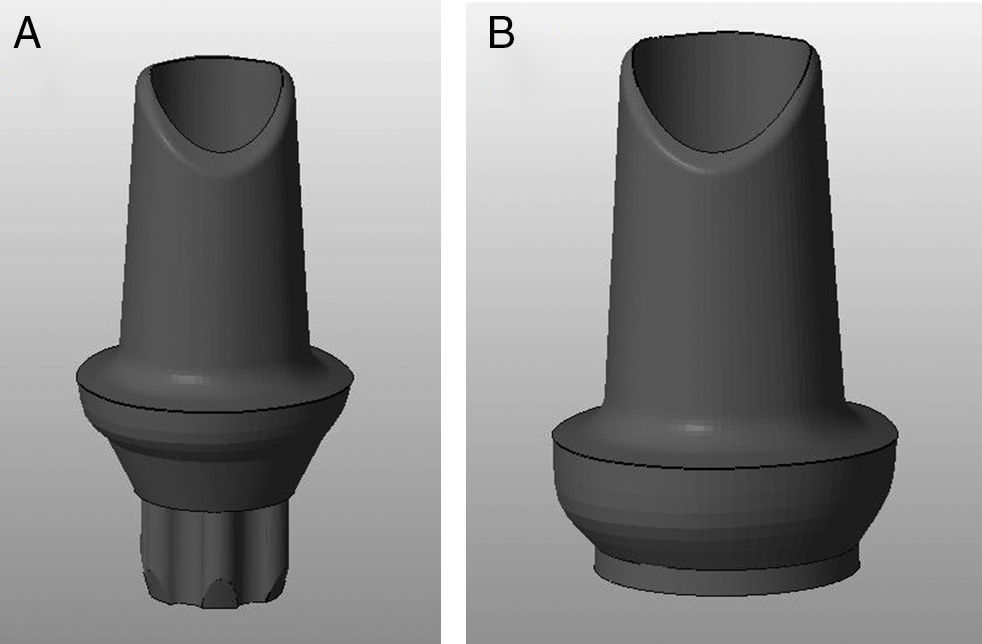
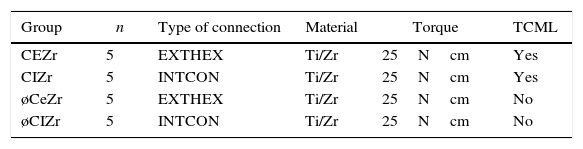
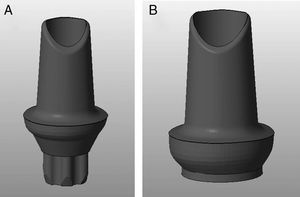
Materials and methodsTwenty 3Y-TZP zirconia abutments (Syntesis®, Phibo®-Dental-Solutions, Barcelona-Spain) were selected and equally divided into two groups (Figures 1–2):
- –
CEZr, composed of abutments for externally hexed implants (BNT®, Phibo®-Dental-Solutions, Barcelona-Spain).
- –
CIZr, composed of abutments for implants with an internal conical connection (Aurea®, Phibo®-Dental-Solutions, Barcelona-Spain) (Table 1).
Table 1.Groups of the studied sample.
Group n Type of connection Material Torque TCML CEZr 5 EXTHEX Ti/Zr 25Ncm Yes CIZr 5 INTCON Ti/Zr 25Ncm Yes øCeZr 5 EXTHEX Ti/Zr 25Ncm No øCIZr 5 INTCON Ti/Zr 25Ncm No CE, external connection; Zr, zirconia; Ti, titanium; CI, internal connection; ø group control; EXTHEX, externally hexed; INTC, internal conical; TCML, thermocycling and mechanical loading.
The abutments were designed using a digital design program (3Shape, Denmark), and were then produced for the two types of connections using the Phibo® CAD-CAM system (Barcelona-Spain) (Figures 1–2). The abutments were obtained by milling a pre-sintered block of 3Y-TZP, which then undergoes a final sintering.
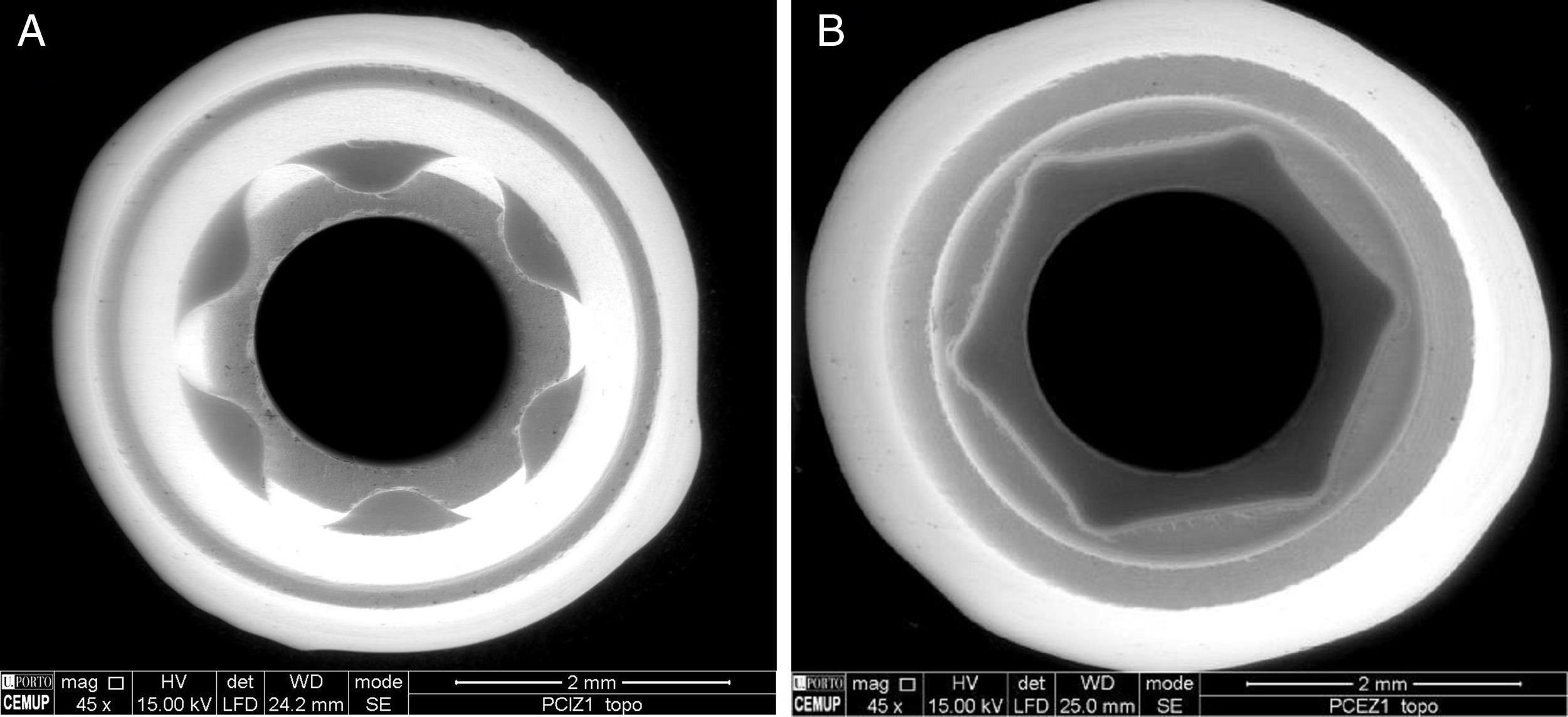
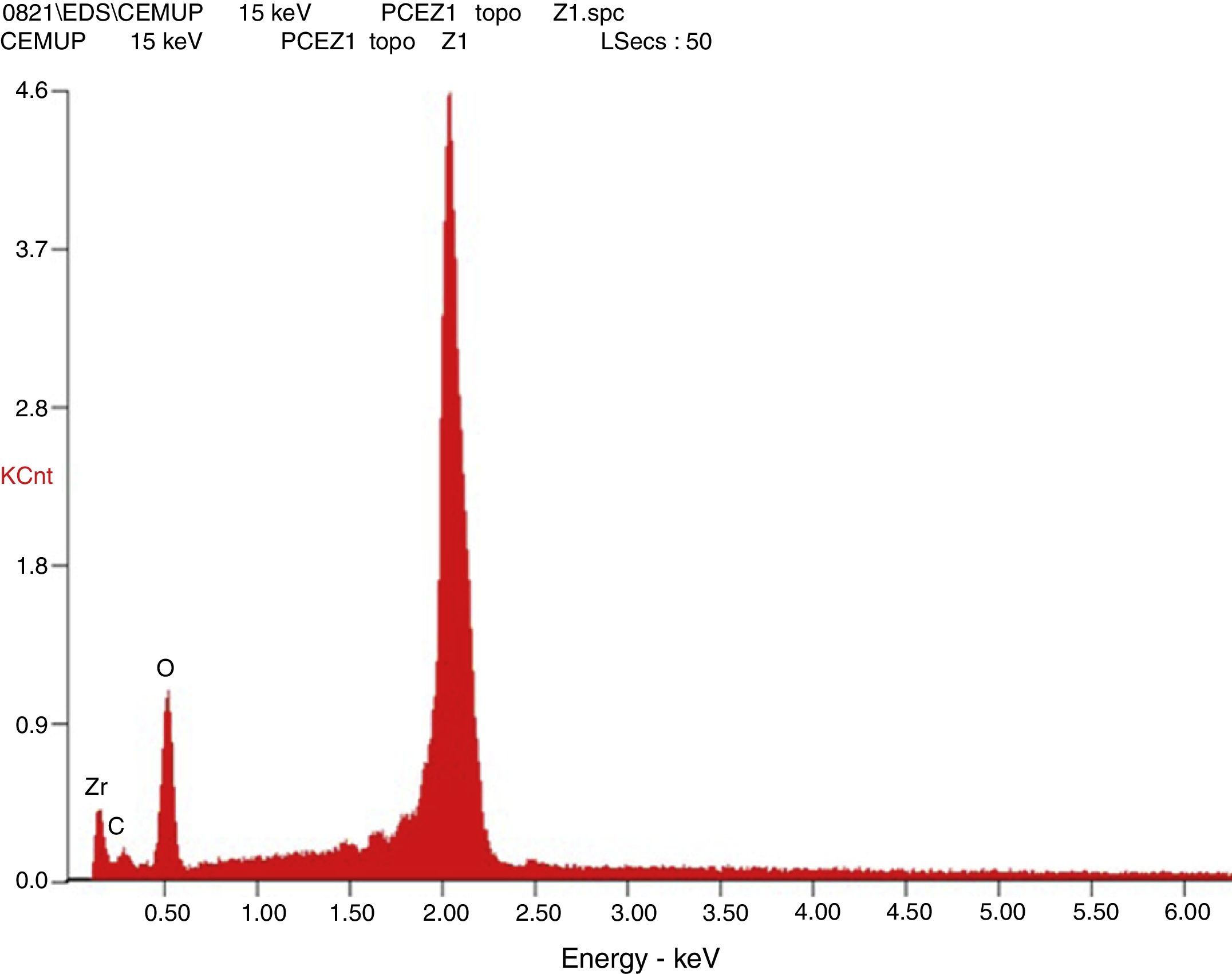
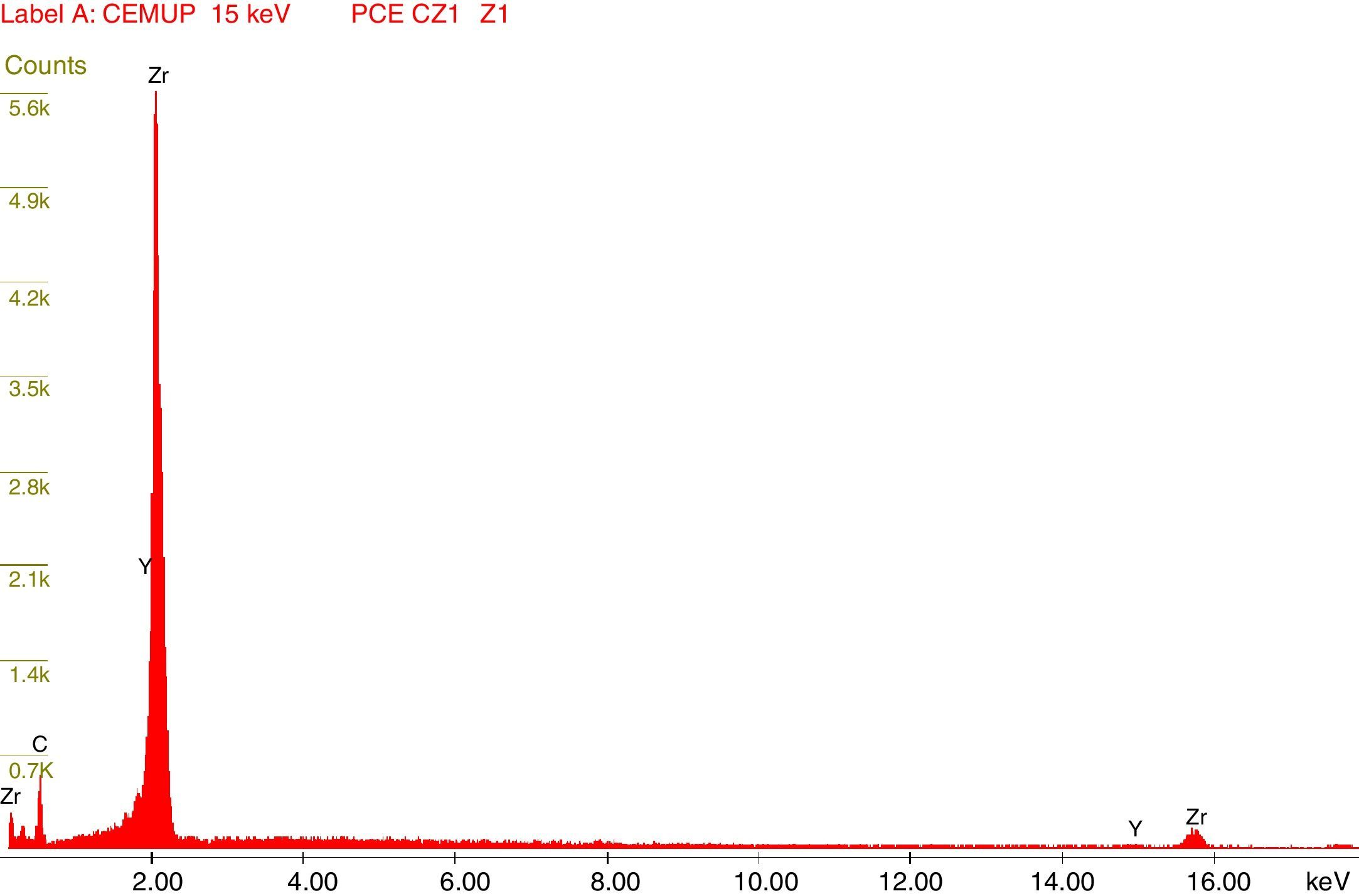
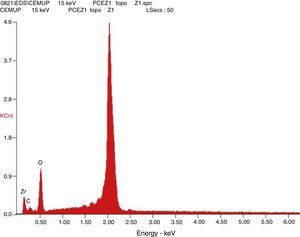
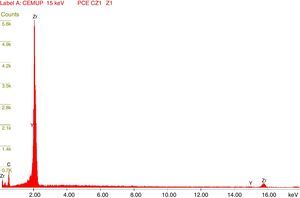
Afterward, the abutments were cleaned in ethyl alcohol for 5min in an ultrasound device (Biosonic® UC50BB, Colténe, Switzerland) and were let to dry in the open air. After this, samples were subjected to X-ray diffraction (XRD) analysis, over the seating platforms, using a diffractometer (Bruker® AXS D8-Discover, Karlsruhe-Germany) with the following parameters: Cu-Kα (λ=1.54060Å) radiation, θ:2θ mode, 15°–80° range, 0.02° increment, and an integration time of 1s. After each diffractogram, 5 samples and 5 controls were analyzed using the EVA® analytical software. The crystalline phases of the samples were indexed according to the International Center for Diffraction Data's database. The phase of 3Y-TZP was identified by chemical analysis using EDS (Energy Dispersive X-Ray Spectroscopy).
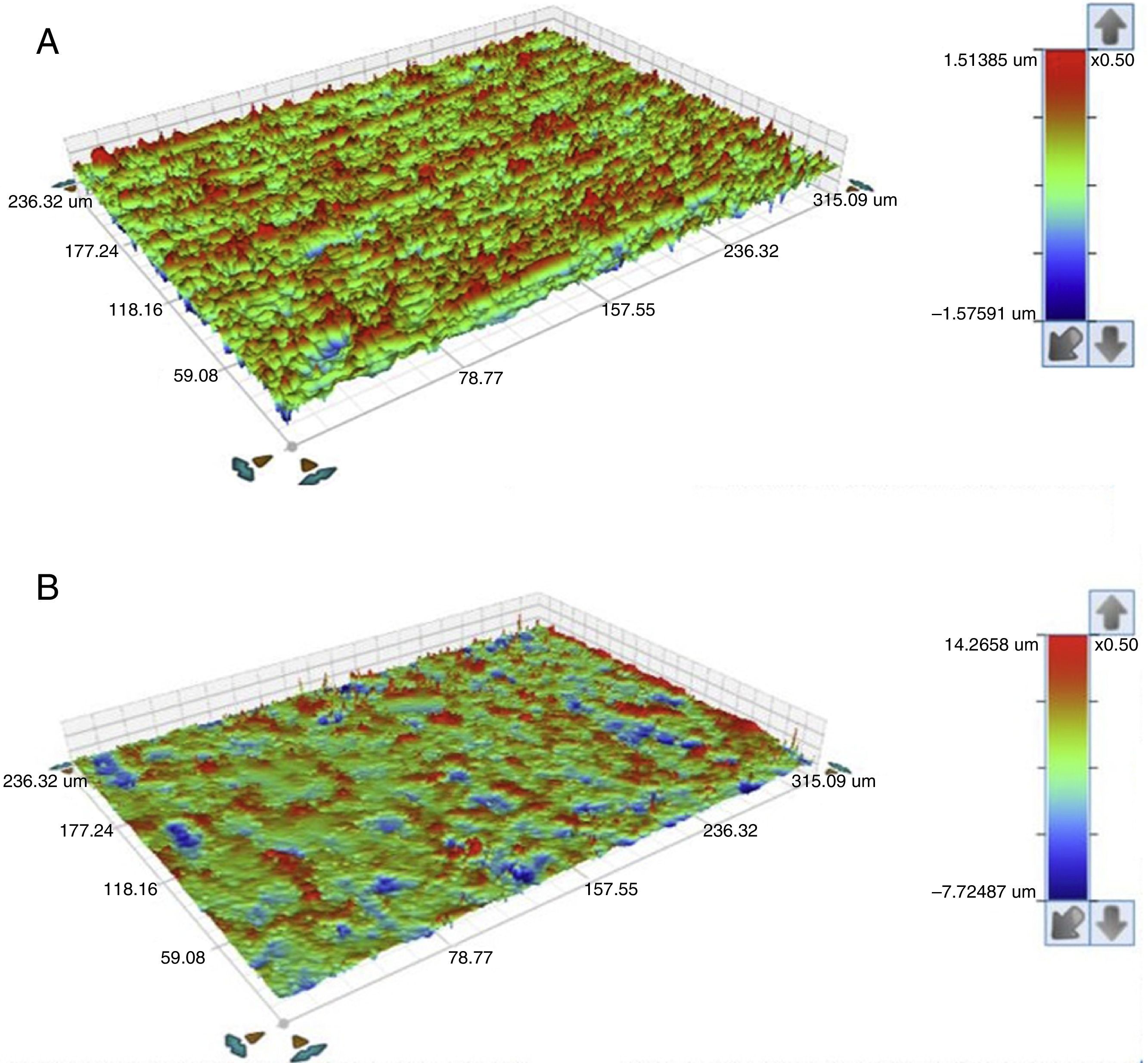
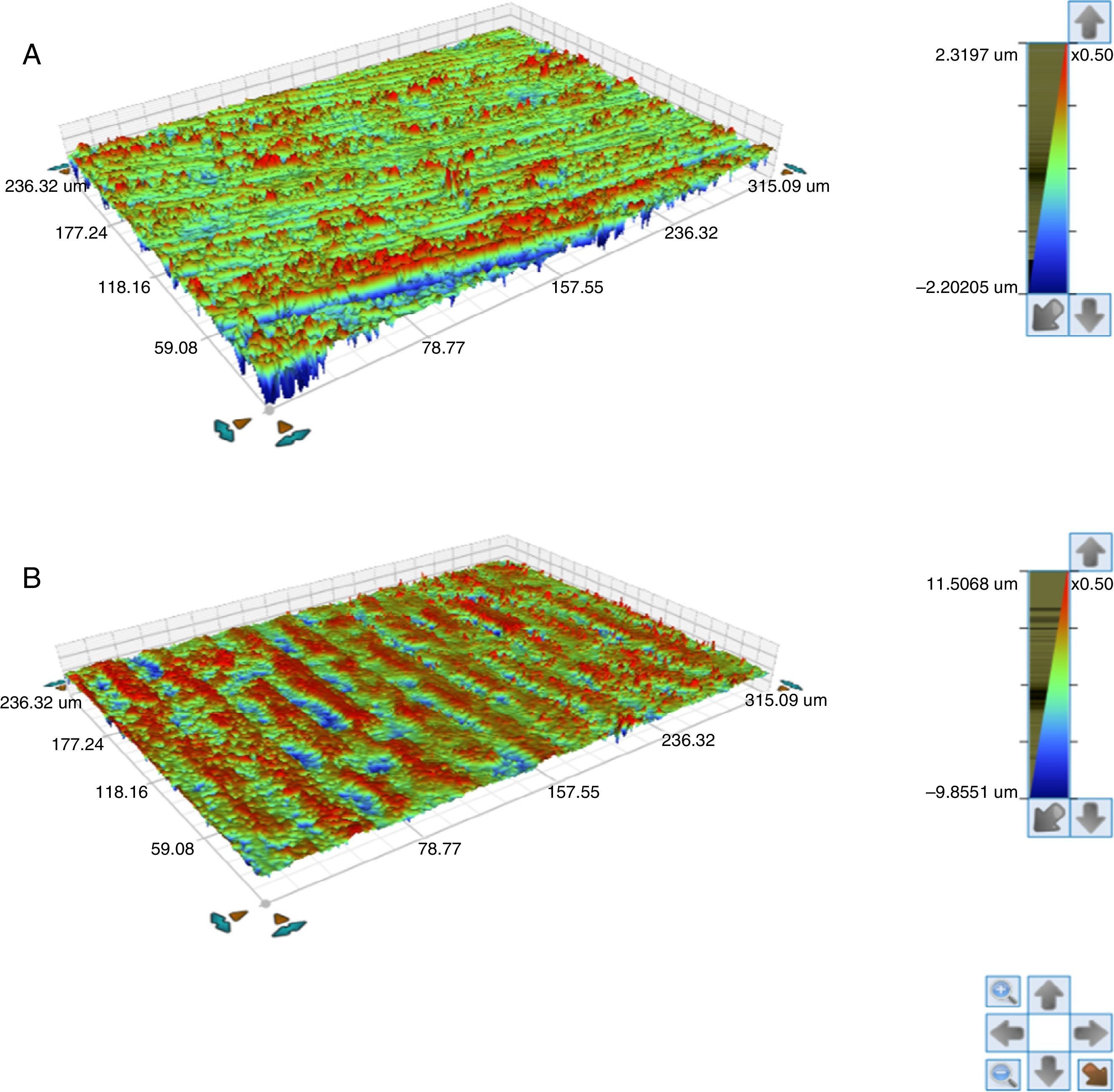
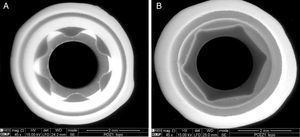
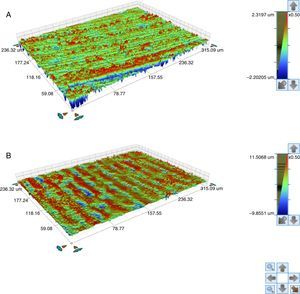
Also an optical interferometry (OI) was conducted on 3 samples of each study group, on the seating surface of the zirconia abutments of both connections to measure the surfaces’ topography by 3D profilometry, using a white light interferometer (Bruker NPFLEX™, Stuttgart-Germany). For each reading in areas of 320μm×240μm, a 10× magnifying lens with a lateral resolution of 1μm was used. The Sa roughness parameter was determined with a two-dimensional Gaussian filter of 80μm.
Then, the samples were subjected to a thermocycler (Ethik Technology®, São Paulo-Brazil) for 5000 cycles of 60s with a temperature range of 5–55°C. For the cyclic loading test, the abutments were torqued into the corresponding implants using a calibrated digital torque wrench (TorqueMeter TQ-8800 Lutron®, China Republic), applying the torque recommended by the manufacturer. All implants were stabilized in a vise.
The abutment/implant sets were embedded in polyurethane resin (Axon® F160, Axson Technologies, France), in a vertical position, keeping a 3-mm distance between the implant's platform and the top of the cylinder, to simulate vertical bone resorption, according to the ISO 14801 standard. Then, these sets were introduced in a thermomechanical fatigue testing machine (Cicladora Termomecânica ERIOS-37000 Plus, São Paulo-Brazil) and subjected to an 88.8N loading with 4Hz frequency for 1,200,000 cycles while submerged in 37°C distilled water.46,47 The loading was applied to the palatal surface of each set, 2mm away from the incisal edge, with a 30° angulation to the long axis of the abutment/implant set.48
After the samples had been detorqued, they were again subjected to XRD, as previously described (Table 2). OI readings were also conducted in the seating platforms after TCML, with the establishment of the Sa parameter.
List of implants and abutments of the samples.
| Material | Description | Manufacturer |
|---|---|---|
| BNT S4 implant ref BNT 04.100 | CE implant with 4.1mm diameter×10mm length | Phibo, Spain |
| Aurea RP implant ref AUR RP 43 100 | CI implant with 4.3mm diameter×10mm length | Phibo, Spain |
| Syntesis Zr BNTS4 abutment | Zirconia abutment for BNT S4 implant with 8mm height×5mm wide | Phibo, Spain |
| Syntesis Zr Aurea abutment | Zirconia abutment for Aurea RP implant with 8mm height×5mm wide | Phibo, Spain |
| Ti screw for BNT S4 ref H19.3450 | Retaining screw for BNT S4 implant | Phibo, Spain |
| Ti screw for AUR RP ref AUR RP 10.1 | Retaining screw for Aurea RP implant | Phibo, Spain |
| Axon F160 | Polyurethane resin | Axon, France |
No statistical test was applied to the data of the XRD technique, because of their descriptive and observational nature. A statistical analysis of the OI data was performed with software (IBM SPSS Statistics v23.0; IBM Corp). For the comparative study of two independent groups used the nonparametric test Mann–Whitney. Significant differences test were used to detect significant differences among the group means (p<0.05).
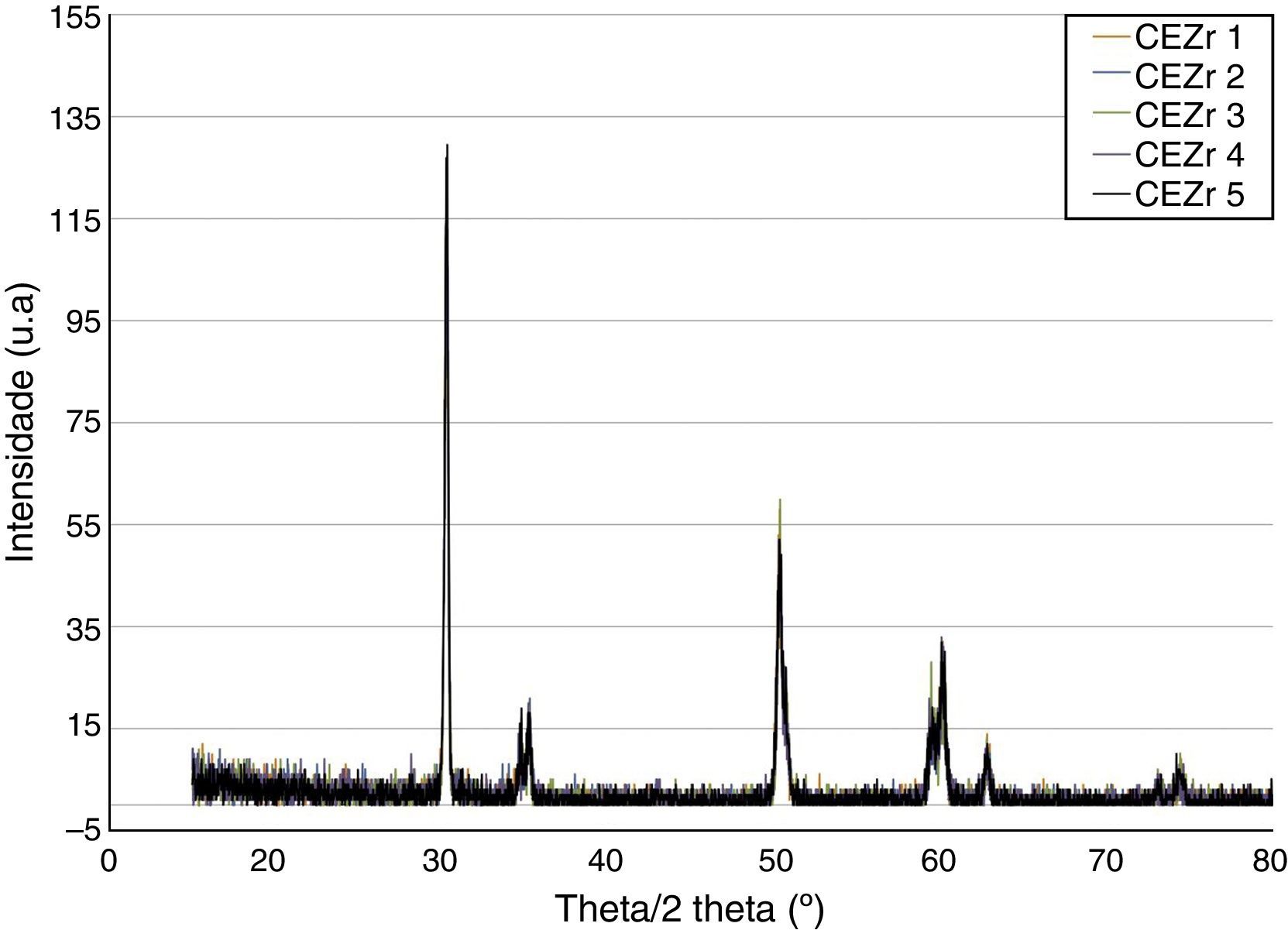
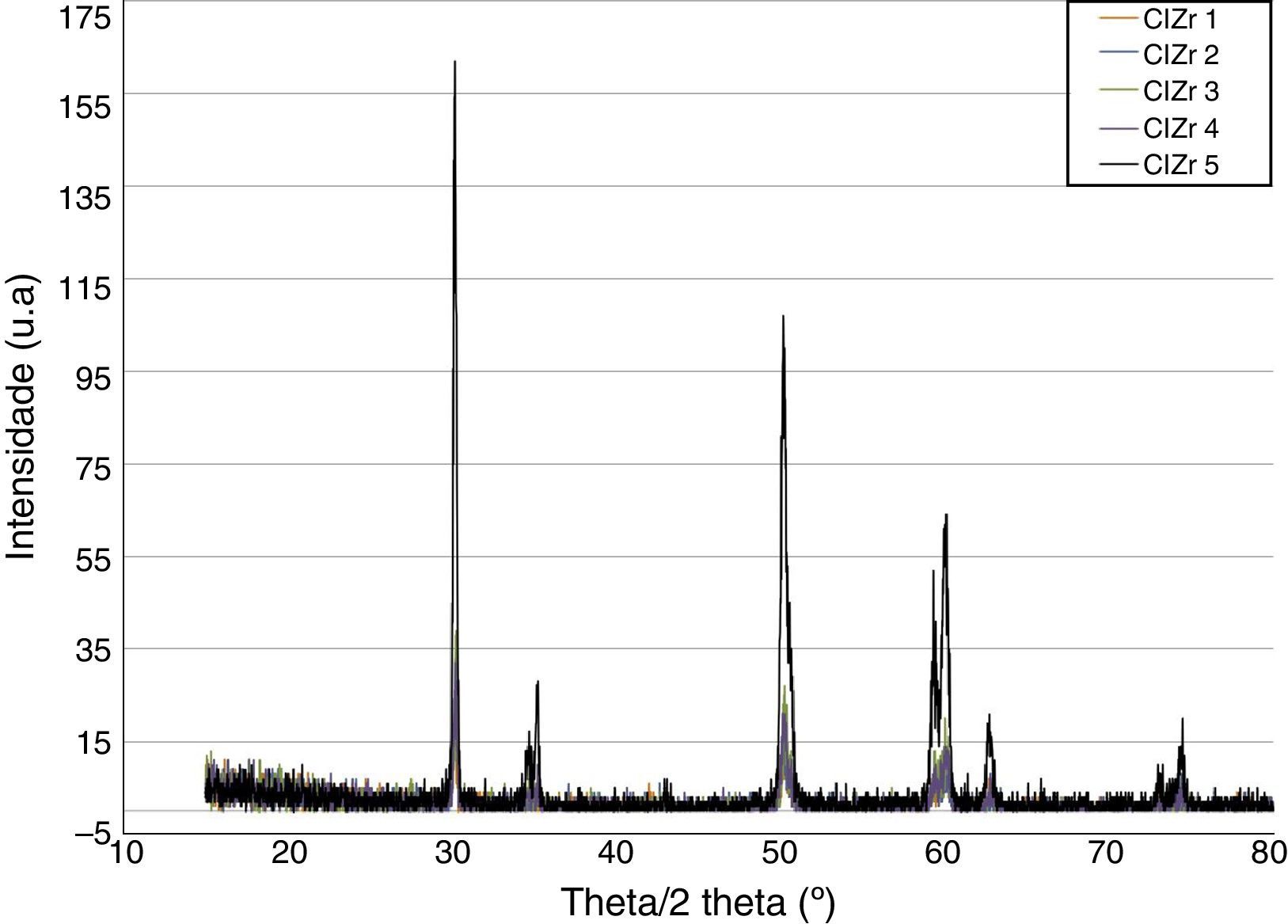
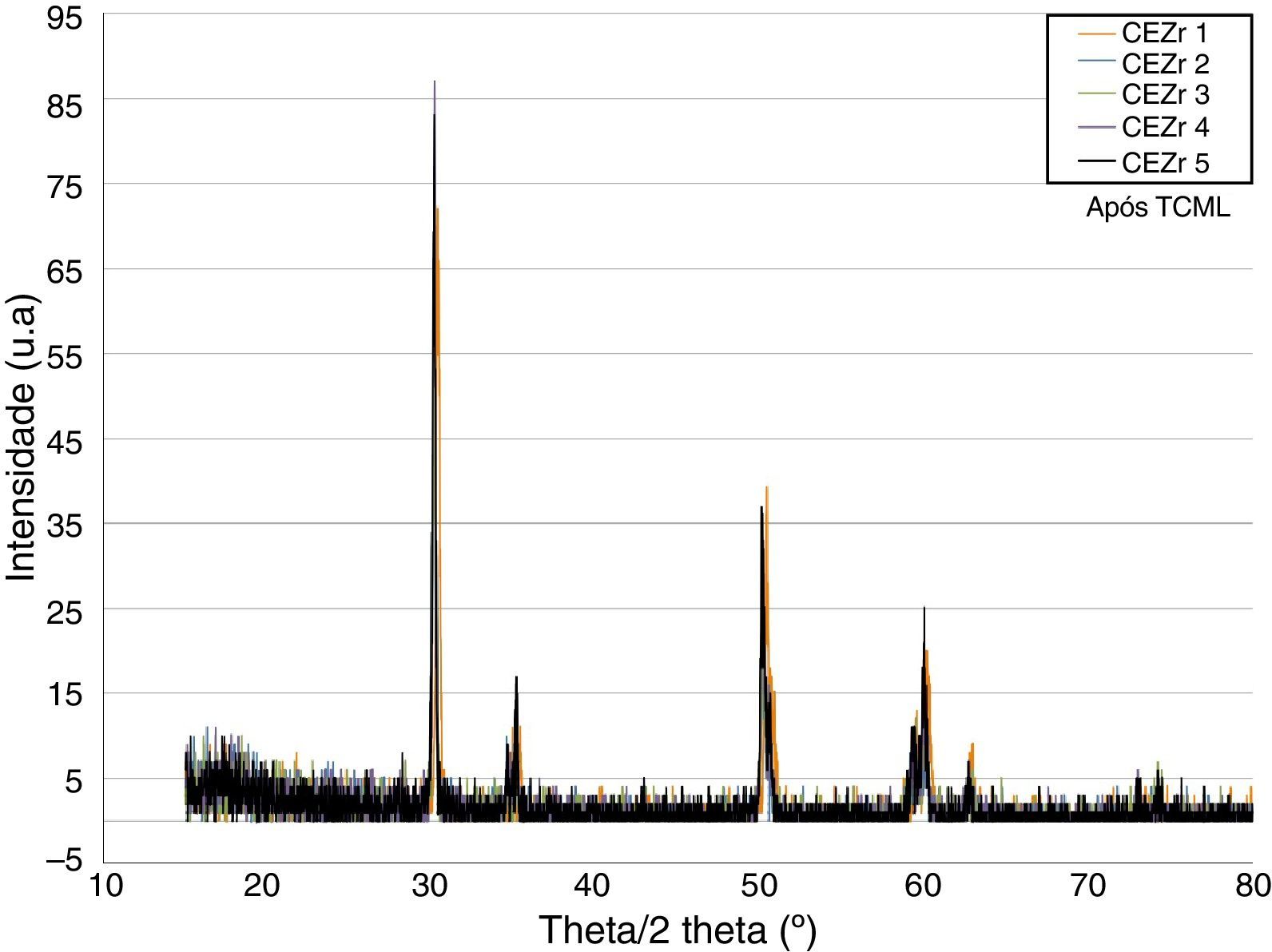
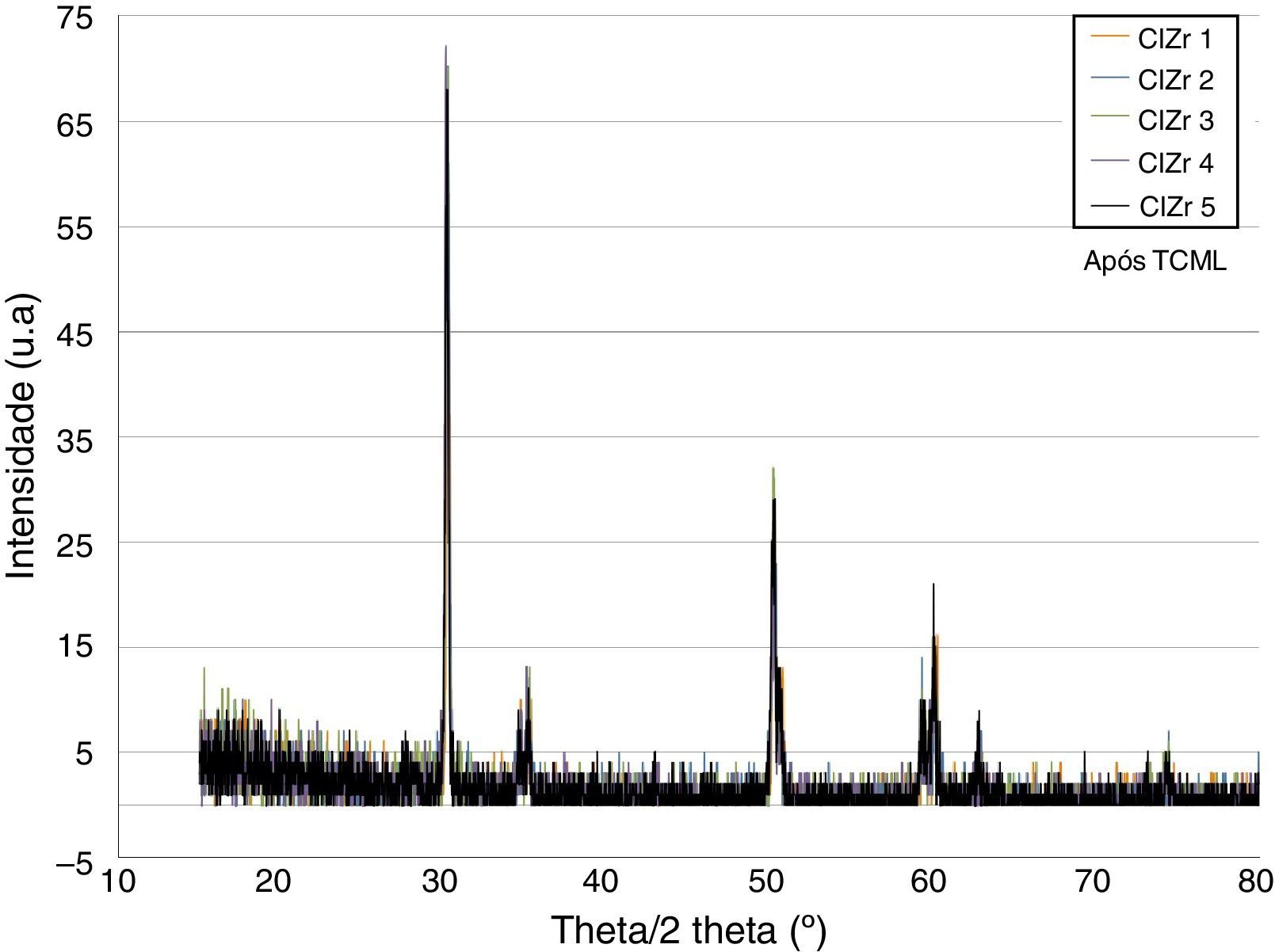
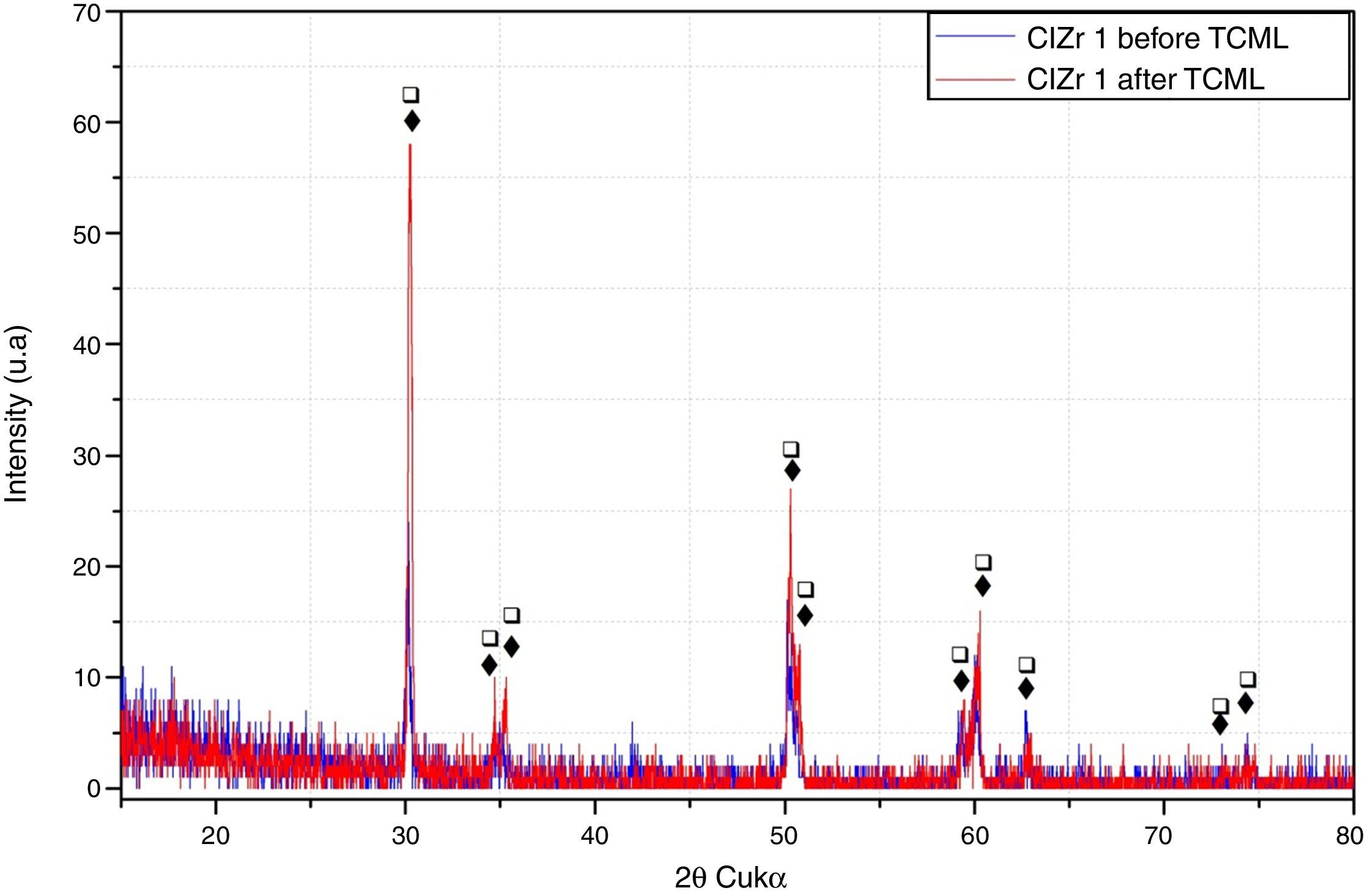
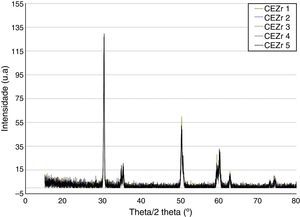
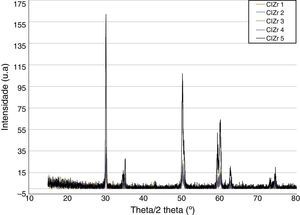
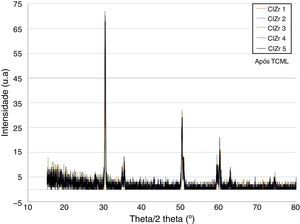
ResultsBefore TCML, the analysis of the diffractograms for phase identification revealed that samples “CEZr 1” to “CEZr 5”, as well as “CIZr1 to “CIZr 5” had peaks that corresponded to the diffraction of a tetragonal phase of 3Y-TZP. No other phase was detected. Diffractograms of the CEZr and CIZr groups showed similar diffraction results (Figures 3 and 4). In fact, the various samples had similar scans, differing only in the intensity of the peaks, and this variation was more evident in the “CIZr 5” sample.
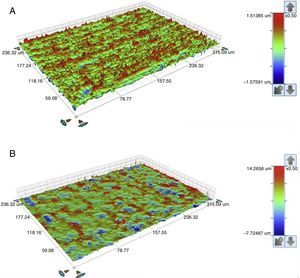
The seating platforms for the zirconia abutments showed a mean Sa of 496nm for external connection abutments and 506nm for internal connection abutments at the initial state (Figure 5).
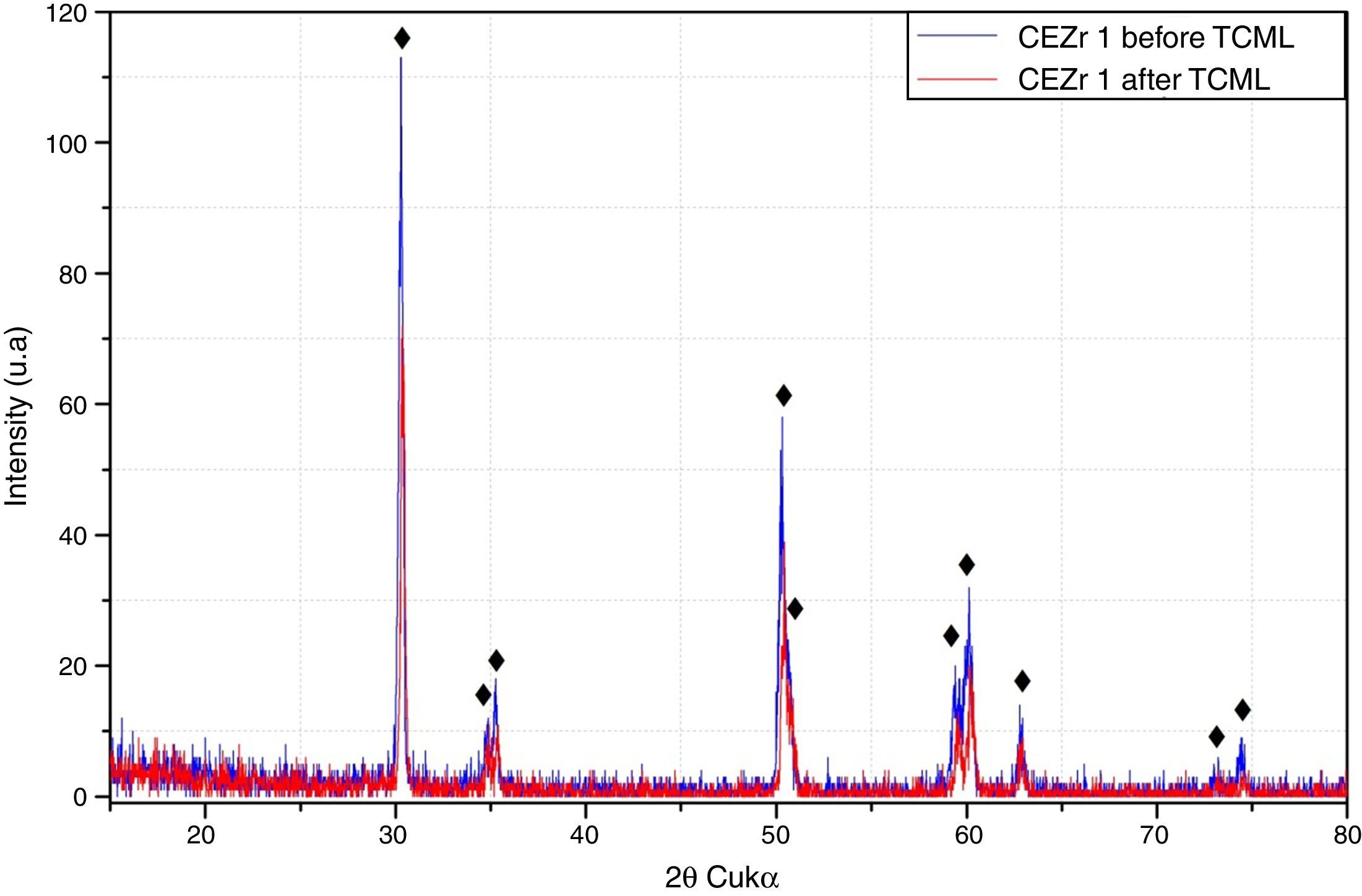
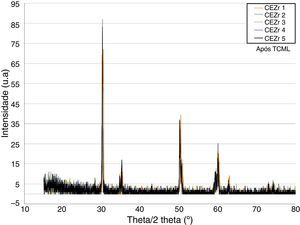
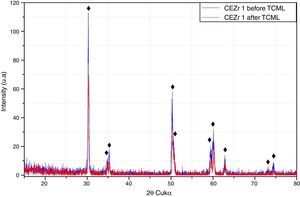
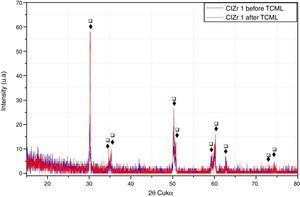
After TCML, the crystalline structure of the samples was assessed by XRD analysis, and the results are presented in Figures 6–9, where the crystalline phases identified in the samples can also be identified through peaks indexation. Figures 6–7 present a comparison of the XRD spectra of the analyses conducted after the TCML of the external and internal connection conditions. All samples showed a similar diffraction pattern. Figures 8 and 9 shows the graphs obtained for each condition before and after TCML.
The results obtained in the XRD characterization of the samples analyzed after TCML, using EDS, revealed the tetragonal phase of 3Y-TZP in every sample, with no evidence of the monoclinic phase (Figures 10–11).
The seating platforms for the zirconia abutments showed it a mean Sa after TCML of 542nm for external connection abutments and 672.6nm for internal connection abutments (Figure 12). Statistically significant differences in the distribution of values Sa (Mann–Whitney test U=57.0, p=0.161>0.05), before and after TCML, and between implants connections (Mann–Whitney test, U=57.0, p=0.053>0.05) were not detected.
DiscussionZirconia partially stabilized is metastable at room temperature, which provides good mechanical properties. However, in the presence of body fluids, it is sensitive to aging, which has negative effects on those same properties. At room and/or body temperature, aging is a slow process but may become relevant a few years later.
The stability of zirconia was assessed to determine if aging had occurred. XRD was used, to comparatively analyze the corresponding diffractograms, before and after TCML, and characterize the detected crystalline phases.
Other analysis methods have been used, such as aging predictions based on subjecting the samples to increased temperatures in an autoclave at 134°C and 2 bars, for 6h to simulate approximately 5–20 years of exposure to body temperature.38,39 Nevertheless, those methods have raised doubts regarding the extrapolation of the process from an in vitro to an in vivo context.
Therefore, the most adequate method seems to be the thermocycling and mechanical loading fatigue assessment.44,46,47 That was the method used in this study, thus corresponding to five years of thermomechanical cycles in the oral cavity.
Different techniques for assessing aging have been described in the literature. This transformation consists in a crystallographic change that is reflected in the surface roughness. Techniques such as XRD, Raman spectroscopy, Atomic Force Microscopy (AFM), Optical Interferometry (OI), and Scanning Electron Microscopy (SEM) are the most used and most referred in the literature. In this study, XRD and OI were used.40 XRD is a classic nondestructive method that is the first option for assessing stability with the characterization of the crystallographic phases from a qualitative and quantitative perspective.
The analysis of the diffractograms before TCML revealed that every sample had similar diffraction patterns, there was no monoclinic phase, and samples were composed of Y-TZP in the tetragonal phase. The diffractogram of Figure 4 showed a greater intensity in the diffraction peak on the “CIZr2” sample, comparing with other samples from the same group, due to the higher degree of crystallinity. After TCML, abutments continued to have similar diffraction patterns, and there was still no monoclinic phase and no signs of aging. Since the monoclinic phase was not detected, no Rietveld analysis was conducted to quantify it. The null hypothesis could not be rejected.
The use of two different connections associated with different stress patterns in the interfaces and micromotion after screwing on the corresponding implants, and their subjection to cyclic loading did not represent a differentiating factor in aging. The comparison of the diffractograms obtained before and after TCML (Figures 8–9) revealed only one difference: 3Y-TZP was detected in the samples of external connection before TCML while zirconium oxide was detected in the samples with internal connection before TCML. However, this difference in the stoichiometry does not imply a change in the crystalline structure, as every sample presents a tetragonal crystalline structure.
Basilio et al.44 detected aging signs through XRD analysis only in half of the of samples of zirconia abutments torqued into external connection implants, subjected to cyclic loading and/or thermocycling. Cotes et al.43 evaluated aging in the disk shape samples and reported an increase in the m-phase contend in all the samples. The different results obtained in this study may be explained by the fact that aging depends on the microstructural features of zirconia, despite all the standardization in the manufacturing process.
Nevertheless, XRD has some limitations. Namely, it is less precise on small changes in fractions of less than 5% of the transformed phase, which occurs particularly in the first stages of aging.40 Another limitation is the fact that XRD is only limited to the surface, not surpassing a depth of a few micra,43 and thus the obtained results may show some variability, depending on the location of the scanning beam in the sample.
The results obtained in this study lack confirmation by other more sensitive techniques for the first stages of aging. Namely, OI and/or AFM, which are other nondestructive techniques that are sensitive to changes in surface topography, as the formation of the monoclinic phase is always accompanied by an increased volume.37,40 On the other hand, using SEM for analyzing in depth the referred changes implies destroying the sample, and the specific preparation technique may contribute itself to the transformation of the crystallographic phase, which can lead to analysis errors.
The surface topography analysis, through the roughness comparison (using the Sa parameter) of the abutments’ seating platforms before and after TCML (using 3D profilometry) showed only a slight increase in both connections after TCML, respectively 46nm in the group CETi and 166nm in the group CIZr. So, this aging method did not promoted surface alterations when compared to control group. Similar results were reported by Cotes et al.43
Nevertheless, the results were not conclusive. Although the equipment had a lateral resolution of 1μm and a vertical resolution of 1nm and the areas of transformation into the monoclinic phase in the zirconia surface appear as small elevations with approximately 2μm height, it was challenging to detect those areas using this technique.43
AFM, which is more sensitive to small surface elevations, might be a good option. However, its scanning area is too small, with approximately 100μm×100μm, and it is challenging to implement it in more-complex geometries, as implant abutments.
Furthermore, it should be noted that the detection of aging in its initial stages is a difficult task that may only be assessed by using various techniques together.
ConclusionsWithin limitations of this study the following conclusion were made:
After a 5-year simulation of its clinical use, the analyzed 3Y-TZP zirconia abutments did not show signs of aging. Also, the connection's geometry — external or internal — does not interfere in aging.
Ethical disclosuresProtection of human and animal subjectsThe authors declare that no experiments were performed on humans or animals for this study.
Confidentiality of dataThe authors declare that no patient data appear in this article.
Right to privacy and informed consentThe authors declare that no patient data appear in this article.
Conflicts of interestThe authors have no conflicts of interest to declare.
Authors gratefully acknowledge the funding of Project NORTE-01-0145-FEDER-000022 – SciTech – Science and Technology for Competitive and Sustainable Industries, cofinanced by Programa Operacional Regional do Norte (NORTE2020), through Fundo Europeu de Desenvolvimento Regional (FEDER).