To develop recommendations, based on best evidence and experience, on pain management in patients undertaking total knee or hip replacement.
MethodsNominal group methodology was followed. A group of experts was selected (five orthopedics, one anesthesiologist), who defined the scope, users, topics, preliminary recommendations, and three systematic reviews: efficacy and safety of pre-surgical analgesia regarding to post-surgical pain, efficacy and safety of pre-emptive analgesia and pre-operative factors of post-operative pain. The level of evidence and grade of recommendation were established using the Oxford Centre for Evidence Based Medicine, and the level of agreement with the Delphi technique (two rounds). The Delphi was extended to 39 orthopedics and anesthesiologists. The whole document was reviewed by all the experts.
ResultsA total of 21 recommendations were produced. They include specific pharmacological treatment, as well as the evaluation and monitoring of patients on this treatment, and post-operative pre-emptive treatment. Agreement above 70% was reached in 19 recommendations.
ConclusionsIn patients undergoing total knee or hip replacement, a proper evaluation, follow-up, pharmacological and non-pharmacological treatment of predictors of poor surgical outcomes should be performed, especially those related to pre-operative pain. This can improve post-operative pain and surgery outcomes.
Desarrollar recomendaciones basadas en la mejor evidencia y experiencia sobre el manejo del dolor en pacientes con artrosis de rodilla o cadera e indicación de artroplastia.
MétodosLas recomendaciones se emitieron siguiendo la metodología de grupos nominales. Se seleccionó un grupo director de expertos (5 traumatólogos y un anestesiólogo) que definieron el alcance, usuarios, apartados del documento, posibles recomendaciones, revisiones sistemáticas y se asignaron tareas. Se realizaron 3 revisiones sistemáticas sobre: la eficacia y seguridad de la analgesia prequirúrgica en relación al dolor posquirúrgico; la eficacia y seguridad de la analgesia preventiva, y sobre los factores prequirúrgicos que influyen en el dolor posquirúrgico. Los expertos redactaron los apartados y generaron las recomendaciones correspondientes. El nivel de evidencia y grado de recomendación se clasificaron según el modelo del Center for Evidence Based Medicine de Oxford y el grado de acuerdo por técnica Delphi (2 rondas). El Delphi se amplió a 39 traumatólogos y anestesiólogos. El documento completo circuló entre el grupo director para su última revisión.
ResultadosSe generaron 21 recomendaciones. Incluye el manejo farmacológico específico, la evaluación y monitorización de estos pacientes que están en tratamiento, y el tratamiento preventivo del dolor posquirúrgico. Existió consenso mayor del 70% en 19 de ellas.
ConclusionesEn el paciente pendiente de artroplastia de cadera o rodilla se debe hacer una correcta evaluación, seguimiento y manejo farmacológico y no farmacológico de los factores que predicen un mal resultado de la intervención, en particular del dolor prequirúrgico. Estas actuaciones pueden mejorar el dolor posquirúrgico y el resultado de la artroplastia.
Total knee (TKA) or total hip arthroplasties are frequent surgical procedures which effectively reduce pain and functional limitation in those patients with arthrosis who undergo them.1,2 Their popularization in the second half of the 20th century resulted in their being considered one of the most relevant surgical advances of that century.3 They are now one of the most extensively used orthopedic surgical procedures in Spain and in high demand from patients.4
This popularization has resulted in a significant number of patients who are dependent upon the public health service having to wait weeks, months or even years for surgery. By December 31, 2012, there were over 30,000 patients on the waiting list for THA or TKA surgery. These patients probably present with high levels of pain and functional limitations due to the fact their arthrosis has progressed.
Although both THA and TKA are considered successful procedures, a satisfactory outcome is not always possible for all patients undergoing this type of surgery,5 and up to 30% of patients do not report major improvements in their quality of life a year after surgery.6 Several studies suggest that patients who experience high levels of pain prior to surgery obtain a worse outcome and are more dissatisfied with surgery after intervention.
The aim of this document is to draw up a consensus, guided by the Delphi methodology which establishes recommendations on the appraisal, monitoring and treatment of pain in this group of patients with arthrosis, who have been referred for total hip or total knee replacement and are awaiting surgery.
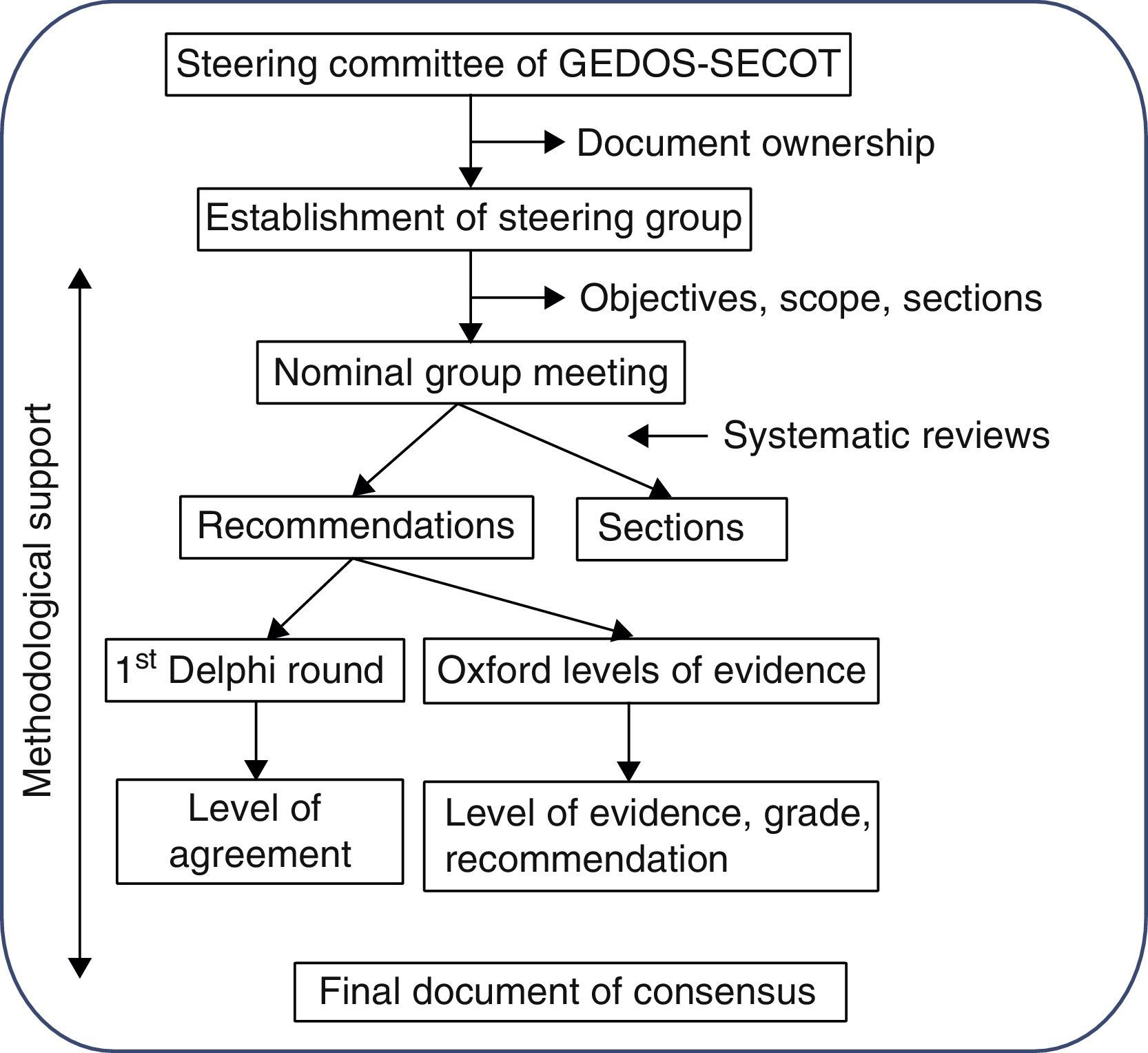
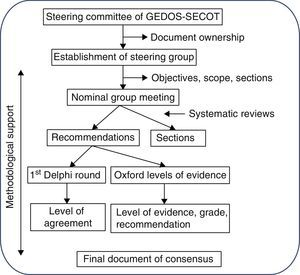
MethodologyThe Delphi methodology was used to establish the consensus. This system is based on presenting a panel of experts with a series of questions, independently and anonymously prepared by a steering group, and through repetitive circulation of the responses of this group, to adjust these questions and establish points of consensus (Fig. 1). The document was entirely prepared by distributing tasks and comments to the parties, aided by three systematic reviews of the literature.
Stage 1: consensus designThe initiative for reaching the consensus was the brainchild of the management committee of the study group of musculoskeletal pain of the Spanish Orthopedic and Traumatology Society (GEDOS-SECOT). Firstly a steering group was appointed comprised of six individuals (the first six authors of this article) with recognized experience in the field of musculoskeletal pain management and also TKA and THA. A methodology experienced in Delphi consensus and systematic reviews was used.
At the first steering group meeting decisions were taken regarding the study sample, scope, objectives and points of consensus. It was also decided that systematic reviews on three specific aspects of pre-surgical pain management would be made. The steering group also decided to wait for the results of the systematic reviews to assign each panelist with the drafting of one or several points of consensus.
Stage 2: systematic reviewsThree systematic reviews were made, one on pre-surgical pain management, another on pre-emptive analgesia and a third on the pre-surgical predictive factors of post-surgical pain. The same review protocol was used in all of these: studies were selected which included adult patients who had been referred for hip and/or knee arthroplasty. These studies (according to the review) also had to analyze the efficacy and safety of the pre-surgical pharmacological, preventative treatment and the predictive or determining pre-surgical factors of post-surgical pain. Finally, only studies with the following designs were included: meta-analysis, systematic reviews, clinical trials, or observational studies. In all the reviews the following reference databases were screened: Medline (from its commencement until May 2013), Embase (from its commencement until May 2013) and the Cochrane Library (from its commencement until May 2013). Mesh and free text terms were used. For each review, two reviewers independently analyzed the articles resulting from the search strategy using different reference databases, and detailed analysis of the articles included in them. The Oxford Quality Scale was used to assess the methodological quality of the included articles. Tables of evidence were created in which the main characteristics of the included studies were described. The results of these systematic reviews were available to the steering group and were used to establish the evidence levels of the relevant recommendations.
Stage 3: consensus questionnaire preparationDifferent points of the consensus were assigned to each member of the group. Once an initial draft of recommendations had been completed, the points and recommendations were combined and edited for style. The complete document was then circulated among the steering group and several corrections and comments were made. After several repetitions of these procedures a final document was obtained.
Stage 4: Delphi surveyA group of experts comprising of 39 traumatologists and anesthesiologists was established. The steering group was included in it, as were other experts who had not participated in the drafting of the document, as suggested by different members of the steering group (see Appendix 1). They were sent the questionnaire with the complete recommendations and instructions online and anonymously, so that they could indicate their level of agreement with each of the recommendations on the consensus document. Level of agreement was expressed using a Likert scale of 1 (totally disagree) to 10 (totally agree). Agreement was understood to exist if the recipient voted seven or above. The aggregated Delphi results were shown to all panelists (modified Delphi). The recommendations with a level of agreement below 70% were reassessed and, if necessary, reedited and voted for in a second round.
Stage 5: final consensus documentThe final document was drafted using all this information. For each recommendation, and with the help from the methodology, a level of evidence (LE) and a level of recommendation (LR) was assigned, in compliance with the recommendations for medicine based on the Oxford Center for Evidence Based Medicine.7
ResultsThe steering group drafted 21 recommendations which included a sentence of summary (see Table 1) and an explanatory paragraph with justifications and references. Almost half of them refer to pain management, including pharmacological and non-pharmacological measures, five referred to pain assessment, four to treatment monitoring and two focused on pre-emptive analgesia for the immediate post-operative period in these patients.
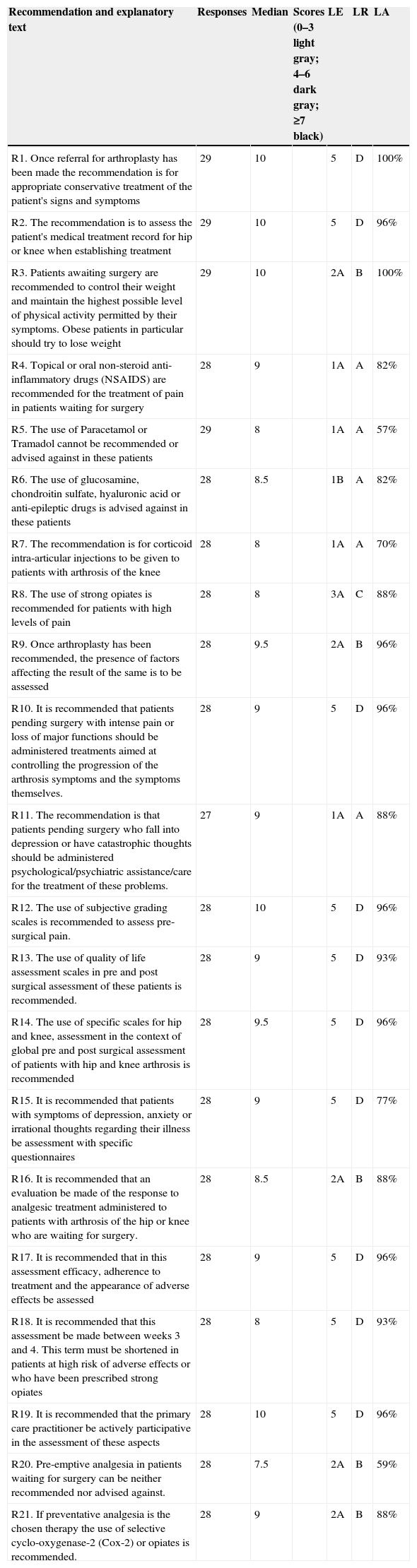
Recommendations and main results reached in the Delphi study, including evidence level, level of recommendation and level of agreement.a
| Recommendation and explanatory text | Responses | Median | Scores (0–3 light gray; 4–6 dark gray; ≥7 black) | LE | LR | LA |
|---|---|---|---|---|---|---|
| R1. Once referral for arthroplasty has been made the recommendation is for appropriate conservative treatment of the patient's signs and symptoms | 29 | 10 | 5 | D | 100% | |
| R2. The recommendation is to assess the patient's medical treatment record for hip or knee when establishing treatment | 29 | 10 | 5 | D | 96% | |
| R3. Patients awaiting surgery are recommended to control their weight and maintain the highest possible level of physical activity permitted by their symptoms. Obese patients in particular should try to lose weight | 29 | 10 | 2A | B | 100% | |
| R4. Topical or oral non-steroid anti-inflammatory drugs (NSAIDS) are recommended for the treatment of pain in patients waiting for surgery | 28 | 9 | 1A | A | 82% | |
| R5. The use of Paracetamol or Tramadol cannot be recommended or advised against in these patients | 29 | 8 | 1A | A | 57% | |
| R6. The use of glucosamine, chondroitin sulfate, hyaluronic acid or anti-epileptic drugs is advised against in these patients | 28 | 8.5 | 1B | A | 82% | |
| R7. The recommendation is for corticoid intra-articular injections to be given to patients with arthrosis of the knee | 28 | 8 | 1A | A | 70% | |
| R8. The use of strong opiates is recommended for patients with high levels of pain | 28 | 8 | 3A | C | 88% | |
| R9. Once arthroplasty has been recommended, the presence of factors affecting the result of the same is to be assessed | 28 | 9.5 | 2A | B | 96% | |
| R10. It is recommended that patients pending surgery with intense pain or loss of major functions should be administered treatments aimed at controlling the progression of the arthrosis symptoms and the symptoms themselves. | 28 | 9 | 5 | D | 96% | |
| R11. The recommendation is that patients pending surgery who fall into depression or have catastrophic thoughts should be administered psychological/psychiatric assistance/care for the treatment of these problems. | 27 | 9 | 1A | A | 88% | |
| R12. The use of subjective grading scales is recommended to assess pre-surgical pain. | 28 | 10 | 5 | D | 96% | |
| R13. The use of quality of life assessment scales in pre and post surgical assessment of these patients is recommended. | 28 | 9 | 5 | D | 93% | |
| R14. The use of specific scales for hip and knee, assessment in the context of global pre and post surgical assessment of patients with hip and knee arthrosis is recommended | 28 | 9.5 | 5 | D | 96% | |
| R15. It is recommended that patients with symptoms of depression, anxiety or irrational thoughts regarding their illness be assessment with specific questionnaires | 28 | 9 | 5 | D | 77% | |
| R16. It is recommended that an evaluation be made of the response to analgesic treatment administered to patients with arthrosis of the hip or knee who are waiting for surgery. | 28 | 8.5 | 2A | B | 88% | |
| R17. It is recommended that in this assessment efficacy, adherence to treatment and the appearance of adverse effects be assessed | 28 | 9 | 5 | D | 96% | |
| R18. It is recommended that this assessment be made between weeks 3 and 4. This term must be shortened in patients at high risk of adverse effects or who have been prescribed strong opiates | 28 | 8 | 5 | D | 93% | |
| R19. It is recommended that the primary care practitioner be actively participative in the assessment of these aspects | 28 | 10 | 5 | D | 96% | |
| R20. Pre-emptive analgesia in patients waiting for surgery can be neither recommended nor advised against. | 28 | 7.5 | 2A | B | 59% | |
| R21. If preventative analgesia is the chosen therapy the use of selective cyclo-oxygenase-2 (Cox-2) or opiates is recommended. | 28 | 9 | 2A | B | 88% |
NSAIDS: non-steroid anti-inflammatory; COX-2: selective cyclo-oxygenase-2 inhibitors; SD: standard deviation; LA: level of agreement; LR: level of recommendation; LE: level of evidence; R: recommendation. Scores: number of experts votes in each score range.
The recommendations were sent to a total of 34 traumatologists and five anesthesiologists for an appraisal of their level of agreement. 29 of them in total voted (74% response rate). The main Delphi results are shown in Table 1, including their level of evidence (LE), level of recommendation (LR) and level of agreement (LA) with recommendations. Based on the established criterion, a sufficient level of consensus was reached on 19 recommendations. Sufficient consensus was not reached on two recommendations (numbers five and 20). The panel weighed up the possibility of reediting them and submitting them for a second round of voting but since the idea conveyed in them was very clear, it was finally decided not to do this since the same responses would be expected even if reedited.
The final recommendations text is as follows:
These recommendations refer to a very specific patient group with hip and knee problems. The majority of them present with severe arthrosis leading to painful symptoms and major functional loss. Furthermore, different treatments have been tried regularly which have failed for one reason or another. They therefore have a significant symptomatological profile associated with conservative treatment failure which has led the traumatologist to refer them for arthroplasty. With this profile they are not good candidates for direct application of the different clinical guideline recommendations for arthrosis treatment on these joints.
Recommendation 1. Once arthroplasty is indicated, the recommendation is to initiate appropriate conservative treatment in keeping with the patient's symptoms and signs. (LE 5; LR D; LA 100%).
This treatment must be maintained whilst intervention is delayed, which may on occasions be for prolonged periods. There is a two-fold aim to this treatment: to improve the patient's quality of life, reducing their suffering until surgery may be performed, and also place them in the best position possible to realize the expectations that athroplasties generate.
Recommendation 2. On establishing this treatment, the recommendation is to assess the clinical record of treatment received for their knee or hip pathology (LE 5; LR D; LA 96%).
Meticulous analysis of these previous treatments is essential in order for the new treatment to be target specific. The reasons for the failure of previous treatments must be analyzed and taken into consideration: lack of efficacy, adverse effects or insufficient therapeutic compliance.
Recommendation 3. The recommendation is that patients pending surgery should control their weight and maintain the highest possible level of physical activity, symptoms permitting. Obese patients in particular should try to lose weight (LE 2a; LR B; LA 100%).
Increasing physical activity and reducing weight are effective therapies for pain control and the loss of function in patients with arthrosis.8–10 Both pain level11–16 and functional limitation levels17,18 were inversely correlated with the final outcome of arthroplasty. Although obesity has no specific harmful effect, in the final functional outcome of a knee or hip replacement, it is related to a higher level of post-operative local and systemic complications.19 The use of specific knee braces or insoles is not to be recommended8 in patients with predominantly medial articular compromise.
Recommendation 4. The use of topical or oral NSAIDS for pain management in patients awaiting surgery (LE 1a; LR A; LA 82%) is recommended.
Oral non-steroid anti-inflammatory drugs are proven to be effective in the management of arthrosis symptoms. Cox-2 selective inhibitors have a higher gastrointestinal safety profile than traditional NSAIDS with a cardiovascular safety profile similar to Ibuprofen and Diclofenac20; moreover, their lack of effect on platelet aggregation means they can be used until surgery is performed.21 Administration must adhere to the general rule of “the shortest time possible at the lowest dose possible”. Topical NSAIDS have a comparable efficacy profile to that of oral NSAIDS with fewer systemic adverse effects,22 but are effective for a limited period only.23
Recommendation 5. The use of Paracetamol or Tramadol can be neither recommended nor advised against in these patients (LE 1a; LR A; LA 57%).
Until recently, Paracetamol was considered the keystone to treatment initiation for arthrosis. However, new dose recommendations to limit the maximum dose to 3g daily and the lack of good quality evidence to support its efficacy in arthrosis,8,24 particularly arthrosis of the hip, together with the fact that these patients usually present with intense symptomology, have resulted in its role in these patients being limited. Tramadol has proven efficacy for the treatment of pain in arthrosis8 but its efficacy/adverse effect profile in these patients is poor25 and improvements in pain levels are not linked to improvements in functional capacity.26
Recommendation 6. The use of glucosamine, chondroitin sulfate, hyaluronic acid or anti-epileptic drugs is advised against for these patients (LE 1b; LR A; LA 82%).
The efficacy of glucosamine, chondroitin sulfate and hyaluronic acid in patients with arthrosis has been a subject of constant debate in the last decade8,23,27; however, the most recent clinical guidelines and systematic reviews suggest that their role is limited, particularly in this group of patients who present with advanced arthrosis and major symptoms. Although the use of anti-epileptic drugs (Pregabalin and Gabapentin) in the treatment of advanced arthrosis has been proposed in relation to a central neuropathic/awareness component in patients with advanced arthrosis of the knee,28 there is no evidence that supports their usage today.
Recommendation 7. Intra-articular corticoid injections for patients with arthrosis of the knee are recommended (LE 1a; LR A; LA 70%).
Although its role in treatment of arthrosis of the knee is under discussion,8,23 and although duration is limited, since relief is fast and effective this therapy may be useful for the control of symptoms in the group of patients awaiting surgery who have significant symptoms which do not respond to NSAIDS.
Recommendation 8. In patients with high levels of pain the use of strong opiates is recommended (LE 3a; LR C; LA 88%).
The role of opiates in chronic non-ontological pain is highly controversial in general29,30 and in arthrosis in particular,8,23,27 due to the difficult balance between efficacy and adverse effects. In this specific patient group, when there are intense levels of pain and they do not respond to other conservative therapies, the fact that treatment is suggested for a limited period of time (limited due to a possible improvement in symptoms or the surgery itself) means that strong opiates play a major role.
Recommendation 9. Once the patient has been referred for arthroplasty, it is recommended that the presence of factors affecting its outcome be assessed (LE 2a; LR B; LA 96%).
The identification of factors which may affect the outcome of arthoplasty is essential for two reasons. Firstly, in patients with factors which are predictive of poor outcome which can be modified prior to surgery, such as extreme sedentariness, level of pain11–16 or functional limitations17,18 or in patients with central sensitization/neuropathic components, depression31–34 or catastrophic thoughts, an active effort must be made to change them (see the following two specific recommendations) Secondly, patients with factors predictive of poor outcome which are not modifiable prior to surgery, such as advanced age, female gender, low socio-economic status and the presence of associated low back pain should be informed appropriately of this when giving their informed consent to surgery.
Recommendation 10. It is recommended that patients pending surgery with intense pain or loss of major functions should be administered treatments aimed at controlling the progression of their arthrosis symptoms (LE 5; LR D; LA 96%).
This patient group has an increased risk of an unsatisfactory outcome from arthroplasty and there are safe and effective alternative therapies for the control of their symptoms which could have a positive effect on results of surgery. The post surgical outcome is worse for patients who present with the most severe pre-operative pain11–16 or functional limitations.17,18 To be effective, the chosen treatment should be prolonged up until surgery takes place.
Recommendation 11. Patients pending surgery who fall into depression or have catastrophic thoughts should be administered psychological/psychiatric assistance/care for the treatment of these problems (LE 1a; LR A; LA 88%).
The outcome from arthroplasty is worse for patients with depression31–34 or catastrophic thoughts34,35 and there is a higher rate of surgical failure. There are several therapies available which are effective for modifying catastrophic thoughts and curing depression. These therapies should be specified, prescribed, and assessed by a mental health specialist. Prudent use of these interventions may have a clear positive effect on the outcome of arthroplasty.36
Recommendation 12. The use of subjective scales for assessment of pain levels is recommended for pre-surgical pain assessment (LE 5; LR D; LA 96%).
The patient provides information about the intensity of their pain, and this is useful for initial assessment of the patient and of the results obtained from the different surgical interventions. The visual analog scale (VAS) is the most frequently used.37
Recommendation 13. The use of quality of life evaluation scales pre and post surgery are recommended in these patients (LE 5; LR D; LA 93%).
These scales assess not just the pain but also other dimensions of the experience of pain, such as emotional, cognitive and social aspects. There are multiple scales, although the questionnaire 36-Item Short Form Health Survey (SF-36), or its shorter version, the SF-12, are valid in Spanish and are commonly used in literature. There are also more specific scales which evaluate the quality of life of patients with hip and knee arthrosis (The osteoarthritis knee and hip quality of life questionnaire, OAKHQOL38).
Recommendation 14. The use of specific scales of hip and knee assessment, in the context of global pre and post operative assessment of patients with arthrosis of hip and knee (LE 5; LR D; LA 96%).
The Western Ontario and McMaster University Osteoarthritis Index (WOMAC) includes 24 items, which assess three dimensions: pain, joint stiffness and physical function.39 It is one of the most popular self-administered scales for measuring pain and function in the lower extremities. Its use requires minimum training and takes only 5–10min to complete. It has been validated and translated into Spanish.40 Furthermore, it includes a VAS scale for pain and a Likert type scale of five pain response levels. Other scales are also used for the knee (Knee Society Score [KSS]41 and Oxford knee scale42) and for the hip (Harris scale [HHS],43 the Merle d’Aubigné/Poste scale44 and the Oxford hip scale45).
Recommendation 15. It is recommended that those patients with symptoms of depression, anxiety or irrational thoughts be assessed with specific questionnaires (LE 5; LR D; LA 77%).
There are consistent data on the existence of psychological predictors of poor post-operative outcome.31–35 Depression, general anxiety and catastrophic thoughts are associated with a worse outcome, particularly in knee arthroplasty.31–35,46 The Pain Catastrophizing Scale (PCS)47 and the Hospital Anxiety and Depression Scale (HADS)48 have been used in patients with hip and knee arthrosis and arthroplasty.49,50
Recommendation 16. It is recommended that an evaluation of the response to analgesic treatment administered to patients with hip or knee arthrosis awaiting surgery be completed (LE 2a; LR B; LA 88%).
Since there are many available therapeutic alternatives with different efficacy/safety profiles,8,23,27 the doctor's first choice is not always appropriate for a specific patient. An assessment of response to treatment must be made to accurately adjust therapy and ensure good control of pain and functional loss. This may have a positive effect on the outcome of arthroplasty.11–18
Recommendation 17. That this assessment evaluates efficacy, treatment adherence and the appearance of adverse effects (LE 5; LR D; LA 96%).
Assessment of efficacy is essential, since it is the cornerstone when attempting to improve the functional outcome of arthroplasty and improve the quality of life of the patient up until surgery.11–18 Assessment of treatment adherence is essential, since in arthrosis this is low51 and is directly related to the clinical efficacy of a therapy.52 The detection of possible adverse effects is key to safeguarding the patient's safety.53
Recommendation 18. This assessment is to be made between weeks 3 and 4. This term must be shortened in patients at high risk of adverse effects or in those who have been prescribed strong opiates (LE 5; LR D; LA 93%).
Response to the majority of available pharmacological interventions usually stabilizes between the second and fourth week, resulting in this being an appropriate moment for evaluating efficacy. Likewise, the adverse cardiovascular effects of some therapies are apparent in the first 3 weeks. In those patients where side effects are of the greatest concern (mainly patients with known risk factors or those who have been prescribed strong opiates, where side effects are more frequent), a more rigorous and early assessment may be more effective.
Recommendation 19. It is recommended that the primary care practitioner participate actively in the assessment of these aspects (LE 5; LR D; LA 96%).
The general practitioner is the essential pillar of the Spanish health system. Their accessibility to the patient, their knowledge of medical records and skill in handling both symptoms related to the illness and the identification of adverse effects produced by prescribed medication, makes them an ideal professional for this evaluation. If doubts, complications, or unpredictable events arise, it is recommended that any information and decision-making be shared with the patient's traumatologist of referral.
Preventative treatment of post-surgical painPre-emptive analgesia is defined as that aimed at reducing peri-operative pain, which is administered hours or days prior to surgery.
Recommendation 20. Pre-emptive analgesia is neither recommended nor advised against in patients awaiting surgery (LE 2a; LR B; LA 59%).
The use of analgesics immediately prior to surgery aimed at reducing post-operative pain and post-operative analgesic requirements has a clear physiopathological basis; notwithstanding, their actual clinical efficacy is highly controversial.54–56 Although different studies have demonstrated efficacy in reducing post-operative analgesic requirements, it has not been demonstrated that they consistently reduce the pain perceived by patients.
Recommendation 21. If preventative analgesia is the chosen therapy, the use of selective cyclo-oxygenase-2 (Cox-2) or opiates is recommended (LE 2a; LR B; LA 88%).
In general NSAIDS have proved to be effective in replacing post-operative analgesic requirements, but Cox-2 have a more consistent efficacy profile and offer the advantage of not altering platelet aggregation. Opiates are also effective. Pregabalin may be effective in controlling the neuropathic component of post-operative pain but it is not effective in the control of nociceptive pain. Other drugs which may play a role are corticoids, Ketamine and magnesium sulfate.
DiscussionThere are several characteristics particular to the management of patients who are waiting for a hip or knee replacement. This document has established several basic recommendations on which there is a clear consensus. They all focus on the fact that the moment when surgery is indicated is crucial to in-depth patient assessment. The presence of factors which may have an effect on the outcome of arthoplasty must be established and acted upon. Patients must be encouraged to keep active and lose weight if necessary. They should also seek help if they have psychiatric problems or catastrophic thoughts. A key factor in the prognosis of intervention is the pre-operative level of pain and functional limitations. Traumatologists must therefore make an assessment of pain, of joint function, and their patient's quality of life. They also need to monitor the effect that treatment may have on these parameters, in conjunction with the primary care physician. When treating pain, NSAIDS (in particular the selective cyclo-oxygenase-2 inhibitors for their gastrointestinal safety), strong opiates (in intense pain and subject to tighter control) and intra-articular corticoid injections play a clear role.
The Delphi methodology was used to reach this consensus. This methodology is a structured communication strategy initially developed as a systematic and interactive precision method based on a panel of experts.57 It has several intrinsic limitations: on the one hand, the level of quality of the evidence exposed is level 5, expert recommendations. In this case, an attempt has been made to control this limitation, wherever possible, by means of three systematic reviews and the most recent systematic reviews in literature.8,23 This has made it possible to present many recommendations with higher levels of evidence: 5 of the recommendations are level 1, four are level 2 and one is level 3, leaving just 11 at level 5. The Delphi system also tries to obtain “recommendations from experts” where more evidence of higher quality is not possible. Moreover, a Delphi consensus is as weak or robust as the steering group and the group of experts, and they were selected from eminent members of SECOT and GEDOS with specific interest and experience in musculoskeletal pain management. Despite these limitations, this methodology has been chosen by different scientific associations as an appropriate tool for establishing therapeutic recommendations58–60 and establishing opinions about controversial clinical issues.61 Within the group of experts selected, 74% assessed the recommendations presented by the steering group, a percentage in keeping with other documents of consensus.60
In 19 of the 21 recommendations a level of consensus of at least 70% was obtained. This is the universally acknowledged rate for this type of methodology.62 The two recommendations for which no consensus was reached were expressions of the lack of consensus among the steering group in the light of available evidence, and it is therefore difficult to express agreement or disagreement. This is reflected by the spread of responses collected in both cases (Table 1). In the light of these results, it was considered that modifying these two recommendations would not lead to a consensus and no second round was therefore presented.
Predicting factors of poor surgical outcomeThere was uniform consensus regarding the need to identify the factors which may affect the outcome of surgery and which are susceptible to surgery: being overweight, the presence of a mental illness or catastrophic thoughts. In particular, the presence of intense pain and functional limitation. The experts consulted considered it appropriate to assess the presence of these factors and there was consensus regarding the need to act on them. Recommendations referring to the identification of predictive factors are based on quality evidence (levels 1 and 2), and the experts were therefore in agreement with the available evidence. With regard to the management of these factors, good quality evidence is available to prove the effectiveness of weight loss, actions taken for mental health problems and catastrophic thoughts (level 2). With regard to treatment for pain and functional loss, in order to improve surgical intervention, a consensus was reached between the steering group and the other experts (level 5) for its recommendation, despite the limited good quality evidence to support it. This is justified by the major negative impact that pre-operative pain and functional limitation has on the outcome of arthoplasty, which has been well documented, and that this factor is relatively easy to act upon effectively.
Assessment of pain and patient follow-upThere was clear consensus that it is essential to evaluate the presence of the before-mentioned predictive factors of poor outcome, identify their magnitude with the appropriate tools and monitor the effect that proposed surgery would have on them. There is agreement among the experts consulted that this evaluation of efficacy, safety and adherence to primary care attention plays a clear role. This part of the consensus also essentially reflects the opinion of the steering group (evidence level 5) upheld by the larger group of experts.
Pharmacological treatment of pain and functional limitationsThe levels of consensus about which drugs were most appropriate in treating pain and functional limitation in patients with hip and knee arthrosis awaiting surgery were generally high. There was over 80% consensus on the use of NSAIDS and strong opiates and on the use of chrondroprotectors, hyaluronic acid and antiepileptic drugs not being suitable for this specific patient group. The use of intra-articular corticoid injections had a 70% level of consensus, with four experts expressing disagreement (≤3), although the recommendation was based on those recently established by different international clinical guidelines.8,63 The absence of consensus regarding the use of Paracetamol or Tramadol in these patients clearly reflects genuine doubts regarding the role of these drugs on the management of patients with arthrosis pending surgery. In general, all these recommendations are substantiated by high quality (level 1) studies which are widely available and familiar to experts. For this reason the level of general agreement was not unexpected.
Pre-emptive analgesiaNeither the steering group nor the group of experts reached a clear agreement on the role of pre-emptive analgesics in hip and knee replacement surgery. Although there is a growing body of evidence available which suggests that several protocols of pre-emptive analgesia are effective in this patient group, its effect on the levels of post-operative pain or on the post-operative analgesic requirements is not sufficiently consistent for a definite recommendation to be made. However, there was clear consensus that opiates and selective inhibitors of cyclooxigenase-2 were the most appropriate drugs if pre-emptive analgesia was to be used. The steering group and the group of experts also agreed with the available evidence (level 2a) on this point.
The referral of a patient with hip or knee arthrosis for surgery signals the end of conservative management of the degenerative process of that joint. However, the patient will present with pain and functional limitation until surgery and more frequently than we would wish, the outcome of arthroplasty is not as good as expected.6,64 At this time, the surgeon has to decide what he/she could do to improve the outcome of the arthroplasty to reduce pain after surgery. It is essential to identify and modify the factors affecting this outcome and also to treat the patient's pain in an appropriate manner prior to surgery.
Level of evidenceEvidence level V.
Ethical responsibilitiesProtection of human beings and animalsThe authors declare that no experiments were performed on humans or animals for this investigation.
Data confidentialityThe authors declare that no patient data appear in this article.
Right to privacy and informed consentThe authors declare that no patient data appear in this article.
Conflict of interestsDr. Ruiz-Iban received fees from MSD (consultant) in this investigation. He also received consultant fees from the following companies: Biomet, Bristol-Myers Squibb; Grunenthal, MSD; fees for teaching activities from the following companies: Astelas, Biomet, Bristol-Myers Squibb; Grunenthal, MSD, Pfizer, Smith and Nephew y Zambon; financing for research projects from: Biomet, Grunenthal and MSD. Dr. Oteo received fees from MSD (consultant) for this investigation. Dr. Loza received fees from MSD (methodology) for this investigation. She also received financing for the research projects from: Pfizer, TiGenix, Novartis, Roche, Abbvie and MSD. Dr. López Millán received fees from MSD (consultant) for this investigation. He also received fees as a consultant from: Baxter, Ferrer Pharma; for teaching activities from: Astelas, MSD, Pfizer, Baxter, Ferrer Pharma, Jonhson and Jonhson, Lilly, Mundipharma; and financing for research projects from: Medtronic. Dr. Díaz Heredia received fees from MSD (consultant) for this investigation. He also received fees for teaching activities from: Biomet, Grunenthal, MSD, Pfizer, Smith and Nephew; financing for research projects from: Biomet, Grunenthal and MSD. Dr. Maculé received fees from MSD (consultant) for this investigation. He also received fees as a consultant from: Biomet, MSD; for teaching activities from: Biomet, MSD, and Smith and Nephew; financing for research projects from: Smith and Nephew. Dr. Torner received fees from MSD (consultant) for this investigation and received fees for teaching activities from: Puy Synthes, Smith-Nephew, Waldemar-Link. Dr. Gil Garay received fees from MSD (consultant) for this investigation. He also received fees as consultant from: Stryker y Sanofi; for teaching activities from: BMS, Pfizer, Bayer, Lilly, Boehringer Ingelheim; financing for research projects from: Smith and Nephew, Biomet.
Our thanks go to Dr. Alejandro Tejedor, coordinador of the musculaskeletal pain group of the Spanish Society of Family Physicians and Community (SEMFYC), his support to the steering group in preparing the recommendation relating to the role of primary care physicians.
José Luis Martínez de los Mozos, Gonzalo Gómez del Álamo, Pablo García Portabella, Laura Ameneiro Romero, Francisco Chana Rodríguez, Rodrigo García Crespo, Carlos Juandó, Antonio Darder, Carlos Revenga, Juan Pedro Rodríguez Álvarez, Natalia Ruiz Mico, Adolfo Galán Novella, Pedro Carpintero Benítez, Clara Hernández Cera, Antonio Montero Matamala, Antonio Jover, Blas González, Eva Patiño Rodríguez, Miguel Marín Paredes, Manuel García Alonso, Pablo Puertas, Jesús Palencia Ercilla, Carlos Molano Bernardino, Ricardo Cuellar, José Antonio Guerrero, Jorge Díaz Heredia, Fausto González Lizán, Sergi Sastre, Santos Moros, Joaquín Fores, José Manuel García Pequeruz, Alejandro López Pardo, Francisco Lozano Moreno, Óscar Romanillos Arrollo, Vicente León Muñoz.
Please cite this article as: Ruiz Ibán MA, Maculé F, Torner P, Gil Garay E, Oteo-Álvaro A, López Millán JM, et al. Consenso SECOT-GEDOS sobre el control del dolor prequirúrgico en artrosis de rodilla y cadera. Rev Esp Cir Ortop Traumatol. 2015;59:186–199.