Structural bone allografts have become an alternative in the treatment of limb bone tumours with a chance of limb-saving surgery. We present an observational retrospective study on the use of structural bone allografts in bone tumours of the long bones in our hospital between January 1993 and January 2010, with a sample of 37 patients subjected to this surgical technique. After obtaining clinical information from our sample we applied the Mankin and EVACOM HUVA functional scales with excellent, very good and good results in 84%, and with the radiological information we applied the International Symposium on Limb Salvage (ISOLS) osseointegration scale, with 95.6% of excellent results after 24 months. These results demonstrate that structural bone allografts are a valid and reproducible technique in patients with destructive long bone tumours.
Los aloinjertos óseos estructurales han supuesto una alternativa al tratamiento de los tumores óseos de miembros, con posibilidad de cirugía de conservación del mismo. Presentamos un estudio retrospectivo observacional del manejo de los aloinjertos óseos estructurales en tumores óseos de huesos largos en nuestro hospital, durante los años 1993 a 2010, en el que obtenemos una muestra de 37 pacientes subsidiarios de esta técnica quirúrgica. Mediante la obtención de datos clínicos de la muestra aplicamos las escalas de funcionalidad de Mankin y EVACOM HUVA con resultados excelentes, muy buenos o buenos del 84%, y con los datos radiológicos aplicamos la escala de osteointegración ISOLS con un 95,6% de resultados excelentes a los 24 meses. Estos resultados nos muestran que los aloinjertos óseos estructurales constituyen una técnica válida y reproducible en pacientes con tumores óseos destructivos de huesos largos.
The constant evolution of medicine has led to the emergence and development of new technologies that have improved the results obtained in the treatment of different conditions. As in other medical disciplines, the initial approach in the management of oncological surgery is to preserve life and limit damage, while maintaining the functionality of the affected limb insofar as possible. However, modern medicine also requires optimisation of quality of life by avoiding complications attributable to treatment and minimising the use of mutilating procedures such as amputations.1 In this sense, one of the most important advances of the past 50 years has been the development of bone and tissue banks, whose impact is such that a significant number of surgeries performed today at the best orthopaedic surgery centres are made possible by the availability of bone and tendon allografts.2 Thus, in recent years, the progress of chemotherapy, new technologies for cryopreservation of tissues and the development of new surgical techniques and instruments for the treatment of tumours have changed the approaches in oncological bone surgery, making amputation less and less frequent and enabling techniques which preserve affected limbs.3
Tumour surgery usually involves extensive resections requiring complex reconstructions. Some alternatives proposed for such cases are endoprostheses, as well as autologous or allogeneic bone transplants.1
The use of bone defect reconstruction techniques at our centre began in the year 1993, with the use of structural allografts. This was the treatment of choice in reconstructive tumour surgery due to the satisfactory results obtained from the functional point of view. The objective of this work is to conduct a descriptive study through which to assess our experience and results obtained in the management of structural bone allografts, both in regard to surgical technique employed and to tumour condition.
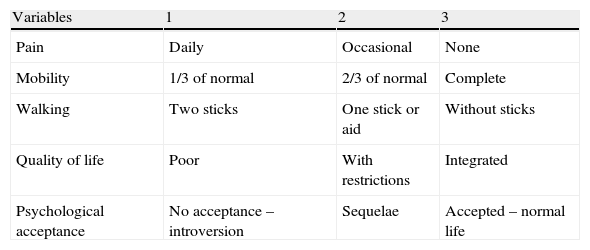
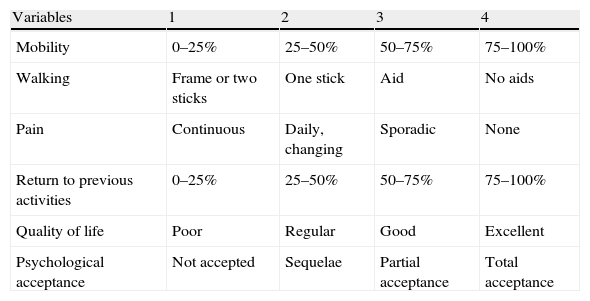
Materials and methodsAll patients included in the study were diagnosed with benign or malignant destructive bone tumour and were treated through the use of structural bone allografts to compensate the bone defect created after resection. They were all monitored at our institution for a mean period of 60 months (range: 5–153 months). We conducted a descriptive, retrospective, observational, longitudinal study of a case series, during the period from January 1st, 1993 to January 1st, 2010. The group consisted of 37 patients diagnosed and treated entirely at Hospital Universitario Virgen de la Arrixaca. We analysed the outcomes of treatment with allografts depending on the surgical technique employed, morbidity, complications, Mankin4 and EVACOM HUVA (Table 1 and Table 2) functionality and satisfaction scales, ISOLS5 osseointegration scale (Table 3), tumour recurrence rates, patient survival and allograft survival according to risk factors, following the protocols established by the hospital to access data from medical records in order to carry out this type of research publication and dissemination to the scientific community.
Variables to be measured according to the Mankin scale and their scores.
| Variables | 1 | 2 | 3 |
| Pain | Daily | Occasional | None |
| Mobility | 1/3 of normal | 2/3 of normal | Complete |
| Walking | Two sticks | One stick or aid | Without sticks |
| Quality of life | Poor | With restrictions | Integrated |
| Psychological acceptance | No acceptance – introversion | Sequelae | Accepted – normal life |
Excellent: 13–15 points; good: 9–13 points; regular: 6–8 points; poor: 3–5 points.
Variables to be measured according to the EVACOM HUVA scale and their scores.
| Variables | 1 | 2 | 3 | 4 |
| Mobility | 0–25% | 25–50% | 50–75% | 75–100% |
| Walking | Frame or two sticks | One stick | Aid | No aids |
| Pain | Continuous | Daily, changing | Sporadic | None |
| Return to previous activities | 0–25% | 25–50% | 50–75% | 75–100% |
| Quality of life | Poor | Regular | Good | Excellent |
| Psychological acceptance | Not accepted | Sequelae | Partial acceptance | Total acceptance |
Excellent: 22–24 points; very good: 19–21 points; good: 15–18 points; regular: 12–14 points; poor: 8–11 points; very poor: 4–7 points.
We used the log-rank test (Mantel–Cox) to calculate the differences between the various aspects studied, while other estimates were computed using Fisher's exact method and the Pearson Chi-square test, considering a value of P<.05 as statistically significant.
We excluded from the study those patients with malignant limb bone tumours who were not candidates for limb conservation surgery and those diagnosed but not treated at our centre.
The case series comprised 41 cases, of which 4 patients were finally excluded; 2 because they were referred to another facility for treatment (iliac osteosarcomas, a priori susceptible of conservative surgery), 1 because he was intervened at a centre in another region and 1 case who suffered cardiotoxicity and died at the beginning of neoadjuvant chemotherapy treatment.
Of the 37 patients in the series, 18 were males (48.6%) and 19 were females (51.4%), with a mean age of 29.27 years (range: 14–74 years), with the most common decade being the second, in 35.13% of cases.
Patients in our study group had no family history of cancer, except for 1 case of chondroblastic osteosarcoma with a history of bone osteosarcoma of unknown cause in a first-grade relative. In terms of personal history, we noted the metastasis of breast carcinoma with history of primary breast carcinoma in 4 cases in our series, all of them were treated by radical mastectomy and chemotherapy–radiotherapy according to protocol.
Clinically, 94.6% of patients suffered pain during their first consultation, 45.9% reported a subjective increase in limb size and 78.4% presented functional impairment. On physical examination, 37.8% of patients presented inflammation, 48.6% presented enlargement of the affected limb and 81.1% presented functional impairment with respect to the contralateral limb.
We found 3 cases in the humerus (8.1%), 18 cases in the femur (48.6%), 11 cases in the tibia (29.7%) and 5 cases in the radius (13.5%). The 3 cases developed in the humerus all appeared in the proximal region (8.10%); in the femur, 3 cases were proximal (8.10%), 3 cases were medial (8.10%) and 12 cases were distal (32.43%); in the tibia, all 11 cases were proximal (29.72%), and in the radius, all 5 cases were distal (13.51%).
The mean time to first consultation in our specialty was 3.69 months (range: 0.1–12 months); the mean time to diagnosis of bone neoplasm was 2.13 months (range: 0.1–24 months), while the mean time to completion of surgery was 6.89 months (range: 0.3–48 months).
With respect to complementary examinations: cortical insufflation was the most common finding in conventional radiology, in 94.6% of cases; in computed tomography (CT-scan), cortical fracture was found in 89.2% of cases and medullary destruction in 64.9%, whereas in magnetic resonance imaging (MRI) the mean tumour length was 7.81cm (range: 3.5–15cm), with soft tissue infiltration being observed in 37.8% of cases, physeal involvement in 50% of patients younger than 17 years and no patients presenting neurovascular involvement.
The extension study of primary bone tumours was negative in 100% of cases using conventional radiology, CT-scan, MRI and bone scintigraphy scans.
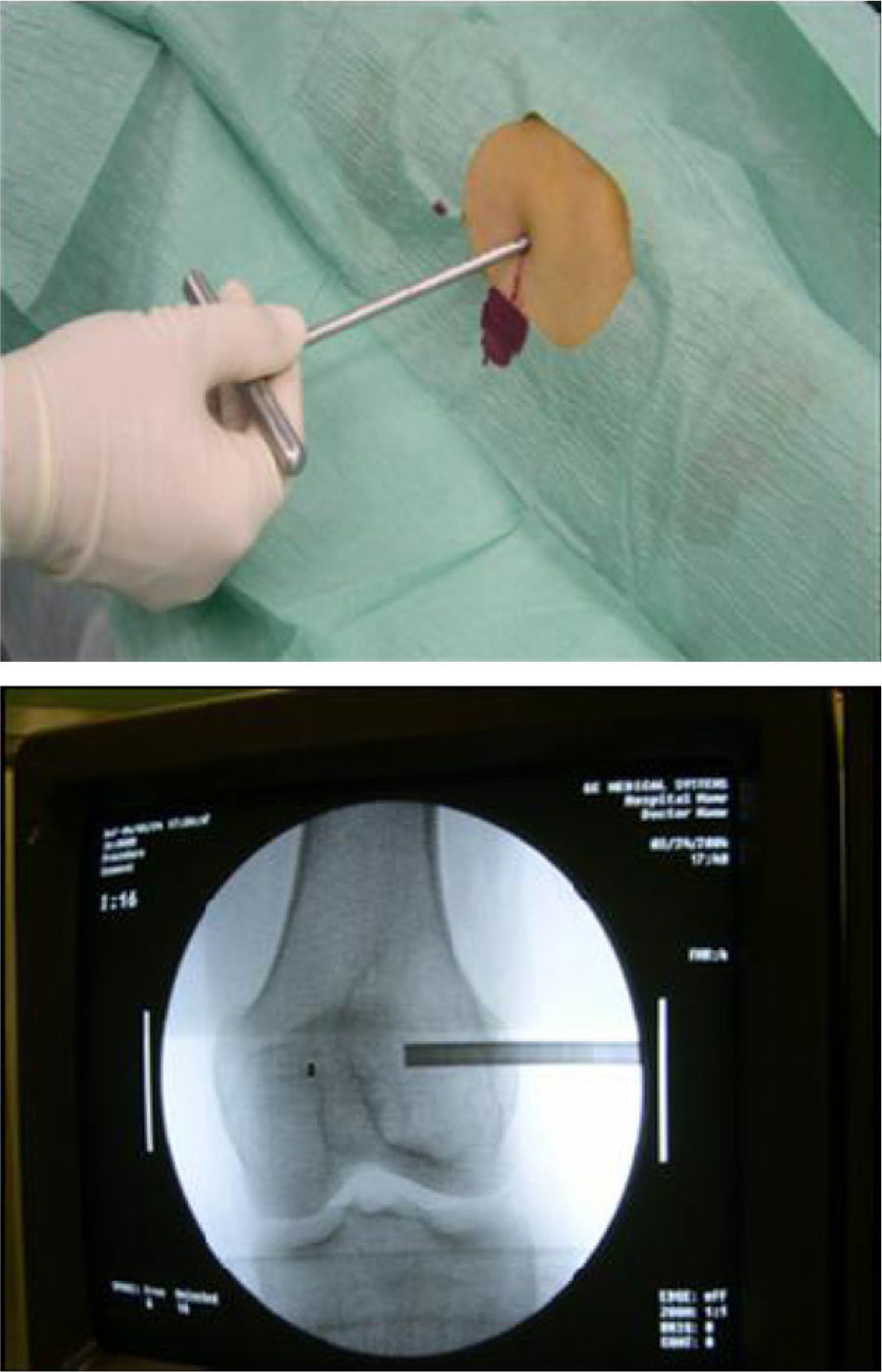
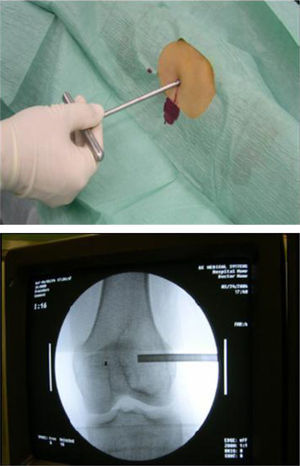
From the anatomopathological point of view, 97.3% of patients underwent preoperative percutaneous biopsy with a trocar guided by radioscopy under local anaesthesia and sedation (Figs. 1 and 2). Only 1 patient required an open biopsy (2.7%), since we failed to obtain sufficient material for diagnosis.
Our series comprised 17 osteosarcomas (45.9%), 6 of which were conventional osteosarcomas (16.2%), 7 were parosteal osteosarcomas (18.9%), 2 were chondroblastic osteosarcomas (5.4%), 1 was a periosteal osteosarcoma (2.7%) and 1 was a telangiectatic osteosarcoma (2.7%). There were 10 cases of giant cell tumour (27%), of which 5 cases were conventional (13.5%) and 5 cases were associated with an aneurysmal component (13.5%). There were 4 cases of metastatic breast carcinoma (10.8%), 3 cases of chondrosarcoma (8.1%), 2 cases of Ewing sarcoma (5.4%) and 1 case of malignant osteoblastoma (2.7%).
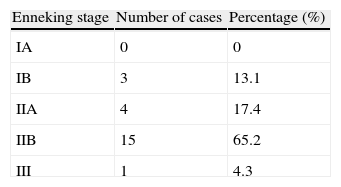
According to the Enneking stages,6 out of the 23 bone sarcomas in our study we classified 13.1% as stage IB, 17.4% as stage IIA, 65.2% as stage IIB and 4.3% stage III (Table 4).
Only 24 tumours in the series were subsidiary of chemotherapy (62.2%): 17 osteosarcomas, 4 cases of metastatic breast carcinoma, 2 Ewing sarcomas and 1 high-grade chondrosarcoma. We excluded the 10 giant cell tumours in the series (27%), 2 chondrosarcomas which were not eligible for chemotherapy (8.1%) and the only case of malignant osteoblastoma (2.7%).
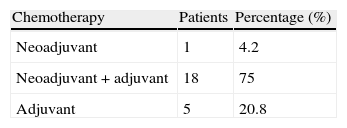
The types of chemotherapy (CHT) employed were: neoadjuvant in 4.2% of cases, adjuvant in 20.8% and neoadjuvant+adjuvant in 75% (Table 5). The most common complications derived from CHT were nausea and vomiting in 95.8% of cases, with a mean hospitalisation period due to complications of 11 days.
As for the method employed, all allografts in our series were structural bone allografts from multiorgan donors and were submitted by the Tissue Bank of Alicante, which belongs to the Organ and Tissue Bank Network of Valencia, which was in charge of processing and preserving the specimens.
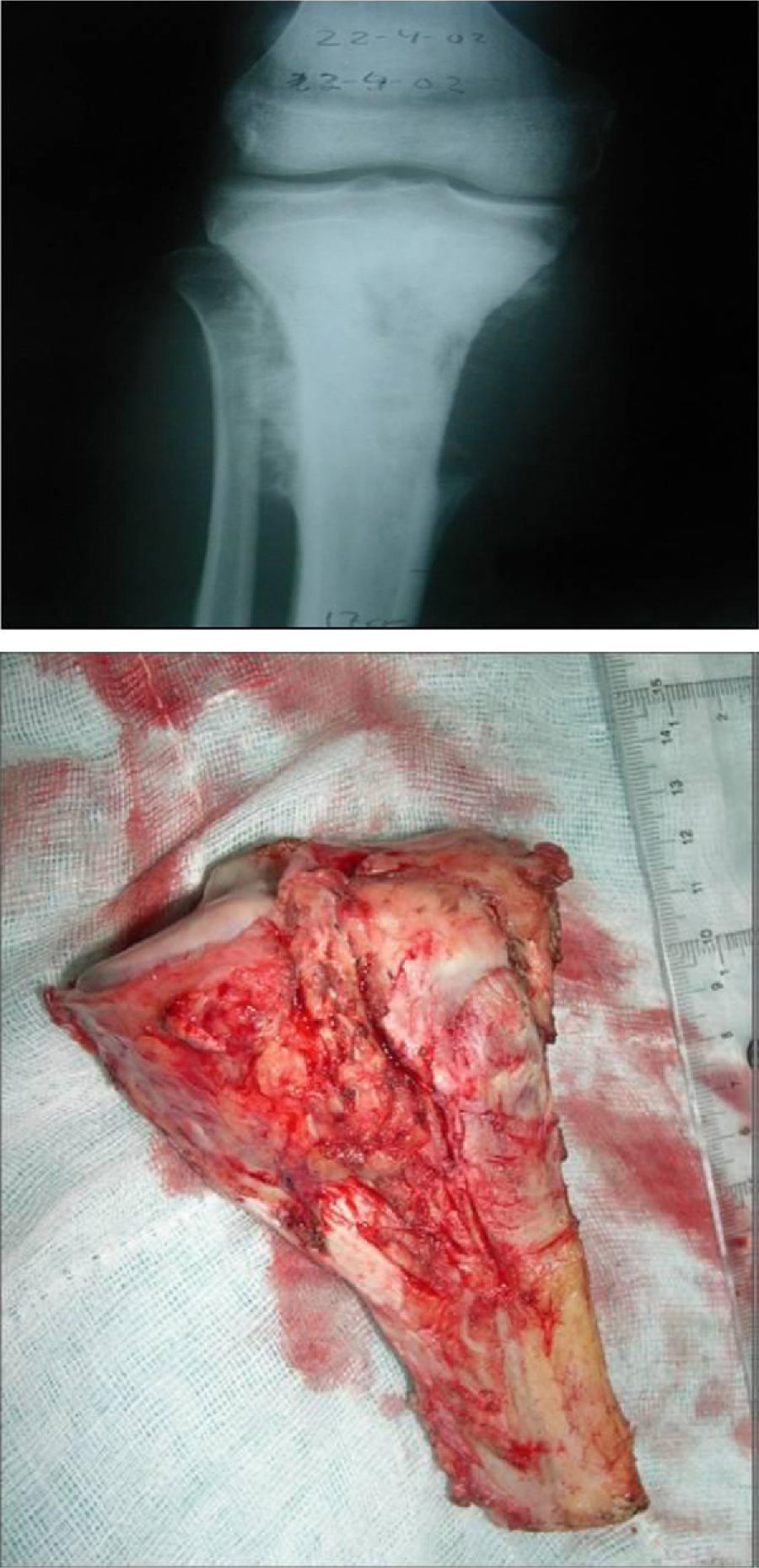
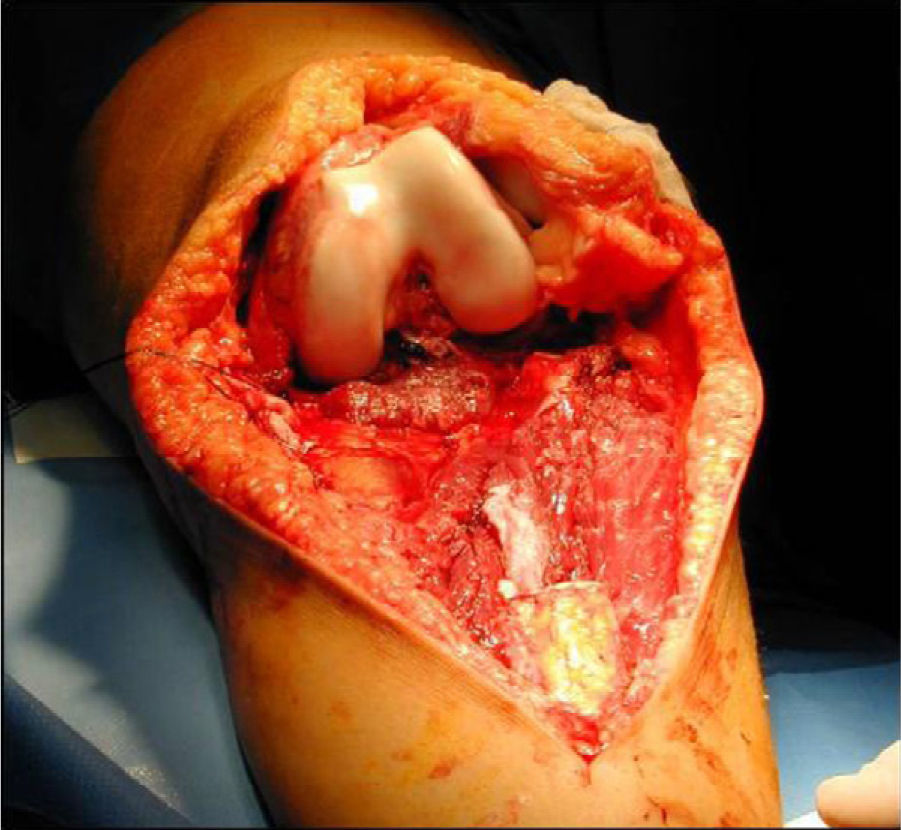
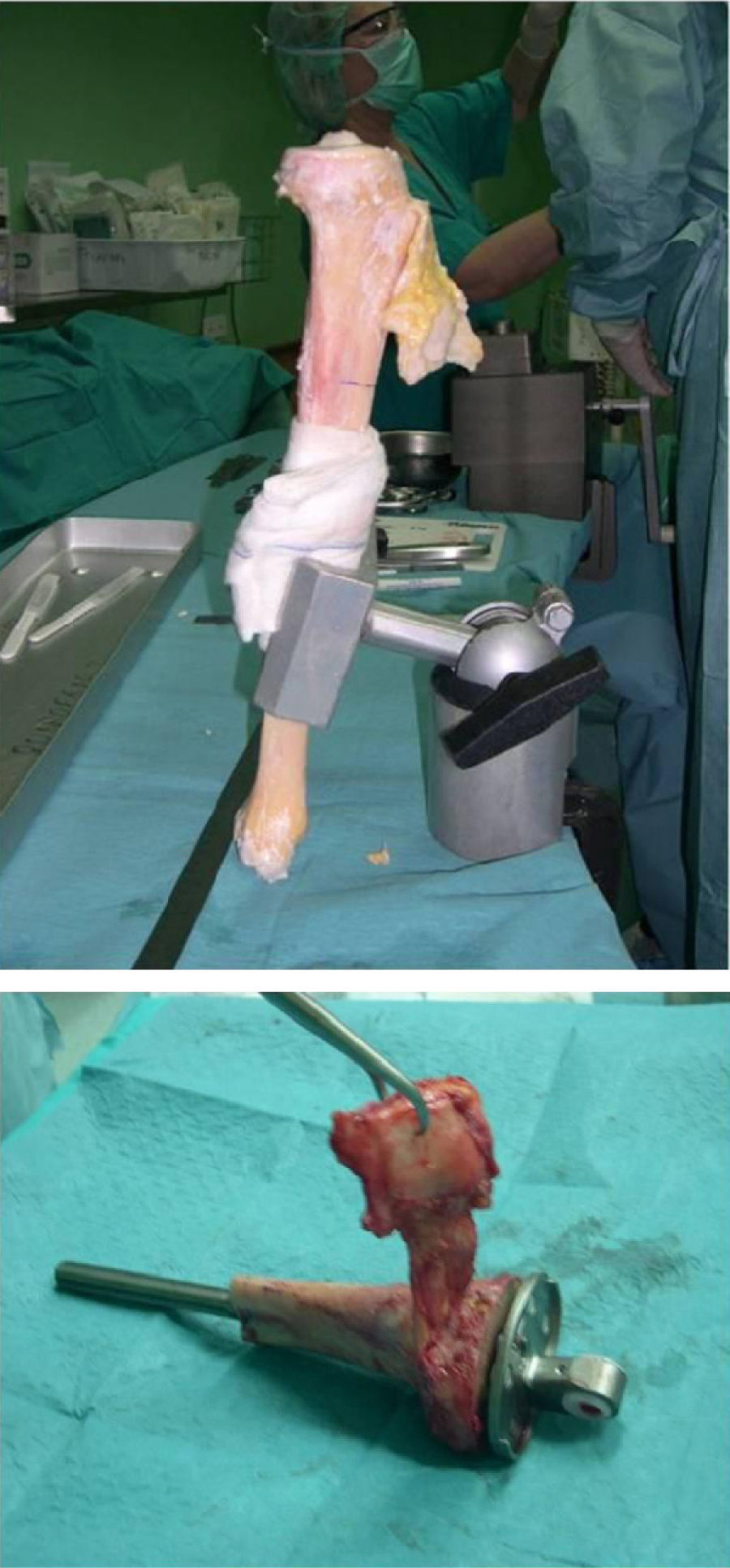
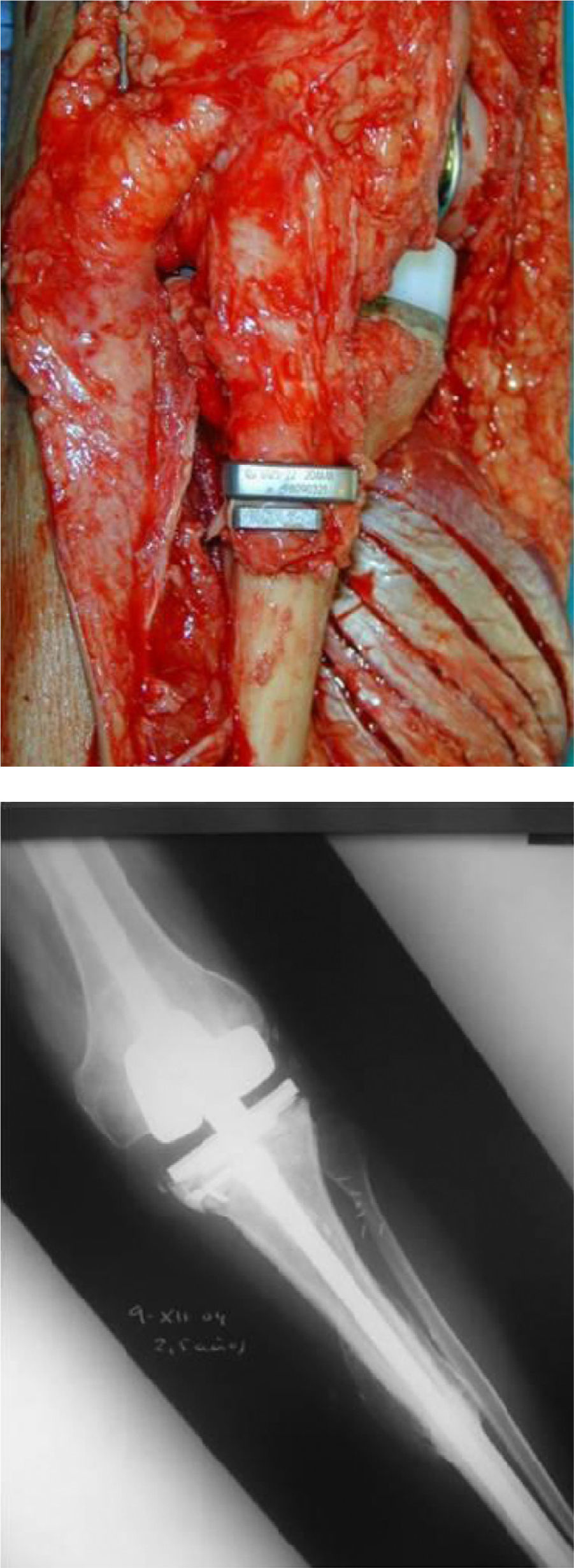
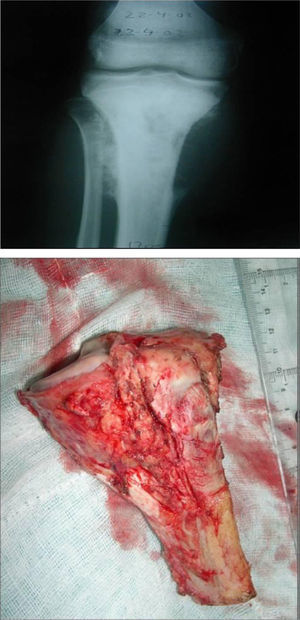
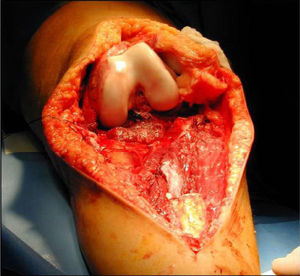
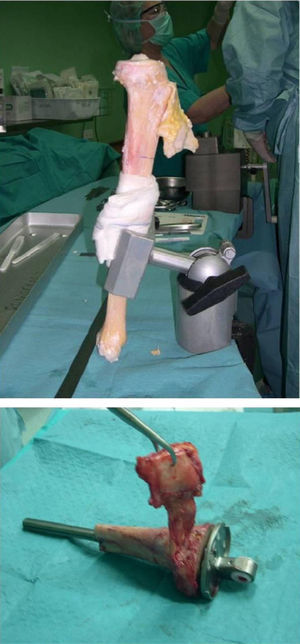
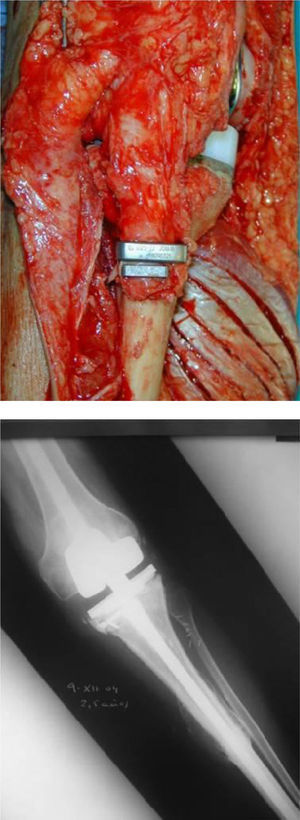
Once patients were diagnosed with a destructive bone tumour of long bones, they were examined by a Tumour Committee, which then decided the attitude to adopt and determined if patients were subsidiary of limb conservation surgery. In this case, after thorough preoperative planning and obtaining informed consent, a block resection of the tumour was performed with wide surgical margins between 2.5 and 5cm. The incision included the biopsy area and also took a biopsy of the intramedullary area adjacent to the osteotomy, presumed healthy. In another surgical field, a second team carried out the preparation of the structural bone allograft, once the defect was accurately measured. After this was prepared, various techniques were employed to stabilise it, depending on the location of the bone tumour (Figs. 3–9).
In our series, the mean size of tumour resection was 14.7cm (range: 4.5–30cm). The mean safety margin of proximal bone resection was 4.3cm (range: 1–10cm) and of distal bone resection was 4.9cm (range: 1–10cm). The biopsy of the spinal canal adjacent to the osteotomy in all malignant tumours was negative in 100% of cases.
Allograft stabilisation was performed with an endoprosthesis in 59.5% of cases, with an intraspinal pin in 5.4%, with a plate in 16.2%, with an intraspinal pin plus endoprosthesis in 10.8% and with endoprosthesis plus plate in 8.1%.
A cadaver graft was used in the host–allograft interface in 54.1% of cases, in 24.3% we associated a freeze-dried allograft to the cadaver graft and in 21.6% we did not use a graft. A plastic cover was used in 13.5% of cases, with 4 of these being in the proximal tibia using a rotation muscle flap of the medial gastrocnemius and 1 case in the distal fibula using a vastus lateralis myocutaneous flap.
We required a mean transfusion volume of 3IU (range: 1–9IU), with a mean operative time of 6.3h (range: 4–7.5h) and a mean hospital stay of 11 days (range 4–40 days).
We observed the following complications in patients included in the study:
- (a)
Surgical wound infections in the immediate postoperative period: these were observed in 2 patients (5.4%), with both cases being superficial skin infections caused by Staphylococcus epidermidis and both being treated through intravenous antibiotic therapy with vancomycin and daily local cures.
- (b)
Fractures: there were 2 cases of incomplete fracture of the proximal tibia (5.4%) and 1 complete case in the distal femur (2.7%), all being caused by diameter discrepancies between endoprosthesis–allograft–host bone. They were resolved through delays in waiting time for full load on the limbs (4 months in incomplete fractures and 5 months in complete fractures).
- (c)
Pseudarthrosis (no evidence of allograft–host union after 6–8 months or mobility of focus with atrophic or hypertrophic callus): this developed in 5 patients (13.5%), with 4 patients requiring reoperation for curettage, association of a new graft and stabilisation. One of them remained in therapeutic abstention due to lack of symptoms and died from oncological causes after 10 months.
- (d)
Breakage of material: this was observed in 2 cases (5.4%), both requiring reoperation.
- (e)
Prosthetic loosening: this took place in 5 cases (13.5%), all requiring prosthetic replacement.
As for the results in terms of functionality, the following scales were applied:
- –
Mankin scale4: with 8.1% of poor outcomes (removal of the allograft, limb amputation or death as a direct result of a local recurrence), 8.1% of regular results (requiring external support for walking, pain or functional limitation interfering with work and daily life), 32.4% of good results (no evidence of disease, scarce limitation of function, without pain and without need for external support) and 51.4% of excellent results (no evidence of disease or pain, normal function with no limitations).
- –
Assessment scale for bone allografts in musculoskeletal oncology surgery of Hospital Universitario Virgen de la Arrixaca (EVACOM HUVA): this internal scale of our centre obtained very poor results (4–7 points) in 5.4% of cases, poor (8–11 points) in 2.7%, regular (12–14 points) in 8.1%, good (15–18 points) in 18.9%, very good (19–21 points) in 13.5% and excellent (22–24 points) in 51.4%.
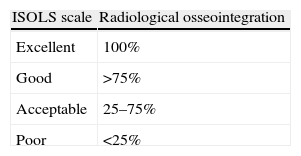
Depending on the radiological allograft–host osseointegration, we classified the results following the ISOLS scale,5 which classifies allograft–bone unions in reference to the percentage of radiographically visible radiolucent line as: excellent (osteotomy line not visible), good (union >75% with osteotomy line still visible), acceptable (union between 25 and 75%) and poor (no evidence of callus or union <25%). The results obtained were as follows:
- •
After 3 months, 83.8% of patients showed a poor outcome in conventional radiology, with 16.2% having an acceptable result.
- •
At 6 months, 46.7% showed a good result in terms of allograft osseointegration, with 37.2% of acceptable results and 16.1% of poor results. The latter included patients with pseudarthrosis and 1 patient with delayed consolidation. The measurement could not be made in 2 patients because they died due to oncological causes.
- •
At 12 months, 50% showed an excellent result, with 37.5% of good results, 9.4% of acceptable results and 3.1% of poor results. The latter included 1 patient with initial pseudarthrosis reoperated 9 months later and with no radiological evidence of osseointegration. The measurements could not be made in 5 cases; in 3 cases due to death from oncological causes and in 2 cases due to patients changing their country of residence.
- •
At 18 months, 88.5% showed an excellent result, with 11.5% of good results. Radiological consolidation could not be measured in 11 patients; in 5 cases due to death from oncological causes, in 2 cases due to change of residence and the remaining 4 patients failed to attend the review.
- •
At 24 months, 95.6% showed excellent results, with 4.4% of good results. The measurements could not be made in 14 cases; in 6 cases due to death from oncological causes, in 2 cases due to change of residence and the remaining 6 patients failed to attend the review.
With respect to the rate of tumour recurrence, the overall rate was 29.7% (11 patients), with bone sarcomas in 8 patients. Of these, 7 cases (87.5%) were stage IIB and 1 case (12.5%) suffered parosteal osteosarcoma in stage III. The mean time to recurrence was 11 months (range: 3–42 months). We observed that allograft failure occurred mainly during the first year after surgery, remaining stable thereafter.
The overall rate of metastasis was 29.7%, with a mean time from diagnosis of 21 months (range: 5–54 months) and a mean time of 17 months since surgical treatment (range: 3–52 months). There were 2 cases of conventional osteosarcoma, 2 cases of parosteal osteosarcoma, 2 cases of Ewing's sarcoma, 3 cases of metastatic breast carcinoma, 1 case of periosteal osteosarcoma and 1 case of chondrosarcoma. The most common location was the lung, in 63.6% of cases. Metastases were treated with palliative treatment in 72.7% of cases and with surgical treatment in 29.7%. Regarding the 8 bone sarcomas of the 11 metastatic patients, stage IIB was predominant in 75% (6 cases), along with 1 case (12.5%) suffering stage III parosteal osteosarcoma (12.5%) and 1 case (12.5%) suffering stage IIA chondrosarcoma.
In relation to the survival of the patients in our series, on January 1st, 2010 and after a mean follow-up period of 60 months (range: 5–153 months), the overall death rate of patients was 32.4% (12 cases), with 67.6% (25 cases) of patients being alive. In all cases (100%), death was due to oncological causes in malignant tumours, unrelated to treatment by bone allografts, resulting in 70% of deaths between 3 and 5 years after surgery.
Overall survival of allografts in our series was 70.3%, with a higher percentage of technical failures in the first year, which subsequently remained stable and showed an absence of failures.
Regarding the results in terms of allograft survival according to different risk factors, following the Enneking stage we obtained a worse overall survival of allografts in bone sarcomas with Enneking stage ≥IIB with higher rates of tumour recurrence, in a statistically significant manner (P<.05). Similarly, we did not obtain statistically significant results in terms of tumour aggressiveness (benign–malignant), postoperative infection, periprosthetic fissures, fractures, pseudarthrosis, breakages of material, prosthetic loosening, stabilisation of the allograft with endoprostheses, plates or pins or type of chemotherapy used, probably due to the small size of the sample.
DiscussionStructural bone allografts have represented an alternative in the treatment of limb bone tumours with the possibility of limb conservation surgery. We did not find any differences in the studies reviewed with regard to male/female ratio, age at presentation, medical history, clinical presentation and physical examination.
In our series, the mean time since patients reported the onset of symptoms until consultation with a traumatology specialist was 3.6 months (range: 0.1–12 months). Moreover, the mean time elapsed since patients were assessed by a doctor until a definitive pathological diagnosis was obtained was 2.1 months (range: 0.1–24 months), thus adding up to a mean time since the beginning of symptoms until diagnosis of 5.7 months. This delay in diagnosis is decreasing with respect to the review of publications7 and with respect to series published at Hospital Virgen de la Arrixaca in Murcia,8,9 which shows an improvement in the early diagnosis of these lesions. This improvement is closely linked to the creation of bone tumour reference centres and the establishment at such centres of Tumour Pathology Expert Committees from different specialties, who individually plan tactics for obtaining a definitive diagnosis and subsequent treatment with the least possible delay. However, despite this improvement, these diagnostic delay figures should make us reflect about how to strengthen the awareness of primary care physicians for suspicion and early diagnosis of these lesions, since 65% of the cases in our series were at Enneking stage IIB. This figure is similar to that found in the published literature10 and it implies a worse prognosis and increased tumour aggressiveness with respect to early stages.
The most commonly affected joint was the knee, in 62% of cases, coinciding with the data provided by authors such as Mankin11 or Muscolo,12 with tumours rates around the knee of 60–70%. This location is most likely related to an association between age/location/period of bone growth, usually affecting young people with greater bone growth in the juxta-articular physis of the knee, where growth is more pronounced.
Treatment by bone allografts applied to patients in our series required an adequate surgical stabilisation according to tumour location, with endoprosthesis stabilisation being the most frequent in our series, in 78% of cases, since the knee was the most common location in the tumours studied, in accordance to the results of various series.13
Regarding the functionality of allografts implanted in patients in our study, this was evaluated using the EVACOM HUVA and Mankin scales. According to the Mankin scale, and after a mean follow-up period of 60 months with 67.6% of patients being alive (25 cases), our study obtained excellent and good results in almost 84% and only 16.2% of regular or poor results, all of which were due to local recurrence of oncological causes and not derived from the allografts. Without these oncological problems we would have, hypothetically, obtained excellent and good results close to 100% in our series, superior to those obtained by Mankin in his series with 85% of excellent results,14 although not superimposable due to the small sample size.
As for the EVACOM HUVA scale, the sum of excellent, very good and good results was 84%. As in the Mankin scale, the negative results derived from the use of allografts were due to oncological causes in the form of tumour recurrences. These EVACOM HUVA scale results were not comparable to other series evaluating the functionality of allografts, since this scale is only intended for internal use at our hospital. Nevertheless, it has very good correlation with the Mankin scale and offers more information regarding patient satisfaction and quality of life.
Regarding the functionality of allografts measured by the Mankin and EVACOM HUVA scales, we found no statistically significant differences with respect to protocols and type of chemotherapy used, or with respect to tumour aggressiveness (benign–malignant) or the type of allograft stabilisation employed. We did find a statistically significant relationship (P<.05) with better results in both scales, with higher allograft survival and better radiological evaluation of allografts in the ISOLS scale. There was a relationship, although not statistically significant due to the small sample size, between the functional results and Enneking staging, with worse results in the Mankin and EVACOM HUVA scales for Enneking stages IIB or above, due to the higher rate of tumour recurrence and worse survival of the allografts.
These functionality results of patients who had received structural allografts, combined with the similarity with results from large series published by Enneking,15 Muscolo,12 Fox,16 or Donati,13 show that bone allografts constitute a valid and reproducible technique for patients with destructive bone tumours of long bones, enabling a better quality of life and psychological acceptance than other tumoural surgery techniques such as limb amputation or reconstruction with megaprostheses, as shown in the study published by DiCaprio and Friedlaender.17
Similarly, in addition to assessing the functionality of patients, we evaluated the osseointegration of allografts. This was measured radiologically according to the scale of the ISOLS which is based on the radiolucent line visible in conventional radiology. In our study, after a mean follow-up period of 60 months, we obtained results which improved substantially over time, with no excellent results at 3 months (acceptable in 16%), no excellent results at 6 months (good in 47% and acceptable in 37%), 50% of excellent results at 12 months (good in 37.5%), 88.5% of excellent results at 18 months and 95.6% of excellent results at 24 months. These results were similar to those obtained by San Julián18 in his publications and show that bone allografts require a minimum of 6 months to show radiographically evident signs of good prognosis regarding osseointegration. The best radiological results are obtained 12 months after surgery.
These radiological results show statistically significant differences (P<.05) in relation to:
- (a)
Functional results obtained on the Mankin and EVACOM HUVA scales: with better functionality of intervened patients regarding a better radiological allograft osseointegration.
- (b)
Location: radiological consolidation, understood as a good or excellent ISOLS result, with a mean result of 4 months faster in allografts placed at the metaphysoepiphyseal level than those placed at the diaphyseal level.
Despite having similar results to the various series, we found differences, although not statistically significant due to the small sample size, regarding the administration or not of chemotherapy, with allograft consolidation being faster in those tumours which did not receive chemotherapy.
We measured allograft survival depending on the oncological results obtained. In our study we observed 30% of tumour recurrence after a mean period of 11 months, which shows that the highest percentage of relapses occurred within the first year, thus requiring periodic reviews, and with very high allograft survival after this first year.
In our study, we found statistically significant differences (P<.05) among patients with local tumour recurrence in terms of the probability of tumour recurrence in Enneking stages IIB or above. There were also differences, although not statistically significant due to the small sample size, in the probability of metastasis according to Enneking stage, with higher rates in stages IIB or above. These considerations, together with those discussed previously regarding delays in diagnosis of bone tumours, illustrate the importance of adequate patient selection19 and of ensuring early diagnosis and treatment in order to initiate conservative treatment of limbs in early stages and decrease the recurrence rate of the technique.As for the survival of the patients in our series, after a mean follow-up period of 60 months the overall survival rate on January 1st, 2010 was 68%, with a death rate of 32%, of which 100% were due to oncological causes. In our series, only 1 survivor presented recurrent metastatic disease, thus we obtained a disease-free interval of 50 months and a survival interval (from diagnosis of tumour involvement) of 58 months. These data are similar to those reported in the large series of Muscolo,12 Mankin14 and Fox16 and present better survival rates than the series of Donati,13 which shows that the reconstruction technique using bone allografts is a valid surgical option for patients with appropriate conditions, which does not affect the survival of patients compared with more aggressive techniques such as amputation.1,7
Level of evidenceLevel of evidence IV.
Ethical disclosuresProtection of human and animal subjects. The authors declare that no experiments were performed on humans or animals for this investigation.
Confidentiality of Data. The authors declare that no patient data appears in this article.
Right to privacy and informed consent. The authors declare that no patient data appears in this article.
Conflict of interestsThe authors have no conflicts of interest to declare.
Please cite this article as: López-Martínez JJ, et al. Tratamiento mediante aloinjertos óseos estructurales en resecciones por tumores óseos de huesos largos. Revisión de 37 casos. Rev Esp Cir Ortop Traumatol. 2012;56:286–94.