Spinal arthrodesis consists of a combination of a system of mechanical stabilisation of one or more vertebral segments with a biological substance that promotes osteoneogenesis, with the aim of achieving the permanent fusion between areas more or less the same size of these segments.
In spinal arthrodesis, the biological support par excellence is the autograft. However, obtaining this involves a high incidence of morbidity and, in cases of arthrodesis of more than one intervertebral space, the quantity available is usually insufficient. The extraction and implantation time prolongs the surgery, increasing the exposure to and risk of bleeding and infection. For these reasons, there is a search for substances that possess the properties of the autograft, avoiding the morbidity and added surgical time required to extract the autograft.
The biomechanical–biological interaction in vertebral arthrodesis has been studied in this article.
Una artrodesis vertebral consiste en la combinación de un sistema de estabilización mecánica de 2 o más segmentos vertebrales con una sustancia biológica que promueva la osteogénesis, con el objetivo de conseguir la fusión permanente entre zonas más o menos extensas de dichos segmentos.
En una artrodesis vertebral, el aporte biológico por excelencia es el autoinjerto; sin embargo, su obtención genera una alta incidencia de morbilidad y, en casos de artrodesis de más de un espacio intervertebral, la cantidad disponible suele ser insuficiente. El tiempo de extracción e implantación prolonga la intervención quirúrgica, aumentando la exposición y riesgo a sangrado e infección. Por ello, actualmente hay una búsqueda de sustancias que posean las propiedades del autoinjerto evitando la morbilidad y tiempo de cirugía añadido que genera extraer el autoinjerto.
En este trabajo se estudia la interacción biomecánica-biología en la artrodesis vertebral.
Technically, spinal arthrodesis is the combination of a mechanical stabilisation system of various vertebral segments and a biological substance that promotes osteogenesis, with the objective of achieving permanent fusion between somewhat large areas of said segments.1–4 In fact, once the parts to be fused have been decorticated, spinal arthrodesis conceptually resembles the treatment of multiple successive fracture sites1,2 via a comprehensive osteosynthesis system that joins the fragments and uses the basic principles of osteosynthesis (Fig. 1).
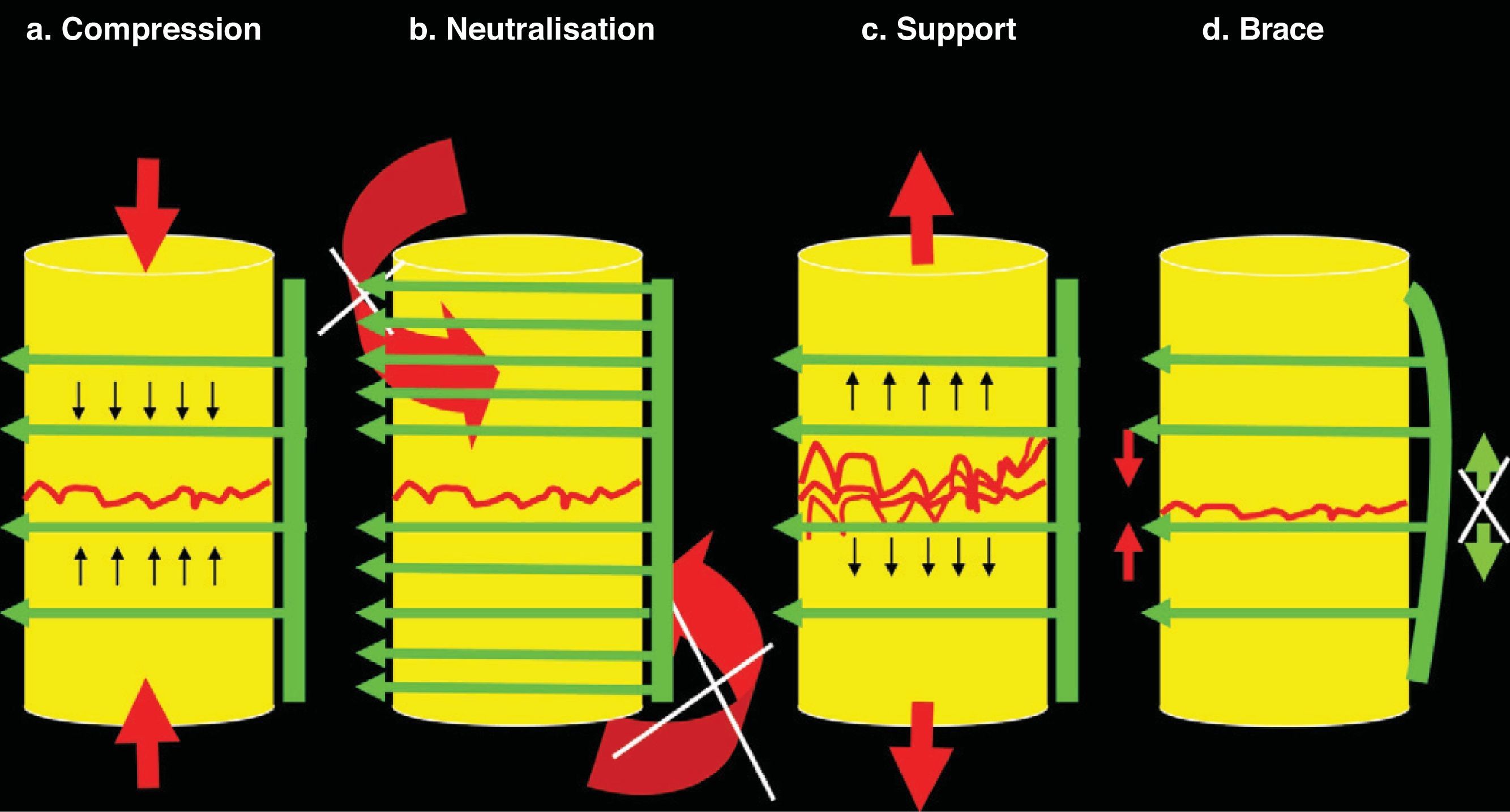
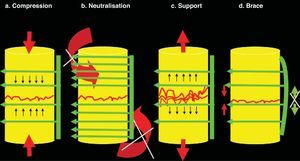
Basic principles of osteosynthesis. In any osteosynthesis, be it of long, short or spinal bone, at least 4 fundamental principles of osteosynthesis should be applied. (a) Compression: Compression consists of being submitted to fracture from confluent forces of contrary vectors. When there is considerable space between the superior and inferior fragments—or between a superior and inferior vertebral body (intersomatic space)—a tricortical graft should be inserted and will undergo compression (between the 2 vertebrae), but will elicit support (to these vertebrae, avoiding approximation to each other). This produces great stability, but disappears after approximately 3–4weeks. Because of this, neutralisation, at least, should be added. (b) Neutralisation: Neutralisation prevents rotational and tangential forces (shear) that displace the fragments from each other. It should be applied after other principles. It is not possible to apply compression after neutralisation because the fragments do not advance towards each other until joining. Neutralisation should always be applied after compression. (c) Support: The support principle maintains fragment height. It is applicable in chip fracture cases on the osteosynthesis shaft or in compression fracture cases on the vertebral body after maintaining height through posterior traction with a pedicle system. (d) Brace: The brace principle combines anterior compression on bone (or graft) with posterior distraction prevention thanks to the implant. The most characteristic example is the olecranon needle-wire system. It is applied to the spine when 360° arthrodesis is performed with an autograft or intersomatic substitute plus the posterior pedicle system.
It is agreed that the most stable osteosynthesis system for posterolateral spinal arthrodesis is a pedicle instrument with an autograft. However, in intersomatic cases, the most stable one is a guided system with support-neutralisation through plates or bars joined to the vertebrae with bolts, coupled with a compression-support intersomatic stabilisation element, like a cortical autograft.1–3 In other words, a biological contribution is necessary with the mechanical construct in order to achieve tissue fusion,5 as without it, the material will end up breaking due to fatigue or the construct will fail. Furthermore, bone formation with element-tissue fusion should be sufficiently robust to withstand mechanical load.6 In some cases, posterolateral fixation is combined with anterior, which is known as 360° arthrodesis (Figs. 2 and 3). Therefore, in any therapeutic action, biomechanical and biological planning should occur together so as to successfully reach the treatment objective.1–3
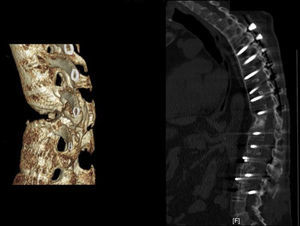
Applying the brace principle with an increase in neutralisation. (a) 3D CT Image of a diabetic patient with ankylosing spondylitis in haemodialysis treatment. (b) Anterior approach with intersomatic tricortical autograft on the iliac crest via an anterior pathway combined with posterior instrumentation. Unlike the previous case, the number of posteriorly fixated vertebrae has been extended due to the characteristic spinal osteoporosis of this illness. The biomechanical construct is stable because it compresses anteriorly, but it will be subject to posterior failure if few levels are fixed due to poor pedicle bolt anchorage.
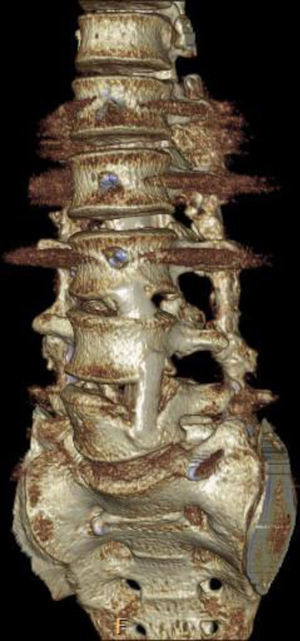
Applying the brace principle with the fibula autograft. The brace principle applied to a patient with tuberculosis at various lumbar levels. Intersomatic fibula autograft that provides good support with interfragmentary compression of the grafts between vertebrae. Combination with fusion via posterior pedicle instrumentation in 360°. After more than a year of follow-up, the fibula autografts are fused to the vertebrae, as can be seen.
Some clear examples of this are shown in scoliosis, ankylosing spondylitis (Fig. 2) and tuberculosis (Fig. 3). In scoliosis, posterolateral consolidation is not uncommon, with breaks in the osteosynthesis bars. It is much more frequent and premature when only 1 posterolateral bar is used, compared to the current pedicle system of using 2 bars. Nevertheless, breaks in the 2-bar system keep appearing, although they occur over several years once the material breaks down due to fatigue. In ankylosing spondylitis, given that the spine acts as a shaft, it is necessary to apply a long instrument that increases neutralisation as the screws may be poorly anchored in porous vertebrae and the mechanical load on a short instrument could result in failure. This type of osteosynthesis is similar to that of a neutralisation plate being applied to the shaft, given that spinal interlocking is technically impossible. However, posterolateral spinal fusion is special in that it is not intended to replace the original anatomy, but rather to form a heterotopic bone bridge where no bone previously existed, which could be the origin of the high clinical failure rate observed.7,8
In spinal arthrodesis, the quintessential biological contribution is the autograft. However, procuring such a graft generates a high incidence of morbidity and, when more than 1 intervertebral disc space is fused, the quantity available is usually insufficient. In addition, the extra time needed to extract and implant it prolongs the surgical intervention, increasing exposure and risk of bleeding and infection. The search for substances that possess the osteogenic properties of the autograft arises in light of the necessity for a biological contribution that promotes therapeutic bone formation, avoiding the inconveniences of autografts.
Alternative substances to the autograft are commonly known as bone replacements. A replacement is a substance that acts as another, while a substitute is a substance that, having properties similar to those of another, can take its place.5 The designation of bone replacement leads to the conclusion that it consists of a substance that, having its properties, acts as a bone. Seeing as no substance exists that possesses live cells—the fundamental characteristic of a bone—it can be asserted that bone replacements do not exist. What do exist are substitutes that have some bone characteristics, but not the fundamental: the cells.6
The biological contribution, be it the autograft or a poorly named replacement, should ideally possess osteogenic, osteoinductive and osteoconductive capacities, while not provoking immunological responses.9
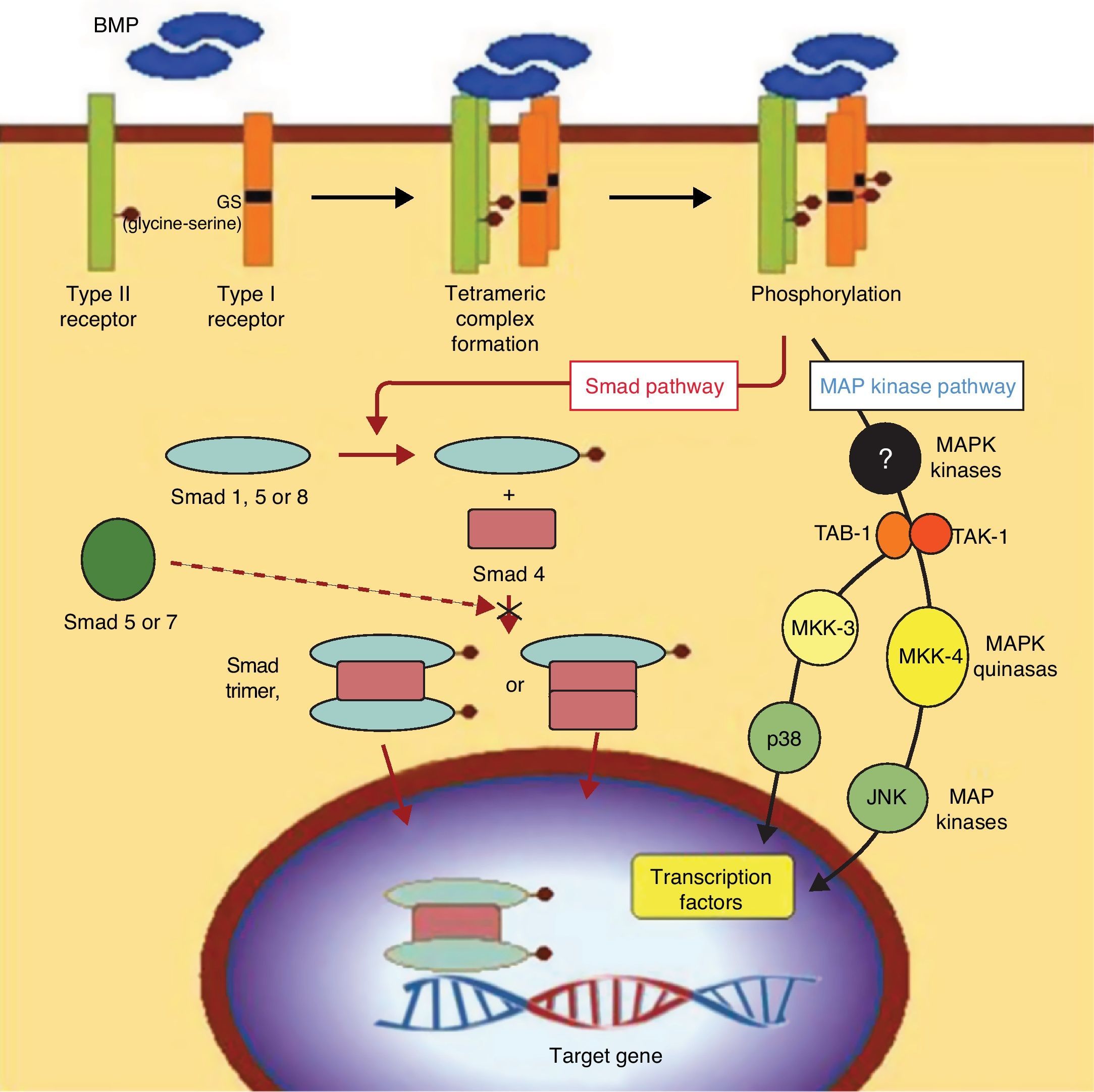
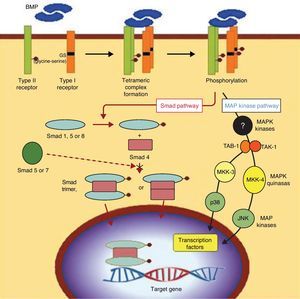
OsteogenesisOsteogenesis is the fundamental factor in efficient consolidation of arthrodesis. It consists of the differentiation between mesenchymal stem cells (MSC) and cells of osteogenic lineage, which are called preosteoblasts.10 This involves a complex process at whose base is the action of proteins from the TGF-beta family, called bone morphogenetic proteins (BMP). These proteins, acting on the specific receptors of the MSC membrane, trigger a cascade of intracellular signals regulated by other proteins that are elements of transcription, called “Smad” proteins (Fig. 4). In reality, various types of cells with different grades of differentiation are included under the name preosteoblasts. The cells range from undifferentiated MSC to differentiated osteoblasts that synthesise the osteoid substance that encompasses them, then differentiating themselves terminally into osteocytes. These, in turn, remain in the bone gaps as maintenance cells for differentiated bone tissue. When mineralised, the osteoid substance constitutes the bone. Thus, bone is made up of mineralised osteoid substance and cells of osteogenic lineage (MSC, osteoblasts, preosteoblasts and, finally, osteocytes). In addition to these osteoblastic-type cells, there are also osteoclasts in the bone, multinuclear cells of a different line that precede monocytes and pertain to the macrophage line, dedicated to bone resorption. Thus, MSC differentiate into preosteoblasts when they receive BMP in their membrane receptors. However, if they receive another type of signal molecule, they differentiate into other cell lines like chondroblasts, adipocytes or fibroblasts. Because of this, MSC are also called multipotent stem cells. From this common source, they can differentiate into different mesoderm lines that constitute different tissues from the same origin. Therefore, once again, the cells from the osteogenic line are those which produce the osteoid substance that is later mineralised to constitute bone and, without osteoblast cells, there is no bone.1,11
BMP signalling. Signal transduction pathway through BMP receptors. The intracellular domains of the type I receptors present a characteristic GS region (rich in glycine and serine), located in N-terminal position to the serine-threonine kinase regions. After the union of the BMP ligand, the type II receptor phosphorylates the GS region of the type I receptor, which involves a process crucial to signal transduction by the serine-threonine kinase receptors. The Smad cascade is initiated by certain Smad proteins phosphorylated by the type I receptor, as well as by the pathways represented by 2 activated protein-kinase cascades (MAPK). At any rate, the ultimate consequence of this signalling process is the regulation of transcription genes in the cell nucleus.
Osteoinduction is the capacity that certain molecules have to link to the membrane of competent cells (MSC) and induce multiplication and differentiation of the preosteoblast line. These, like others that exercise similar actions in the organism, are known as growth factors (GF). Growth factors are signalling molecules because they transmit the MSC signal that triggers multiplication and ultimately differentiation. There are many GF families that have different effects according to the type and degree of the cellular differentiation. Possibly the most important GF family in bone tissue are the transforming growth factors beta (TGF-beta) and among them, the BMP.12
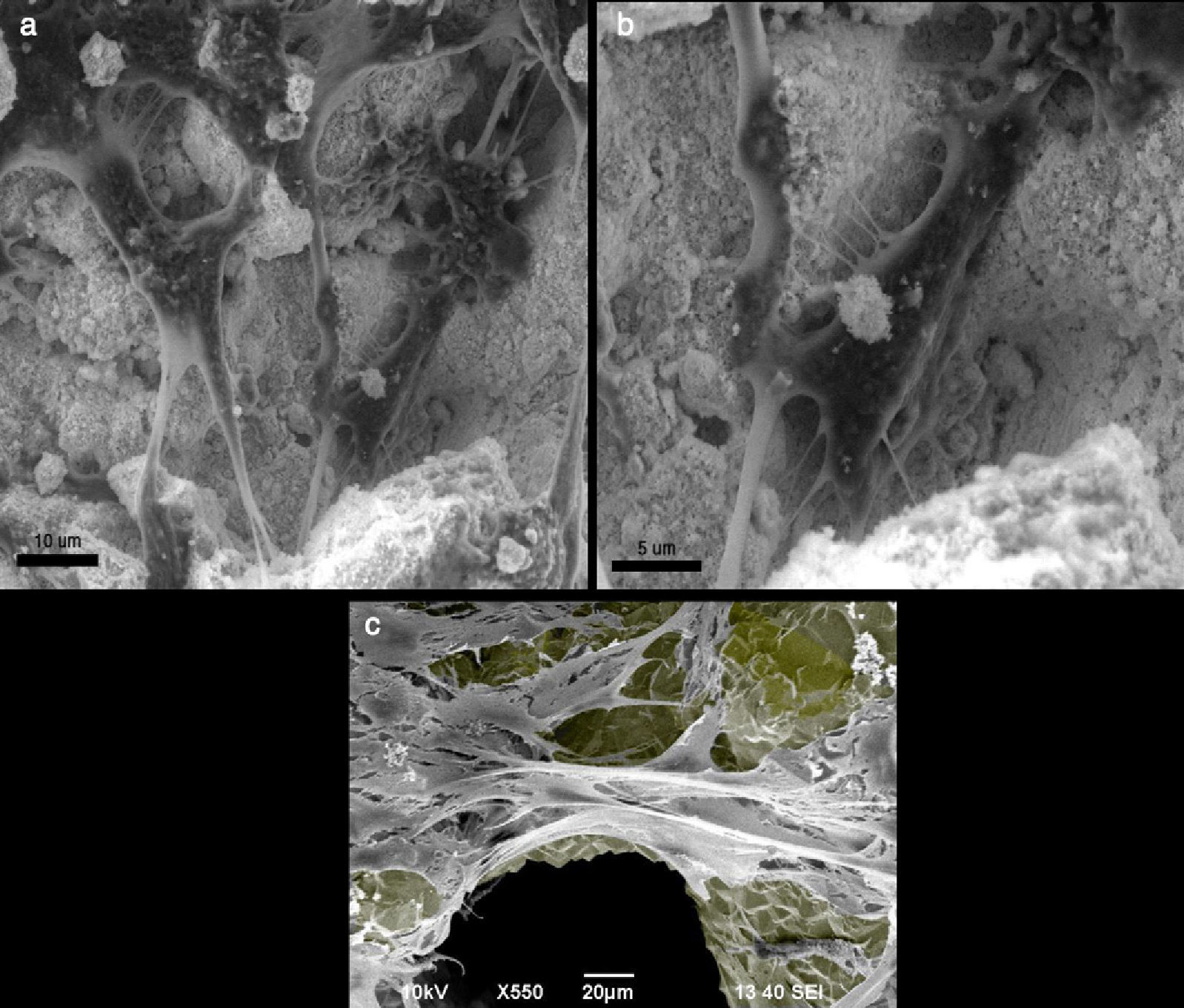
OsteoconductionGiven that the bone is a tissue with an abundant extracellular matrix of which the cellular component is a minority—although essential, as previously mentioned—osteobiology has always tried to favour bone formation using structural supports of varying nature, natural or artificial, but always with the capacity to be colonised by the new cell-formed bone, that is to say, osteoconductive supports. Osteoconduction is the complement to osteoinduction and subsequent osteogenesis. Bone growth must occur on some kind of surface, and better still, on a fundamentally 3-dimensional structure, similar to the physiological bone. The substitutes, which act as scaffolds for the bone to grow upon, are called osteoconductors; they could be an existing bone trabeculum or some artificial material (Fig. 5). A bone substitute is an osteoconductor if its surface is biocompatible with cellular growth and if it possesses a microstructure with an appropriate porosity of 200–400μm. Currently, synthesising a material that is naturally and structurally osteoconductive with the referred characteristics is easy. However, we do not know osteoinduction characteristics as to the number of cells per unit of volume, molecule type, dosage, cadence and order of release. Modern tissue engineering (TE) seeks to respond to these questions by completing ex vivo constructs composed of scaffolds, cells and biomolecules intended to be an approximation to the controlled production of autograft replacements, such that the quantity limitation would not be a problem13 (Fig. 5).
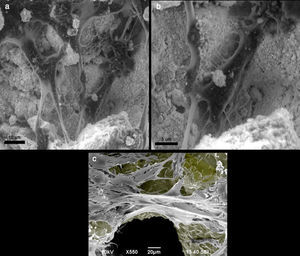
(a and b) Mouse preosteoblast MC3T3-E1 growing in hydroxyapatite crystal, observed with a scanning electron microscope. The cells are fixed with formalin and dehydrated before their observation in the electron microscope. (c) Mouse preosteoblast MC3T3-E1 growing on a titanium trabecular structure, observed by a scanning electron microscope. The colour grey has been added to the titanium surface to highlight contrast.
Osteogenesis and osteoconduction constitute the basis for bone tissue therapy: osteogenesis based in managing the 3 elements of TE—the cells that produce bone, osteoinduction and the biomolecules that appropriately induce cells—and osteoconduction based on the materials which structurally support the growing bone, In the case of TE for bone tissue, it is essential to add not only vascularisation, but also the fifth element, adequate biomechanics, without which the autograft would not progress as desired. Consequently, the current consensus is to call the management of these 5 elements of bone repair “the diamond concept”.14,15 Usually, TE in a broad sense adds the essential vascular element for construct viability, which has special relevance in the case of bone, but not in other situations involving structures designed for cartilage repair. In any clinical situation, if 1 of these 5 variables is lacking, there will be an undesired outcome.14,15
BiomechanicsThe beginning of spinal osteosynthesisThere are many studies concerning biomechanics of the spine. These range from the classic studies by White and Panjabi16–22 to the nearly 7000 bibliographic references that currently appear on Medline under the search “spinal biomechanics”.
In posterolateral spinal arthrodesis, a pedicle instrument is used effectively; in intersomatic fusion, the plate or bars bolted to the vertebrae in support-neutralisation is used in conjunction with a compression-support intersomatic stabilising element.1–4 However, this is purely an analysis leading to the minimisation of the mechanics. The field in which this construct is realised is alive and, as it is biologically active, biomechanics-biology interaction should be kept in mind.1–4
The standard biological contribution is the autograft. Basic physiological knowledge concerning autografts in general, and the spine in particular, has been acquired from animal experimentation, making human inference not completely reliable. Cancellous bone possesses greater cellularity than cortical bone, while the latter is more robust.23 The sponge-like structure allows better osteoconduction for the osteoprogenitor cells and better tissue infiltration by the vascularisation. Vascular invasion of the cancellous autograft begins on the second day after implantation, reducing the mechanical properties of the autograft, getting them back several months later.24 Cortical bone possesses a slower biological life than cancellous; consequently, it takes 6–24weeks to lose 75% of its resistance and mechanical properties,25 later recovering them at 48weeks26 after implantation. Knowing these periods are important for postoperative follow-up and for considering the fusion definitively consolidated. In fact, one of the fundamental problems in assuring that arthrodesis has consolidated lies in the sensibility of diagnostic tests. The diagnostic imaging tests usually used in clinics are of little predictive value in the first few months, but improve their accuracy after the first 12months. Radiographic images increase in predictive value over time; however, CT scans, even though the same is true, possess greater predictive value earlier on. After 12months, CT scans have a very high predictive value.
Consequently, based on the biomechanical and biological properties of cortical and cancellous autografts, posterior arthrodesis always benefits from the cancellous graft because it requires exclusively large osteogenic capacity without mechanical support for the graft. In turn, the cortical graft (iliac crest tricortical or fibula cortical) is suited for intersomatic anterior arthrodesis because of its capacity to support the compression between 2 vertebrae and to serve as support to avoid collapse (Figs. 2 and 3). In anterior approaches to the thoracic or thoracolumbar areas of the spine using thoracotomy, a removed rib could be used as intersomatic structural support. In these cases, if not accompanied by posterior fixation, posterior intersomatic instrumentation should be combined with a plate or bars bolted to the spinal column with a support-neutralisation effect (Fig. 6). The rib fragments constitute autologous cortical grafts, which therefore possess osteogenic capacity but do not have the resistance of the tricortical iliac crest or the pure fibular cortical grafts. Consequently, they cannot undergo complete interfragmentary compression in a way that they put support principles into play; an osteosynthesis system that not only applies neutralisation but also a solid support is thus required (Figs. 2 and 3).1–4 Under no circumstances should the anterior compression of the costal grafts be a stable part of osteosynthesis; and, in the cases in which they are combined with a posterior instrument, the number of fixed spaces should increase to provide greater neutralisation. If the intersomatic graft used is tricortical, a short pedicle instrument of only 2 vertebral segments could be used, but taking care that the graft remains compressed and the instrument, without support, only in neutralisation. In these cases, moulding the bars (for example, giving only a mild lumbar lordosis) can lead to a brace effect that increases stability, the anterior compression being absorbed through the graft and the posterior distraction through the implant (Figs. 2 and 3).1–4 As we will study later, the characteristics of the materials used in constructing the bars allow more or less bar rigidity and plasticity. Furthermore, the anterior cortical graft can be substituted, under the same principles of compression-support, by a synthetic cage. However, this procedure causes important problems, as we will also see later.
Applying the support and neutralisation principles through an anterior approach. Anterior intersomatic approach in a patient with L1 spondylodiscitis. The use of the rib as a stockade, removed in the surgical approach, permits anterior fusion but does not provide sufficient support effect, as does the tricortical graft. Therefore, an anterior plate is added that supports the intervertebral space and neutralises the rotation. In some cases, when synthesis is not stable (as in osteoporotic bone), adding a posterior pedicle fixation is advisable.
In any biomechanical assembly of fracture fixation or spinal arthrodesis, the tissues are subjected to a mechanical load; this means that the cells are as well, through the extracellular matrix. The mechanotransduction mechanism (conversion of mechanical stimuli into molecular release that provokes a cellular response depending on the nature of the stimulus) is not well known, despite that this fact has been empirically known under the name Wolff's law for more than a century. Clinically, it is observed in a long bone when, in certain locations, a fracture or fusion site is not well vascularised and immobilised. The movement provokes, at the same time, non-consolidation and formation of a fibrous tissue, known as non-union or atrophic pseudoarthrosis; this requires removing the fibrous callous, contributing vascular fluid through decortication and reviving the bone ends, contributing live cells through autograft, as well as introducing neovascularisation and adequate fixation. Nonetheless, when the fracture is well vascularised but not immobilised, a non-union site is produced, mainly made up of cartilage tissue. By simply immobilising it adequately, it ends up forming bone tissue, with consolidation of the site. In this way, a mechanical event conditions a biological one.1 The cellular translation of this phenomenon consists in the way the MSC, facing different mechanical stimuli, react by differentiating into different cell lines, be it osteoblastic, chondroblastic or fibroblastic. This is a clear mechanotransduction mechanism and it is achieved more easily on the shaft of long bones than on the spine. This is because, on the spine, the distance between the sites to be fused makes the arrival of vascularisation more difficult. Even if the bone ends have been carefully decorticated, leaving a well vascularised bleeding bed, in the spinal non-consolidation, the lodged tissue is typically naturally fibrous. To observe an “elephant's foot” in the spine, characteristic of the hypertrophic non-union, is exceptional. By virtue of this, the first step of spinal arthrodesis is meticulous decortication of the elements to be fused (whether posterior or anterior) to assure the arrival of vascularisation, and thus cells, to the fusion site. After that, the combination of the biomechanical principles described is fundamental. Notwithstanding this, the fact is that, in practice, decortication with a graft contribution can be postponed to the end of the surgery to avoid bleeding during the entire operation and thus avoid the problem bleeding represents while placing the instrument. Nevertheless, being fundamental, meticulous decortication of the receptor bed up to bleeding bone does not guarantee arthrodesis success, given that the bone ends remain distant from each other and the fragments (vertebrae) remain mobile.
Mechanotransduction has been best studied in sinewy tissue and this knowledge has been inferred to the rest of connective tissues, among them bone. Given that cells are submitted to mechanical stimuli through the extracellular matrix, the molecules released by them trigger a molecular cascade that leads to gene expression that differs depending on the stimulus and, therefore, to a different protein synthesis.27 In fact, protein production of the extracellular matrix increases when higher amounts of growth factors are released due to mechanic stimuli.28,29 Matrix remodelling is influenced by the mechanical stimuli that induce metalloprotease secretion.30,31 Different mechanical loads lead to different proportions and character in the extracellular matrix (this is the simple explanation of Wolff's law). This means that when the osteosynthesis-graft construct is rigid, it triggers a different molecular and cellular response than if it were to show different degrees of mobility. This can be seen in shaft osteosynthesis, according to the system used: a rigid system, like the compression–neutralisation plate, or a flexible system, like the unreamed bolted nail.1–4 On the other hand, in an interface among materials of different rigidities undergoing loaded mechanical stimuli of shear force, biological integration between both materials is impeded. This has been seen in the uncemented interface of the tibial plateau-tibial tray (in knee arthroplasties), of the femoral canal-prosthetic stem and, of course, in that of the vertebral plateau-intersomatic cages. Cementation should be used when there is no primary stability, as it produces immediate integration; however, it will also have a limited number of years of life as this integration is produced in living tissue. Cementing the intersomatic cages is not a procedure studied since the intention is that the cages lead to secondary osteointegration after primary stability.
Therefore, the adequate combination of a biological microenvironment with osteosynthesis is of capital importance. If a good autograft rich in cells is used, but it has an erroneous biomechanical construction, the final result will not be as desired; the same is true if a good osteosynthesis is used with a biologically poor graft, or a simple replacement. This is what occurs with either a defective osteosynthesis or a replacement like a porous calcium matrix, without growth factors promoting osteoinduction or cells osteoinduced by the osteogenic line, especially in elderly patients whose MSC population is substantially scarce.32
The spine is a characteristic example for the necessity of an adequate construct.1–4 The cells respond depending on the osteosynthesis-graft coupling. Given that a biomechanical model of spinal arthrodesis consists of the fixation of multiple fracture sites once the parts to be fixated have been decorticated, the fundamental principles of any osteosynthesis (compression, neutralisation, support or brace) play a fundamental role when the graft, or its replacement, is included as part of osteosynthesis. Tricortical intersomatic grafts or intersomatic cages are examples of this. Spinal arthrodesis has further challenges, as the distance between the posterior elements in the intertransverse arthrodesis, and also the anterior intersomatic space are very wide.1 Together with the interface issue, the cages are biologically problematic given that the distance between the living tissue surfaces is greater, and even if the cage becomes filled with the autograft, the latter loses biological properties easily due to the distance from the healthy vascular bed.1
There is unanimity in that, independently of the biomechanics that combine the autograft and instrument, biology dictates that the graft should be autologous, tricortical or cancellous depending on its biomechanical union. However, in the spine, in cases of fusion on more than 2 levels, the providing autograft in sufficient quantity proves difficult due to graft scarcity, the morbidity that its collection generates and the extra time that its extraction and implantation requires, increasing exposure and risk of bleeding and infection. This has incited a search for biological alternatives with osteoinductive, osteogenic and osteoconductive capacities, avoiding the provocation of immunologic responses.9 The biology of fracture consolidation or arthrodesis is always the same: undifferentiated cells (MSC) multiply and differentiate into others that are able to synthesise osteoid material that mineralises and fuses the segments.
BiologyAutograftFrom a therapeutic point of view, the autograft is the quintessential cell source, accompanied by the fact that it possesses growth factors and, obviously, an osteoconductive structure exactly equal to the unremoved physical bone. The autograft possesses a good volume effect. Nonetheless, the loss of properties (mainly cells) during the autotransplant,33 its limited availability and its morbidity34 necessitate new searches of cell sources with capacities for multiplication and differentiation. A new technique used with the graft, considered less invasive, is the reamer/irrigator/aspirator (RIA) system; this technique apparently provides greater quantities of cells and growth factors than the traditional open graft, thus preventing postoperative pain and morbidity and reducing the length of hospital stays.35–39 However, clinical studies of RIA have yet to assure its validity, and its role in spinal arthrodesis is currently unknown. The quantity and quality of the cells present in the bone marrow aspirate do not confirm osteogenesis. This is because the presence of a significant quantity of haematopoietic stem cells in contrast to MSC and stem cells of mesengenic lineage leaves the latter clearly in the minority, to the detriment of osteoinduction. The MSC rate per unit of volume is very important for the success of the process, since paracrine secretion of MSC-stimulating factors and MSC osteogenic stem cells is of decisive importance in the osteogenic proliferation/differentiation process. In addition, if the aspirate is not done correctly, blood is the majority element, which further dilutes the active biomolecules. Furthermore, bone marrow aspirate possesses a low BMP quantity. In these cases, BMP are essential in triggering the osteoinductive cascade in the fusion area, and above all in the remote ends of the decorticated bone, whose proximity makes osteoinduction easier. Thus, in theory, the prospects for RIA in the spine are not promising.
AllograftEven though some isolated publications have communicated that the allograft (graft from the same species) possesses osteogenic capacities, its true capacity lies in its osteoinductive (having growth factors) and osteoconductive (having bone-like porosity, as it is actually bone without cells) properties. Its use as an alternative to the autograft is based on its availability and the absence of morbidity in its extraction. In return, its capacity to provoke immunological rejection and the probability of transmitting diseases have made many surgeons refuse to use it. However, despite the very high prevalence of use, the risk is extremely low.40–42 Like autografts, cancellous allografts possess better conditions than structural allografts, and the same mechanical characteristics as autografts.43 Growth factors, especially BMP, have a great capacity for attracting target cells (MSC and bone stem cells), but as these cells migrate from the decorticated areas of the host, distance is a limiting factor in these cases. Still, the allograft is currently often used in spinal arthrodesis, due to its availability.
MSC and spinal arthrodesisMesengenesis is the process of multiplication and differentiation of stem cells from the mesodermal lineage by which MSC generate differentiated lines, such as the osteogenic. The MSC–preosteoblast–osteoblast–osteocyte sequence is the start of bone formation and is what occurs in spinal arthrodesis.
Because bone formation is an exclusive property of osteoblasts, it is imperative for there to be a minimum quantity of precursor cells so that there is an adequate quantity of bone. As occurs with any other tissue, the differentiated bone cells are not those that proliferate in the necessary amount, but rather the undifferentiated cells (MSC and osteogenic stem cells) are those that perform cell multiplication (a phenomenon called “amplification) in order to ultimately differentiate into osteoblasts, after going through the intermediate cell types. Signalling molecules—including different cytokines like FGF, PDGF, VGF, IFG and different TGF-beta, of which BMP are the most prominent—regulate this entire process. The fact that less differentiated cells multiply more than the differentiated ones is typical in all tissues. However, it can also be a pathological phenomenon; this occurs especially in cancer when anaplastic cells (less differentiated) multiply more than more differentiated ones, resulting in greater growth and worse prognosis for tumours containing anaplastic cells compared to those with more differentiated cells. In other words, multiplication of less differentiated cells is a physiological mechanism; in cancer, the regulation of this mechanism is altered.
This process is very important in osteobiology because, before the osteogenesis process, the number of osteogenic cells should pass a determined threshold to achieve therapeutic fusion efficiently. To that end, many more differentiated stem cells can recapitulate those less differentiated, travelling an inverse osteogenic path until arriving at a cell lineage capable of multiplying itself significantly. Consequently, they can face the bone reconstruction process in a more efficiently, starting with an elevated number of synthesised cells from bone matrix.44–48 During this entire process, new vessels that act as a source for more MSC appear; recent publications have contributed solid evidence that MSC are of perivascular origin.49,50 This reinforces the importance of decortication up to bleeding bone, be it on a non-consolidation shaft or in the preparation of spinal arthrodesis,1 given that it opens the path for the arrival of MSC; these cells not only trigger mesengenesis but they also have a trophic and immunomodulatory effect essential for the repair function.51
Consequently, MSC are considered the centre of osteogenesis and are the object of intense research for their therapeutic use because they are the only cells capable of (1) differentiating into osteogenic precursors to produce the physiological appearance of bone and (2) conditioning the success of osteogenesis through advanced therapies in a specific repair site. The currently accepted fundamental characteristics defining MSC are those of cell population capable of adhering to the surfaces of culture flasks and, under controlled conditions, differentiating in vitro into osteogenic, chondrogenic, adipogenic, myogenic, tenogenic or stromal cells of haematopoietic tissue.10,52–55 During the last few years, it has been published that MSC are also capable of differentiating into epithelial, endothelial and even neuronal cells,56–58 although the latter is undergoing review.
These cells possess the same genotype and phenotype as their descendents and because of this they maintain “stemness” (capacity to differentiate themselves into the previously indicated lines). These characteristics, however, have come to be known through in vitro studies where the cells can be altered.59,60 Human MSC—in addition to their adhesion to plastic, their role as colony-forming units (CFU) in primary cultures and their capacity to differentiate into osteoblasts, adipocytes and chondroblasts—should express CD73, CD90, CD105, while not expressing CD11b, CD14, CD19, CD34, CD45, CD79alpha or the surface molecules HLA-DR.10,61 Because of this, when it is said that stem cells have been contributed in treating a fracture or fusion site as a product of centrifuging, what is being done is simply the adding of a blood product that has a cell content capable of differentiating into osteoblasts. Besides being unknown, the amount may be minimal, given that under normal circumstances, MSC do not circulate freely in significant quantities in blood.62 Unfortunately, confusing cell transplant practices are currently performed on patients.
The MSC occupy a reduced space (called a “niche”) constituted by a defined space with a microenvironment of molecules that participate in regulating amplification and/or differentiation. In general, the niche possesses parent cells, non-parent cells, extracellular matrix and signalling molecules that interact with the cells in order to modulate their biology.13 One of the best studied niches is that of the haematopoietic stem cells (HSC) of bone marrow, where the regulation of the quiescence, proliferation and differentiation of these cells is highly sophisticated. Osteoblasts from the lamellae, stromal cells, fibroblasts, endothelial cells, etc., all participate.63
Over the last few years, MSC have been used experimentally as a method of promoting osteogenesis in spinal arthrodesis. Mixed models of MSC with a hydroxyapatite transporter and type I collagen have shown high effectiveness in rabbits.64 Nonetheless, protocols have yet to be distributed regarding laboratory standards for cell manipulation or which transporters are best.65,66 New porous metallic materials like titanium and tantalum have also been studied with promising results, especially for the spine. However, the combination of these materials with MSC has yet to show clinical effectiveness67 even though the in vitro studies are very promising.68 In addition, nanomaterials have recently burst into the biomedical research field with this purpose. The combination of nano-hydroxyapatite with collagen, poly(lactic acid) and MSC derived from fat tissue has been studied—although until now only in rabbit subjects—to promote posterolateral spinal arthrodesis, with hopeful results.69 Using the same experimental animal, MSC and bioceramic composites with low intensity ultrasound pulsations have been combined, observing that the ossification was endochondral, similar to that produced in humans.70 Likewise, in the cervical spine, the implantation of allogeneic MSC models with hydroxyapatite and tricalcium phosphate as intersomatic spacers has shown bone formation with good results as far as biosafety, but these results have not exceeded those of the autograft.71 In addition, new spinal arthrodesis models were developed to compare the mechanical behaviour of the fixation using an instrument versus genetically modified MSC.72 These studies, however, were performed with mice using steel needles, a biomechanical model that does not resemble the human at all, making this one of the fundamental problems of all studies performed.8
In addition, relating to experimental spinal arthrodesis, models have been used ranging from autologous powders and intersomatic cages73 to combinations of MSC with hyperbaric oxygen,74 pharmacological manipulations of MSC,75,76 combinations of new transporters,77 genetic transductions of MSC derived from fat tissue,78–80 genetic transfections81,82 or combinations of GF with MSC,83 even observing an inhibitor effect in osteogenesis in cases where cell therapy without a transporter was tested.84
In spinal arthrodesis there is a definite objective of replacing the autograft—with its high osteogenic quality, but also high morbidity—with cell transplant within the TE concept. TE intends to create or induce the formation of a specific tissue in a particular location by selecting and manipulating cells included in matrix structures that provide support, as well as some molecules that modulate cell growth.85 This live structure needs immediate vascular contribution that will continue for the life of the construct. Because of this, spinal transplant techniques have obstacles that have yet to be resolved. For example, the distance that exists between 2 fusion sites makes vascularisation over such a long path impossible. This difficulty is evident in both intertransverse and intersomatic arthrodesis, whether bone substitutes or cages (filled with those bone substitutes) are used. Mixed grafts that combine cellular and vascular contributions from the decorticated bone, with the contributed MSC in an adequate transporter and BMP—of great chemotactic and differentiating power—absorbed in collagen sponges, can represent favourable conditions for osteoinduction in spinal arthrodesis. Until now, BMP associated with collagen scaffold are being supplied in high concentrations, which makes the surgical procedure more expensive. It also introduces a certain degree of insecurity regarding BMP passage to systemic circulation, with the consequential effects not completely ruled out.86–88 Production of BMP with molecular domains that specifically join them to a transporter, without compromising their effectiveness, could be a solution to be explored clinically.89
Growth factorsThe use of growth factors in spinal arthrodesis is centred on BMP, after the failure in using platelet concentrates.90 BMP are multifunctional proteins, within the growth factor superfamily TGF-b,91 which have different effects on many tissue types in the organism and modulate growth factors and embryologic differentiation as well as cell functions. BMP stimulate osteoprogenitor cells from the host bone bed. More than 20 different types have been identified, BMP-2, 4, 6, 7 and 9 being the ones that possess osteoinductive capabilities with a synergic relation between them.92 BMP molecules are combined in vivo in order to form heterodimers, which are thus much more potent in combination than when employed separately.93 The molecular cascade for osteoblastic differentiation seems to be a succession of interactions between BMP and other molecules.94 Consequently, the BMP-allograft combination seems to be an attractive one for lumbar spinal arthrodesis. In addition, the allograft is an excellent transporter and osteoconductor. BMP molecules are relatively soluble, with less soluble transporters being the ones indicated for keeping BMP in the arthrodesis site,95 thus reinforcing the use of the allograft as a transporter. Current strategies of BMP application for spinal arthrodesis in humans involve the proteins being administered without any transporters.
Despite the broadly published experience of using BMP in spinal surgery,1,96–100 and of its security,99,100 in Spain there are no approved indications for the use of BMP in the vertebral column. Consequently, it is used through individually petitioning the Ministry of Health for each patient, labelled as “compassionate use.”
Results from spinal BMP used to vary as far as animal models or human clinical use are concerned. While recombinant BMP-7 shows a superior result to the autograft in canines,101 these results have not been achieved in humans; not even when it is used in isolation as putty.98,99,102 Nonetheless, BMP results are superior to those of any other “bone substitute” and with zero morbidity in comparison to the autograft.98,99 Our group project was based on experimental studies of other authors103; we performed a prospective randomised study with 5years of follow-up using BMP-7 (OP-1, Osigraft Stryker, Kalamazoo®) as an osteoinductive agent together with the allograft as the transporter, also with inductive characteristics. Comparing with using only the autograft in spinal arthrodesis, very superior results were observed, which were also similar to autograft fusion rates. This suggests that BMP use is indicated when lumbar arthrodesis is needed for more than 1 level. Our group has also developed, experimentally, a method of osteogenic capacitation based on 3D cell cultures in collagen gels in the presence of a TGF beta 1, with the addition of a molecular domain that allows the selection of a cell population and in vitro and in vivo bone formation in the experimental animal in which they are implanted.11 The cells selected through this method possess a phenotype much more osteogenic than when this is performed in 2D cultures. In any case, when dealing with BMP it is important to remember that there are different mechanisms of human MSC differentiation into the osteogenic lineage, depending on whether they are induced by BMP-2/4 or BMP-6/7.102
Until now there have been many studies concerning the effectiveness of BMP in cervical arthrodesis1,40 and especially in posterolateral97–100 and intersomatic1,41,104–107 lumbar arthrodesis. Nonetheless, a recent meta-analysis of controlled and randomised clinical trials, which evaluated the clinical and radiographic effectiveness of the autograft and BMP in posterolateral spinal arthrodesis, showed that although the results were superior in the BMP group, the exact role of each BMP, the dosage and the transporter were unknown.108 In addition, while its use in the thoracic and lumbar column was safe,100 it produced an inflammation in the cervical column with such characteristics that the patients had manifested dysphagia and even respiratory insufficiency.98,109–111 Furthermore, foraminal stenosis has not been documented with open-canal surgery.112 Recent studies with compassionate (“off-label”) indications have shown that 92.8% of 340,251 cases of BMP use in spinal surgery present tremendous dispersion regarding the variables (different surgical access paths, primary surgical or review cases, cervical, thoracic or lumbar column locations, different BMP and cage use) that it is difficult to come to any conclusions.113
Intersomatic cagesThe knowledge provided by tricortical intersomatic grafts has been the base for developing different techniques and instrumentations for intersomatic arthrodesis.1–4 Tricortical grafts provide excellent support for superior and inferior vertebrae that subject the graft to interfragmentary compression (Figs. 2 and 3). In addition, an instrument that contributes neutralisation is necessary. When this instrument is posterior via a pedicle system, a brace effect is achieved since the graft supports the anterior compression and the pedicle system supports the posterior distraction. Mechanical resistance of the tricortical autograft to the compression supplied by the superior and inferior vertebrae, joined by its biological activity, has been the base for the design of the intersomatic cages in all their variations of anterior or posterior introduction.
However, in addition to the interface issue, there is a biological problem with the cages given that the distance between the live tissue surfaces to be fixed is very wide. Even if the cage is filled with autograft, the latter easily loses its biological properties because of this distance from the healthy vascular bed. The principle of interfragmentary compression is applied to the cages considering that the cage itself is a bone fragment under compression and it is fused to the superior and inferior vertebrae. The cage assumes the support principle, preventing the segment from collapsing. However, as in any osteosynthesis, a neutralisation contribution is necessary. This consists of an intersomatic bolted plate or bar or, usually, a posterior pedicle instrument. This model assumes that the cage has an elasticity module similar to the superior and inferior vertebrae, as well as an analogous biological richness as it contains the graft within it. Because of this, the cages are constructed with titanium, a metal whose elasticity module is closer to that of bone. They are also built in other high molecular weight materials like PEEK (polyetheretherketone, a semi-crystalline thermostable thermoplastic material commercialised in 1978 to reinforce carbon structures and coat unlubricated mechanical pieces), given that an elasticity module similar to bone is always being sought out. This principle applies to tricortical autografts; however, neither the elasticity nor the biological richness of cages and bone are the same. This means that the cages do not fuse as part of the superior and inferior vertebrae and that they are implants with an intolerable mobilisation rate.1,114–121
MaterialsThe materials used to promote osteogenesis in spinal arthrodesis should possess specific physical properties (for example, pedicle fixation systems), some of which are biological properties (e.g., bone substitutes), while others should have both (e.g., cages). It can therefore be said that there are materials for the biomechanical or biological function of spinal arthrodesis, or for both.6
The most valued physical properties in the orthopaedic use of implants are rigidity, strength, ductility, resistance to corrosion, surface structure and biocompatibility. A material with excessive rigidity brings it closer to rupture, while ductility prevents it. Some materials, like the titanium used in the bars of the pedicle system, combine an adequate degree of rigidity-ductility for their function. An appropriate combination of the properties mentioned is still being sought in the different alloys of titanium and steel.6
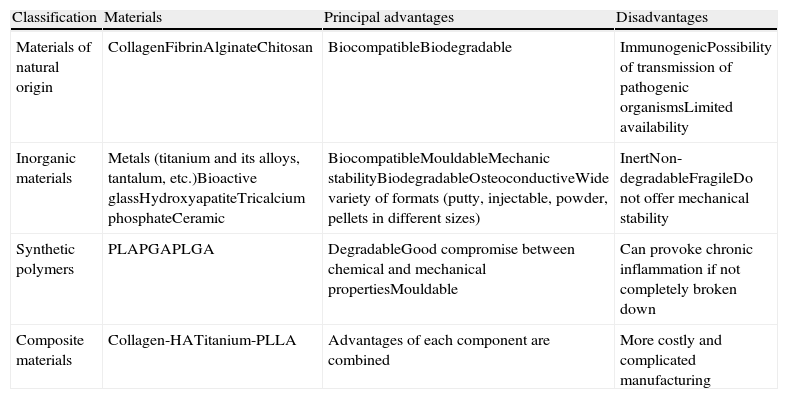
In general, the materials employed in surgery can be divided, according to their compositions, into 4 different groups: natural, inorganic, synthetic polymers and composite materials, in addition to combinations of different materials (Table 1). A material destined for osteosynthesis (for example, a pedicle system) should possess rigidity and strength to maintain osteosynthesis, and some degree of ductility, but not too much, to be moulded to the physiological curvatures of the spine. However, the surface structure does not require making cell adhesion and biocompatibility easier, just so as not to interfere with osteogenesis or host physiology. One could think that a pedicle system could ideally form part of the bar of bone formed in posterolateral arthrodesis, but then one would have to modify the surface and, preferably, change the internal structural and make it more porous. However, this would take away rigidity and strength. On the other hand, an advantage of the pedicle system is that, despite reaching the 3 spine sections (anterior, middle and posterior), it allows a certain elasticity that makes the graft, once consolidated, accept the load and not disappear, an undesirable phenomenon known as stress shielding. Such shielding, well known in the femoral peri-implant bone in hip joint replacements, is not as well known and less foreseeable in spinal surgery.
Principal advantages and disadvantages of the different types of materials employed or proposed as bone substitutes.6
| Classification | Materials | Principal advantages | Disadvantages |
| Materials of natural origin | CollagenFibrinAlginateChitosan | BiocompatibleBiodegradable | ImmunogenicPossibility of transmission of pathogenic organismsLimited availability |
| Inorganic materials | Metals (titanium and its alloys, tantalum, etc.)Bioactive glassHydroxyapatiteTricalcium phosphateCeramic | BiocompatibleMouldableMechanic stabilityBiodegradableOsteoconductiveWide variety of formats (putty, injectable, powder, pellets in different sizes) | InertNon-degradableFragileDo not offer mechanical stability |
| Synthetic polymers | PLAPGAPLGA | DegradableGood compromise between chemical and mechanical propertiesMouldable | Can provoke chronic inflammation if not completely broken down |
| Composite materials | Collagen-HATitanium-PLLA | Advantages of each component are combined | More costly and complicated manufacturing |
HA, hydroxyapatite; PGA, polyglycolic acid; PLA, poly(lactic acid); PLGA, poly(lactic-co-glycolic acid).
The materials destined to promote osteoconduction or osteogenesis (Fig. 5), called bone substitutes, should possess, inexorably, biocompatibility, as well as a surface and structure that allow osteogenesis. However, they are not typically required to be rigid or strong. In some cases, a certain degree of ductility may be useful but it would probably affect the organisation of the internal structure and it would be at the cost of a loss of osteoconductive capacity, which would affect osteogenesis. Consequently, an intersomatic cage would require rigidity and strength to support the vertebrae, accompanied by biocompatibility and an osteoconductive structure. For a cage, which comes preformed with an appropriate design to occupy the intersomatic space, ductility is not necessary. Current cages satisfy all the physical requirements perfectly, yet the loosening problem is not physical but biological; although it derives, in large part, from the fact that their physical properties are different from those of bone. At first, an intersomatic cage shows very solid anchorage. However, as live tissue, the receptor bed progressively modifies its physical properties and the cage ends up loosening. To prevent a cage from loosening, it should have the same rigidity, strength and ductility as bone, allowing bone colonisation in order to integrate itself into the under- and overlying bone as a single physical body.
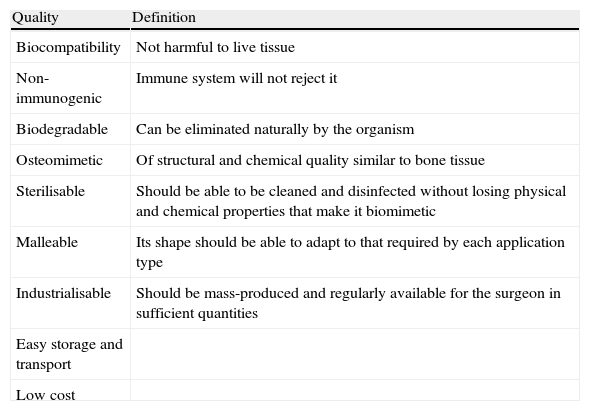
In order to be osteomimetic (having an affinity to be covered in bone), a material should be, ideally, 3-dimensional and porous, ultimately allowing infiltration via blood vessels and mesenchymal and osteoprogenitor cells (Fig. 5). It should have resistance to tension and compression on a level similar to bone itself. It should also have an affinity for osteoinductive growth factors, like BMP. Osteomimetism is complemented by osteoconduction, which is to say by the capacity that a material has to allow bone growth on its surface. The ideal osteoconductive material is that which satisfies all the requirements detailed in Table 2.
Characteristics of osteoconductive materials.6
| Quality | Definition |
| Biocompatibility | Not harmful to live tissue |
| Non-immunogenic | Immune system will not reject it |
| Biodegradable | Can be eliminated naturally by the organism |
| Osteomimetic | Of structural and chemical quality similar to bone tissue |
| Sterilisable | Should be able to be cleaned and disinfected without losing physical and chemical properties that make it biomimetic |
| Malleable | Its shape should be able to adapt to that required by each application type |
| Industrialisable | Should be mass-produced and regularly available for the surgeon in sufficient quantities |
| Easy storage and transport | |
| Low cost |
Given that an osteoconductive material should be capable of being colonised by cells—primarily (if not exclusively) MSC, preosteoblasts and vascular stem cells—the surface properties of the material are of special importance. The surface should offer adequate accommodation for cell adhesion molecules, through which cells will be linked to the material. This accommodation depends on the chemical composition, duration and the micro- and nanoscopic topology of the material surface.121 Calcium and also titanium components have many of the required characteristics (Fig. 5). Both calcium and titanium components are excellent osteoconductors but neither possess osteogenic nor osteoinductive properties, making cells and growth factors necessary. Calcium phosphate is a good osteoconductor, reabsorbed over time. Likewise, thanks to its elasticity module, biocompatibility and availability, titanium is a metal often used for orthopaedic implants. The surface of titanium is excellent for cell growth. Furthermore, titanium can be treated by increasing surface roughness to make the growth area superior.6
Once a titanium implant is introduced into the live body, the implant is immediately covered by a protein biofilm in contact with blood. This biofilm is a provisional matrix for cell adhesion. The surface can determine what protein is absorbed and the orientation of adhesion. Cell adhesion begins within a period of a few minutes, with the process being controlled by the surface characteristics (Fig. 5). In orthopaedic implants, given that they are anodised, the surface is not really titanium but oxide. The metallic ions spread at different speeds in the oxide and oxygen is spread from the oxide to the metal. The biological ions are incorporated into the oxide with absorption proteins that go through changes over time. Protein size, load and stability affect cell arrival and adhesion velocity as much as the interaction with the surface. It can be said that this process is influenced by characteristics of the surface: topography (quantity of adhered cells), chemistry (determines the types of intermolecular strength), hydrophobicity (determines which proteins and how many of each are bound), heterogeneity (different molecular domains with different proteins) and potential (influences the distribution of ions in solution and protein interaction).
The materials, whatever they are, are governed by the same principles of physics, these being based on their atomic structure. The manufacturing process of a material affects its composition and its future performance in relation to its physical properties. Metals are made of crystals positioned in a 3-dimensional structure forming a single network. A typical implant for medical use is constituted of many atoms within a single crystal and many crystals constitute a grain of material. This disposition conditions their properties. The defects that all the crystals have (atom-free areas, dislocations, limits between crystals, cracks, etc.) also participate in the characteristics of the material. Because of this, it is important not to be seduced by the price of the materials but rather by the technical and manufacturing characteristics. If a manufacturing process leaves, for example, small areas free from atoms or cracks, this would not affect the material if not submitted to mechanical load, but it would break if it were submitted to a load. A material can thus be cheap but not cost-effective. The choice of a material should consequently be based on physical requirements and biological necessities. For this choice, the concept of cost-effectiveness should be contemplated. The macroscopic characteristics of implants also decisively influence its performance. For example, the rigidity of a bar in a pedicle system is 4 times less if its section is simply cut by half.
Evaluation of the resultsIn any scientific area, knowledge originates from research, so the design of research studies is very important. There are 2 types of studies in clinical research: observational (the researcher observes, without modifying at any time, what happens over a time period in 2 groups of people that share the same variables before the study, except for a single variable in 1 of the groups being studied) and experimental (the researcher observes what happens in 2 groups of people that share the same pre-study variables, but acts on 1 group by applying a treatment).
Experimental clinical studies are the ones indicated for ascertaining the result of a treatment. Among these studies, controlled randomised clinical trials are the gold standard. A controlled clinical trial is that which ensures that all prior variables are equal to prevent the final results from being influenced by the fact that the groups were not homogeneous. In studies concerning spinal arthrodesis, there are many variables to control, including age, pathological history and previous treatments (whether surgical or pharmacological), using the same type of instrument, follow-up and so on.
Specifically, in relation to bone substitutes, age is an important variable. This is because MSC are fundamental to osteogenesis and, given that elderly patients have a poor MSC population,32 in a small sample size the fact that more or fewer young or elderly patients are included will alter the results. This is one of the most frequent problems found in the results of clinical studies on spines with bone substitutes; patients who “have reached bone maturity” are introduced, which means including, for example, intervals for patients between 20 and 80years old. This example extends to any variable. Other variables—if treatments like anti-absorption pharmaceuticals (or others that interact with osteogenesis) are received or if the same type of instrument is used—are very important, yet they are frequently ignored. To consider a randomised controlled clinical trial to be a quality study, one should respond after critically analysing it, not after seeing the designation alone. It is therefore imperative that studies control the variables, paying attention to their specific characteristics, and increase the number of elements in each group to minimise random error. This is not the norm in studies concerning the biology of osteogenesis and its clinical application, especially in spinal arthrodesis.
Controlled clinical trials should randomise the assignment of individuals into treatment or no-treatment groups in an unbiased way, so that interest in the treatment being better or worse does not intervene. However, consecutive prospective cohorts may produce better information than those simply randomised. Some variables can then be controlled more easily, considering that patients themselves arrive randomly.
In research, as in any other diagnosis, precision and exactness of the methods verifying whether bone fusion occurred are also very important. Assessment via simple X-rays shows poor reliability, being subject to systematic errors. The same is true of the follow-up period, because cortical autografts can fail after 2years and then (if they do not collapse) recover their properties after 4years. Allografts can be reabsorbed before that.1 In a prospective randomised study where OP-1 in putty form was compared to a mixture of autograft plus ceramic pellets of calcium phosphate in posterolateral lumbar spinal fusion, similar results were observed via X-ray at 1year of follow-up. However, the results were radically different when the instrumentation was removed and the fusion mass was explored. Not only was the fusion with OP-1 greater than the control, but the OP-1 group showed worse results in the surgical exploration than in the X-rays.99 Studies with a high-speed spiral computerised tomography (CT) scan also showed greater precision with very high concordance.69 However, due to the radioactive risk, these patients should not be followed up with CT scans systematically.
Inference of the studies of animals to humans is conditioned by the different biological capacities of the ones and the others. Likewise, their biomechanical principles are also different. In the rabbit there are not any significant differences between the fusion provided by autografts or allografts, a circumstance that we know does not occur in humans. This fusion is similar to the use of xenogeneic demineralised bone matrix.84
Definitively, spinal arthrodesis still suffers from a lack of bone substitute that improves upon the osteogenic capacity of autografts and also eliminates its morbidity. Diagnosing fusion is another unresolved problem due to the high radioactive dosage in CT scans, while result assessment requires well-designed prospective studies. Fixation with constructs of high primary stability and autograft contribution continues to be the standard.
Level of evidenceLevel of evidence V.
Ethical responsibilitiesProtection of people and animals. The authors declare that no experiments were performed on human beings or on animals for this research.
Data confidentiality. The authors declare that they have followed their centre's protocols regarding publication of patient information and that all patients included in the study received sufficient information and provided informed written consent to participate in the study.
Right to privacy and informed consent. The authors obtained informed consent from the patients and/or subjects referred to in the article. This document is in possession of the corresponding author.
FundingThe authors appreciate the funding obtained from, among others, DGICYT (SAF99-0133), MCYT (SAF2002-02183), FIS (PI021758), BIO2006-03599, SAF99-0133, Cell Therapy Network RD06/0010/0014, CIBER CB06/01/1015, BIO2009-13903-C02-01, PLE2009-0163, PI10/02529, P07-CVI-2781, PI-0729-2010, and PAID BIO217, Ministry of Health of the Government of Andalusia, Exp. 53/00, Exp. TCMR 0012/2006, PI-0437/2007 and P07-CVI-02781.
Conflict of interestsThe authors have no conflict of interests to declare.
Please cite this article as: Guerado E, et al. Artrodesis del raquis. Ciencia básica. Rev Esp Cir Ortop Traumatol. 2012;56:227-44.