Vascular malformations and tumors, also known as “vascular anomalies”, comprise an extensive variety of lesions involving all parts of the body. Due to a lack of a complete understanding of the origin and histopathology of such lesions, this field has been traditionally obscured by the use of an unclear nomenclature. Knowledge of the classification and clinical and imaging characteristics of this group of lesions is paramount when managing these patients. The objective of this series of two articles is to review the current classification of vascular anomalies, to describe the role of imaging in their diagnosis, to summarize their distinctive histopathologic, clinical and imaging features, and to discuss the treatment options. High-flow lesions were discussed in the first article of this series. In this second article, we will focus on low-flow lesions, including complex syndromes with associated low-flow malformations.
Las malformaciones vasculares y los tumores, también conocidos como “anomalías vasculares”, comprenden una extensa variedad de lesiones en diferentes partes del cuerpo. El origen y la histopatología de estas lesiones no es del todo conocido, por ello este campo se ha visto ensombrecido por el uso de una nomenclatura poco clara. Conocer su clasificación, así como las características clínicas y de imagen es de vital importancia para el manejo de estos pacientes. El objetivo de esta serie de dos artículos es revisar la clasificación actual de las anomalías vasculares, describir el papel que desempeñan las pruebas de imagen en su diagnóstico, resumir sus características histopatológicas, clínicas y de imagen y debatir las posibles opciones terapéuticas. El primer artículo de esta serie versó sobre las lesiones de alto flujo. En este segundo artículo nos centraremos en las de bajo flujo, incluidos los síndromes complejos que asocian malformaciones de bajo flujo.
Vascular malformations and tumors, also known as “vascular anomalies”, comprise a wide variety of lesions involving all parts of the body, and are the most common pediatric soft-tissue tumors.1 Significant confusion exists regarding the nomenclature and classification of such lesions. Vascular malformations can be classified as low- or high-flow, according to their hemodynamics. As previously described, lesions with an arterial component are categorized as high-flow, whereas, when an arterial component is lacking they are categorized as low-flow. In the prior article, we reviewed vascular tumors and high flow vascular malformations. In the current article, we discuss the characteristic histopathogenic, clinical and imaging features of low flow malformations, including venous, lymphatic, capillary and mixed lesions; as well as the recently described fibro-adipose vascular anomaly (FAVA). Complex syndromes with associated low-flow vascular malformations are also presented.
Low flow vascular malformationsVenous malformationVenous malformations (VMs) are the most common peripheral vascular malformations2–6 accounting for more than half of referrals to specialized vascular anomaly centers.2 They are defined as low-flow vascular malformations with an abnormal venous network.6 Histologically, they are composed of dysplastic post-capillary, thin-walled valveless vascular channels with patchy deficiency of mural smooth muscle and a variable amount of hamartomatous stroma. Intramural thrombi may calcify thus leading to phlebolits formation.2,7–9 They range from simple single channel vessel dilatation to multiple spongiform venous lakes which usually drain into adjacent normal veins via narrow tributaries.5 The smooth muscle anomaly is probably responsible for their gradual expansion.6,10
Clinical presentationAs all vascular malformations, VMs are already present at birth, but are usually not clinically evident until late childhood or adulthood, and may enlarge due to hormonal changes during puberty.2 They can be found in the head and neck (40%), the extremities (40%), and the trunk (20%).2
VMs are frequently asymptomatic. On clinical exam they present as sponge like, compressible and non-pulsatile masses5,8,9 which vary in size and shape and may be localized or diffuse. When superficial, lesions typically have a bluish discoloration.2 They characteristically reduce with extremity elevation and local compression and enlarge with dependent position and Valsalva maneuvers. Stiffness and discomfort may occur secondary to hemorrhage and thrombophlebitis.11 Lack of increased local temperature or bruit is characteristic in comparison with high-flow lesions.12
Like all vascular malformations, VMs may infiltrate across multiple tissue planes including skin, subcutaneous fat, skeletal muscle, bones, joints and, internal organs. The involvement of deep structures is underestimated on clinical examination, and potential manifestations include pain, impaired function, and skeletal deformity.5,8,9 As discussed in Part 1, Kasabach–Merritt phenomena characterized by consumptive coagulopathy can occur.13
Imaging featuresOn US, VMs usually present as compressible, anechoic, ectatic venous spaces separated by echogenic septa (Fig. 1a) and with scant monophasic low-velocity flow.12,14 The detection of flow can be enhanced by applying compression or performing the Valsalva maneuver.14 Although rare, predominantly solid lesions and no detectable flow can also be seen.12 This feature may represent the new recognized entity, FAVA, that we will be further discussed later in the manuscript. When venous flow is not depicted, differentiation from a lymphatic malformation can be challenging. Some US maneuvers may be helpful in highlighting some changes in the venous channels. Specifically, these will fill in during Valsalva maneuvers, in dependent position, and drain with compression, elevation of the body part above the level of the heart or when Valsalva is released.2
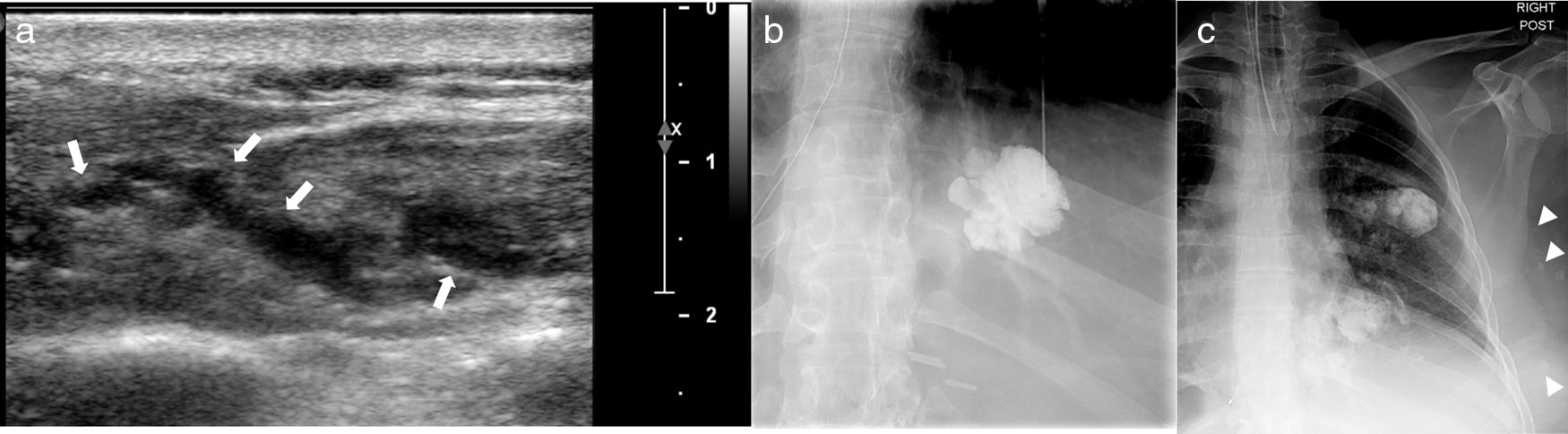
41-Year-old female with venous malformations involving the left-sided chest wall. Ultrasound image (a) reveals a heterogenous subcutaneous lesion containing predominantly anechoic vascular channels (arrows). Image obtained with direct percutaneous injection of contrast material (b) shows diffuse homogeneous enhancement of the lesion. Multiple phlebolites are noted along the left sided chest wall on a post percutaneous contrast image (arrows).
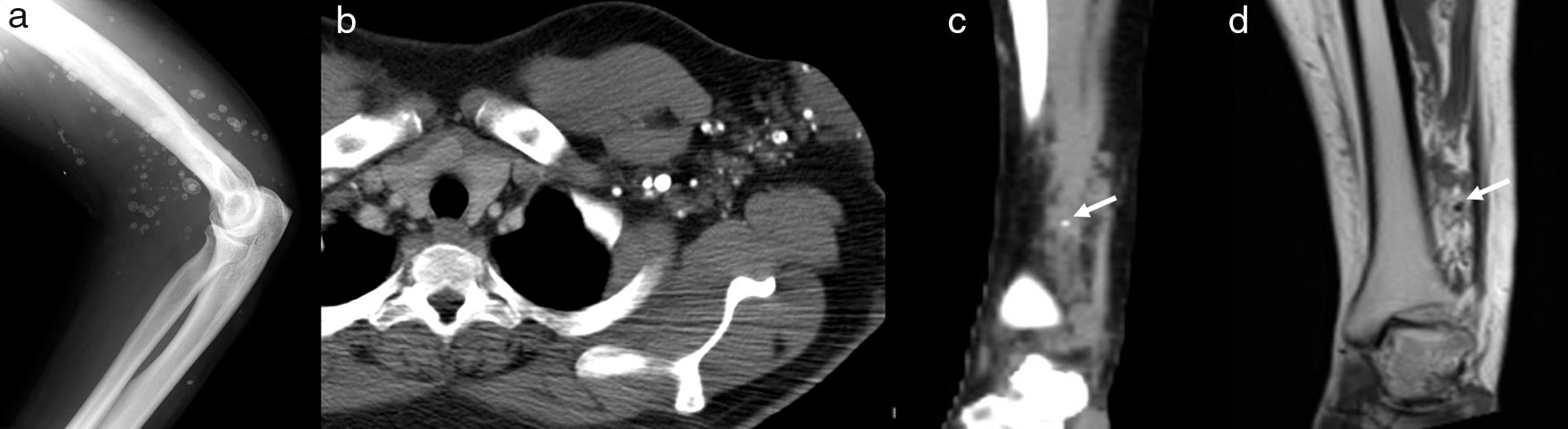
Phleboliths are the hallmark of VMs and are best depicted as small calcifications on radiography and CT (Figs. 1c and 2a–c). They can be seen on US as echogenic foci with posterior acoustic shadowing. Secondary signs of osseous involvement such as bony expansion, osteolysisis, cortical thinning and increased trabeculation can also be seen on radiography and CT.2
On MRI, VMs present as lobulated, non-mass like lesions with low to intermediate signal intensity on T1-weighted images and hyperintensity on fluid-sensitive sequences (Fig. 3). Fat is commonly interspersed within these lesions.15 Heterogeneous signal intensity can be observed on T1-weighted images in case of thrombosis or hemorrhage. The former may result in internal fluid–fluid levels. As previously described, VMs may infiltrate multiple tissue planes and fat suppressed T2-weighted and STIR images provide excellent delineation of the extension of the lesions (Fig. 3).8,15,16 Low-flow malformations are characterized by a lack of arterial and early venous enhancement, and absence of enlarged feeding vessels or arteriovenous shunting.15,17 Recognizing whether a vascular malformation is low-flow is more important than determining exactly whether the lesion is predominantly venous, capillary or lymphatic, in terms of patient management.5,17–20 Gadolinium contrast administration is helpful and often shows slow, gradual, delayed heterogeneous contrast filling with characteristic diffuse enhancement of the slow flowing venous channels on delayed post-contrast T1 weighted images (Fig. 4a).19,21,22
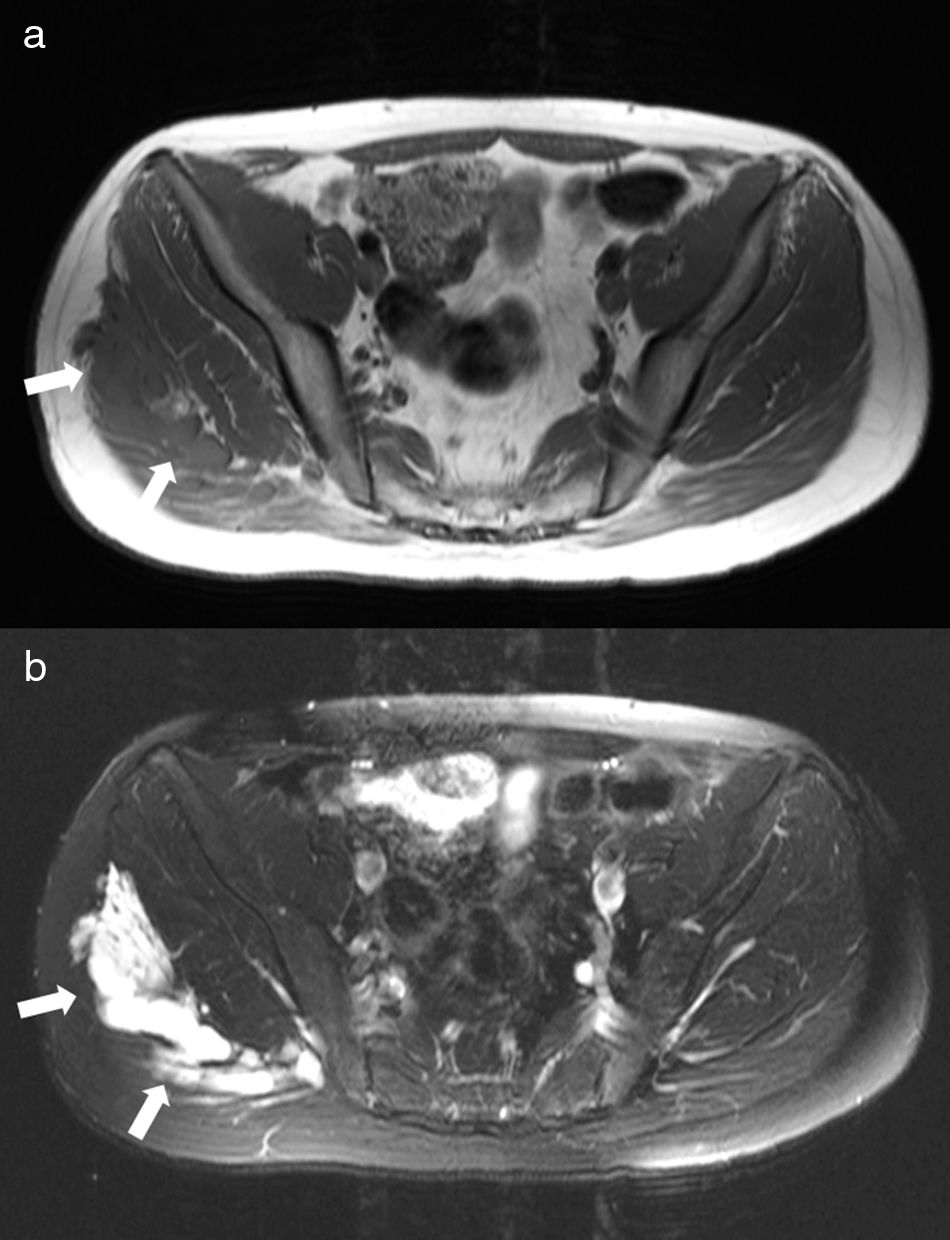
Right gluteal venous malformation. Axial T1-weighted image (a) shows a hypointense lobulated mass involving the right gluteal area (arrows). On axial STIR image (b), the venous malformation is hyperintense and has a multilocular appearance due to abnormal venous lakes separated by thin hypointense septa.
Pleboliths, septations, or thrombosed vessels may simulate flow voids on MRI. Pleboliths and calcifications typically appear as low signal nodular foci on all sequences (Fig. 2d) whereas signal voids related to high flow characteristically appear as high signal foci on GRE sequences and demonstrate contrast enhancement.
TreatmentConservative treatment with compression devices or activity modification is usually the initial treatment. If no improvement is achieved, different treatments can be attempted including percutaneous sclerotherapy, embolization, surgery or a combination of these.2 After treatment, progressive shrinkage of the lesion is usually demonstrated by imaging (Fig. 4b and c).
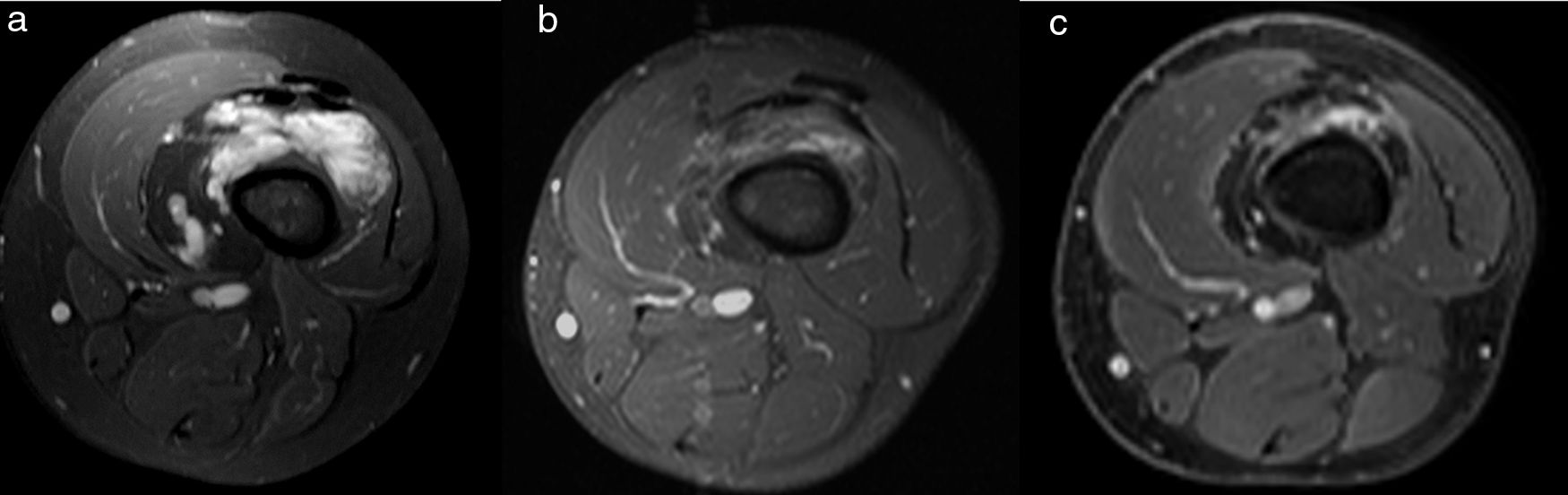
MRI appearance of a venous malformation in the lower extremity after percutaneous scleroteraphy. Delayed contrast-enhanced fat-suppressed T1-weighted images. Baseline image in the 8-year old boy (a) shows diffuse homogeneous enhancement of the lesion involving the right thigh. Progressive shrinkage of the lesion is demonstrated years after treatment (b, c).
Lymphatic malformations (LMs) are the second most common type of vascular malformation after venous malformations.23 LMs consist of chyle-filled cysts lined by flat endothelial cells,9,12,21 surrounded by thickened smooth muscle,12 separated by fibrous septa and isolated from the normal draining lymphatic channels.9,12 The lymphatic endothelial cells characteristically express vascular endothelial growth factor receptor (VEGF-3).
Traditionally misnamed as “lymphangiomas or cystic hygromas”, LMs can be divided into macrocystic, microcystic and mixed types. Microcystic LMs are composed of multiple cysts smaller than 2mm in a background of solid matrix, whereas macrocystic lesions, have larger cysts of variable sizes.21,24 Sometimes, this distinction is based on the sonographic characteristic of the lesion since it will define treatment options; lesions are categorized as macrocystic when the size of the cysts permits a needle to be inserted in.2
Lymphatic malformations are commonly mixed, containing both micro and macrocystic components as well as other types of vascular malformations,1 most commonly venous malformations.
Clinical presentationUnlike VMs, most LMs are identified in the first two years of life. LMs are usually found in the neck (70–80%), especially in the posterior cervical triangle, and axillary region (20%).9,21 Less commonly, the mediastinum, retroperitoneum and the extremities may be involved.11
Upon physical exam, they present as smooth, non-pulsatile, soft tissue masses with a rubbery consistency and without bruit or increased temperature.12 Dermal extension is common, especially with microcystic LMs, and it is seen as numerous small vesicles2 with associated skin thickening and surrounding lymphedema.21 The macrocystic counterparts are seen as smooth, translucent lobulated masses under the normal cutis.25 Unlike VMs, LMs are non-compressible. They can get complicate by infection or bleeding2 thus presenting with tenderness or sudden enlargement of the lesion.7
Imaging featuresMacrocystic LMs appear as thin-walled cystic lesions with posterior acoustic enhancement on US.2 Thin septa are often present. Characteristically, arterial or venous waveforms are absent within the cysts on Doppler US, but may be detected within the septa.2 Unlike VMs, no change in appearance will occur with Valsalva maneuvers, compression14 or change in position.
The cysts in microcystic LMs are often too small to be discernible by ultrasound, and they often present as ill-defined hyperechoic lesions14; the posterior acoustic enhancement suggests the cystic nature of the lesion.2 Absent flow is demonstrated by Doppler.14
On MRI, LMs are usually seen as lobulated, septated masses with intermediate to decreased T1-weighted signal intensity and, like other vascular anomalies, increased signal intensity on T2-weighted and STIR images (Fig. 5). Internal fluid–fluid levels are common (Fig. 5). LMs tend to infiltrate across fat planes and involving multiple adjacent tissues.8,21 The pattern of contrast enhancement on MRI will depend on the type of LM. Microcystic LMs do not usually enhance,21,26 whereas macrocystic LMs exhibit rim and septal enhancement with characteristic lack of internal enhancement of the cystic structures.5,21 Pre-contrast fat-suppressed T1-weighted imaging and post-contrast digital subtraction imaging is paramount to distinguish increased signal intensity secondary to proteinaceous component or hemorrhage within the cysts from contrast enhancement.7 The closely packed, non-perceptible, enhancing septa in Microcystic LMs can occasionally demonstrate solid and diffuse enhancement7 (Fig. 6). Similarly, combined lymphatic–venous malformations may show diffuse inhomogeneous enhancement due to the venous component of the malformation.
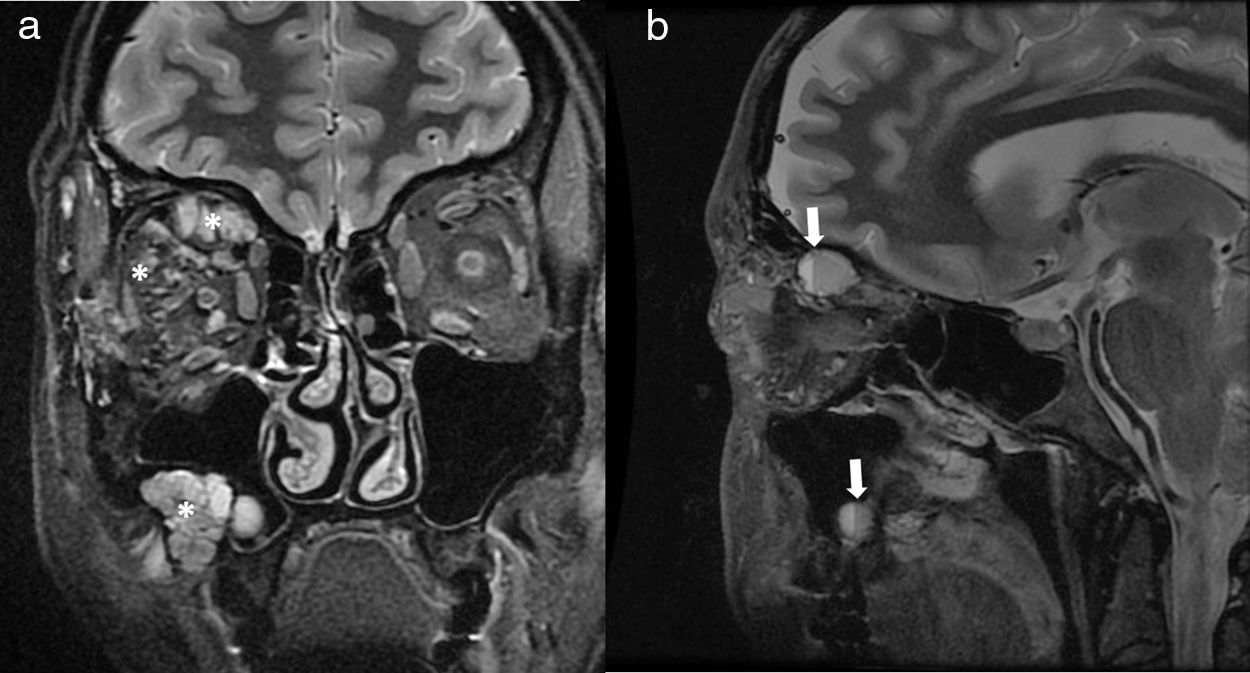
32-Year-old women with macrocystic lymphatic malformation involving the right orbit and right maxillary sinus. Coronal (a) and sagittal T2-weighted images (b) show a multicystic lesion (*) with several internal fluid–fluid levels (arrows) due to hemorrhage. Lack of enhancement was demonstrated on post-contrast imaging (not shown).
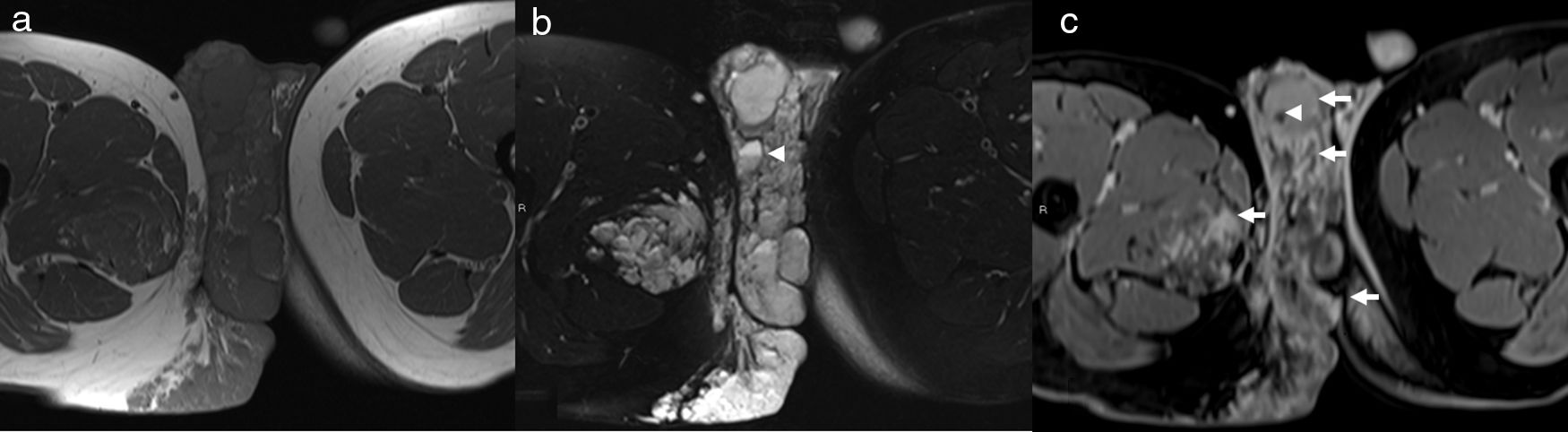
Microcystic lymphatic malformation of the left arm and chest wall in a 5-year-old girl. Coronal STIR image (a) shows a hyperintense, lobulated, septated lesion (arrowheads) involving the subcutaneous (arrows) soft-tissue. The lesion is hypointense on T1-weighted image (b). Delayed contrast-enhanced 3D VIBE (c) image shows mildly increased signal intensity in parts of the lesion due to enhancement of the septa (arrowheads) between the microcysts.
Treatment options include observation, compression therapy, sclerotherapy with ethanol, doxycycline, or bleomycin, drug therapy, surgical excision and laser therapy.
Capillary malformationCapillary malformations (CMs), traditionally named port wine stains, are the least common and the most superficial of all low flow vascular malformations.
Histologically, they are composed of ectatic thin-walled capillary channels surrounded by disorganized collagen.12 They are usually limited to the superficial dermis or mucous membranes; but, on occasions, they may be the hallmark of complex syndromes such as Sturge–Weber, Klippel–Trenaunay, Parkes–Weber or Proteous syndrome.21,25 Those entities are briefly discussed below.
Clinical presentationUnlike VMs, CMs are usually present at birth in around 0.3% of children,21 as a macular pink to dark red12 patch with irregular borders without bruit or local warmth.2 Like LMs, they are usually localized in the head and neck region.25,27 Symptoms may be the result of deeper associated malformations.5,28
Imaging featuresDue to their superficial nature, diagnosis is usually made by clinical exam and history. Imaging is, therefore, not required for their diagnosis but can be sometimes indicated to exclude underlying disorders. Skin thickening is usually the only finding on US.12 MRI findings are also subtle, with skin thickening and occasional increased subcutaneous thickness5,21,29 and faint focal T2 hyperintensity and contrast enhancement.12
TreatmentLaser therapy is the standard treatment. Surgical procedure may be considered when there is overgrowth of soft tissue or bone enlargement.
Mixed low-flow vascular malformationClinical presentationThis group includes capillary-venous malformations, which are combined low-flow malformations formed from dysplastic capillary vessels and enlarged post-capillary vascular spaces, and mixed venous and lymphatic malformations. Clinical presentation depends on location and size of the lesion.
Imaging featuresImaging findings in capillary-venous may be indistinguishable from those of VMs and dynamic contrast-enhanced MRI can be useful for this purpose, as capillary-venous malformations will typically show early enhancement, whereas only delayed enhancement is seen in VMs.21 Mixed lymphatic venous malformations present as partially enhancing multicystic lesions (Fig. 7).
4-Year-old male with mixed venous–lymphatic malformation. Axial T1-weighted image (a) shows a hypointense lobulated mass malformations involving the perineum and extending into the scrotum and right thigh. Axial STIR image (b) shows a well-defined septate hyperintense lesion with few fluid–fluid levels (arrowhead). Delayed contrast-enhanced fat-suppressed T1-weighted axial image (c) demonstrates partial enhancement of the lesion (arrows). A phlebolit is also noted as a hypointense foci on this image (arrowhead). There was no arterial enhancement (not shown).
These lesions are treated with a combination of methods for venous and lymphatic malformations, as described before.
Fibro-adipose vascular anomalyRecently described by Alomari et al.30 a fibro-adipose vascular anomaly (FAVA) constitutes a new vascular disorder with distinct clinical, radiologic, and histopathologic features. It is a complex mesenchymal malformation characterized by fibrofatty infiltration of muscle and unusual phlebectasia that presents with constant severe pain, and contracture of the affected extremity.30 The gastrocnemius muscle is the most commonly affected muscle in FAVA.30 Although this new entity shares some similarities with the more common intramuscular VMs, it is important to recognize FAVA because of a different management approach.
Histopathologically, VMs comprise dilated and disorganized channels with thin, abnormal walls, whereas FAVA is composed of fibrofatty tissue and low-flow vascular malformations (usually VMs) within the muscles and adjacent subcutaneous tissue.30 Occasionally, small capillary and lymphatic components exist in FAVA.
Imaging featuresUnlike VMs, a non-compressible, echogenic mass characterizes FAVA on US.30 On MRI, the dominant fibrofatty solid component is seen with associated phlebectasia characterized by heterogeneous moderately hyperintense signal on T2-weighted images which is less hyperintense than that seen in common VMs.30 Moderate to strong and homogeneous post-contrast enhancement is also seen.30
TreatmentAlthough sclerotherapy can be performed on the generally smaller venous component of FAVA, the dominant solid fibrofatty component is not amenable to this intervention,30 and depending on the severity of symptoms, physical therapy and/or surgical resection may be needed.30 There is a report of image-guided percutaneous cryoablation for control of symptoms in FAVA lesions with significant improvement in pain.31
Syndromes with low-flow vascular malformationsSoft-tissue vascular anomalies associated with syndromes are usually low-flow. VMs or combined LM–VM are found in Blue rubber bleb nevus, Proteus and Maffuci syndromes. As stated earlier, capillary malformation may be the hallmark of Sturge–Weber and Klippel–Trenaunay syndrome.
Blue rubber bleb nevus syndromeBlue rubber bleb nevus, or Bean, syndrome is a rare disorder, first described by Bean in 1958 and characterized by multiple cutaneous (Fig. 8) and gastrointestinal VMs.13 Patients can present with gastrointestinal hemorrhage and bloody stools.13,16,26 Intermittent small bowel obstruction caused by intussusception or volvulus can also be seen.16,26
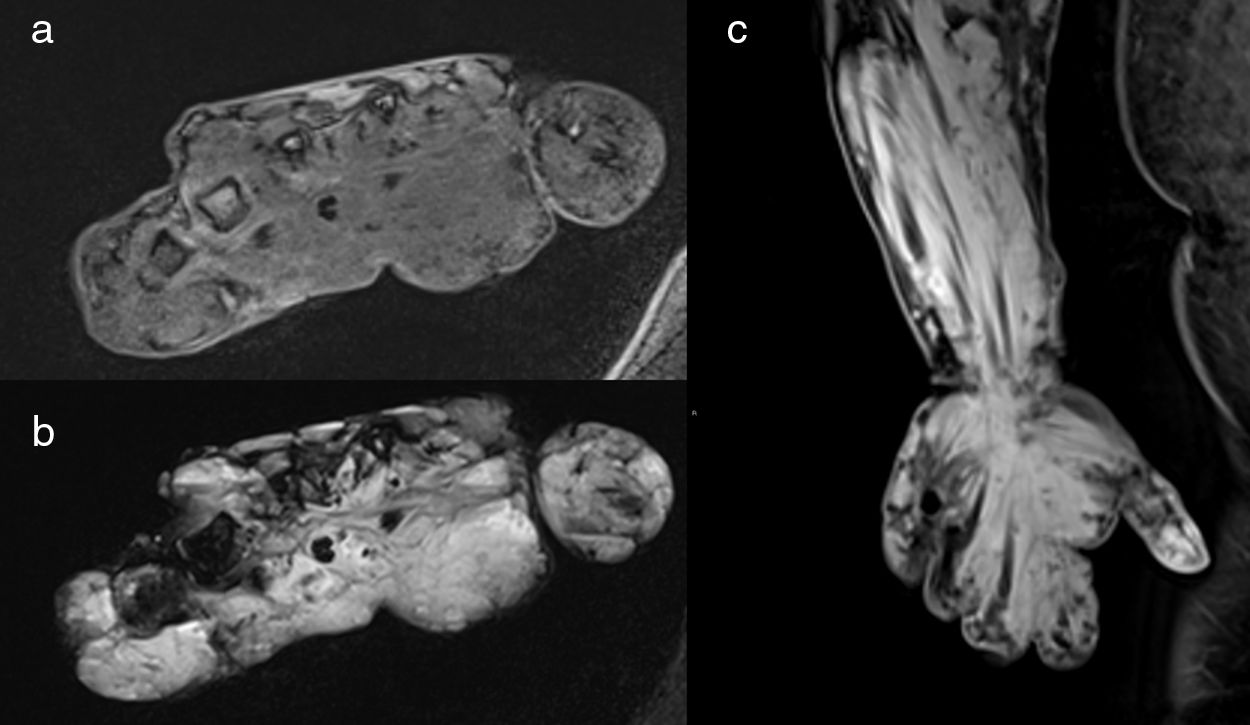
Blue rubber bleb nevus syndrome in a 32-year-old woman. MR images demonstrate an extensive subcutaneous and intramuscular venous malformation involving the left forearm and left hand; the lesion is hypointense on axial T1-weighted fat sat image (a), hyperintense on axial STIR image (b) and demonstrates diffuse delayed enhancement on coronal post contrast image (c).
Proteus syndrome is a rare sporadic condition with complex multisystemic involvement and wide clinical variability. It is characterized by asymmetric overgrowth of the bones, skin, and other tissues, cutaneous and visceral combined lymphatic–venous malformations,8,13,16 bilateral ovarian cystadenomas or a parotid monomorphic adenoma, lung cysts and facial abnormalities.13
Mafucci syndromeMaffuci syndrome is a rare sporadic disorder characterized by diffuse enchondromatosis involving the phalanges of the hands and feet associated with multiple venous2 or lymphatic malformations.16
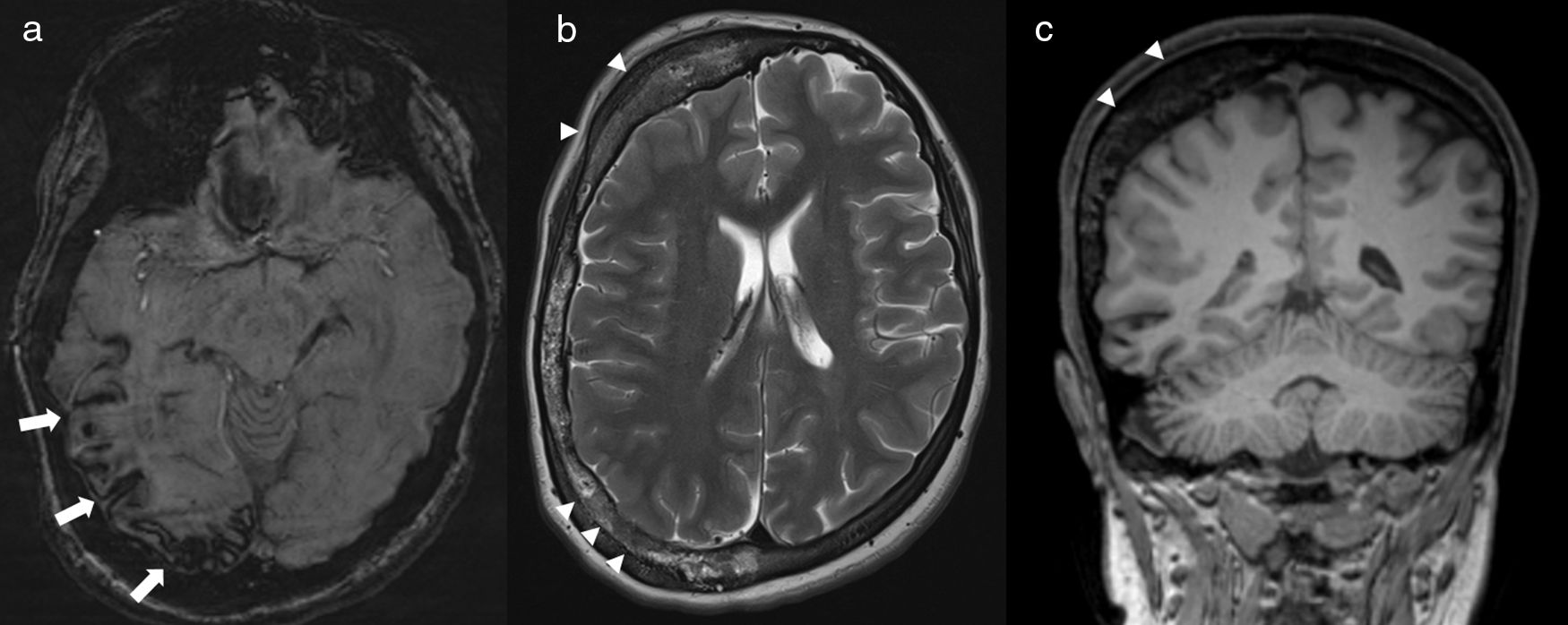
Sturge–Weber syndromeSturge–Weber syndrome, also called encephalotrigeminal angiomatosis, is a neurocutaneous disorder that combines a unilateral capillary malformation in the trigeminal nerve distribution (port wine stain) with a capillary-venous malformation in the pia and arachnoid mater and choroid of the eye, and atrophy and calcification in the subjacent cerebral cortex8,13,16,17 (Fig. 9).
17-Year-old female with Surge–Weber syndrome. Susceptibility weighted image (a) shows atrophy and cortical mineralization involving the sulcus of the right parietal-temporal occipital convexity (arrows), reflecting low vascular malformations in the pia mater. Marked right calvarial thickening is seen on axial T2-weighted (b) and coronal T1-weighted (c) images. Facial capillary malformation was present on clinical exam.
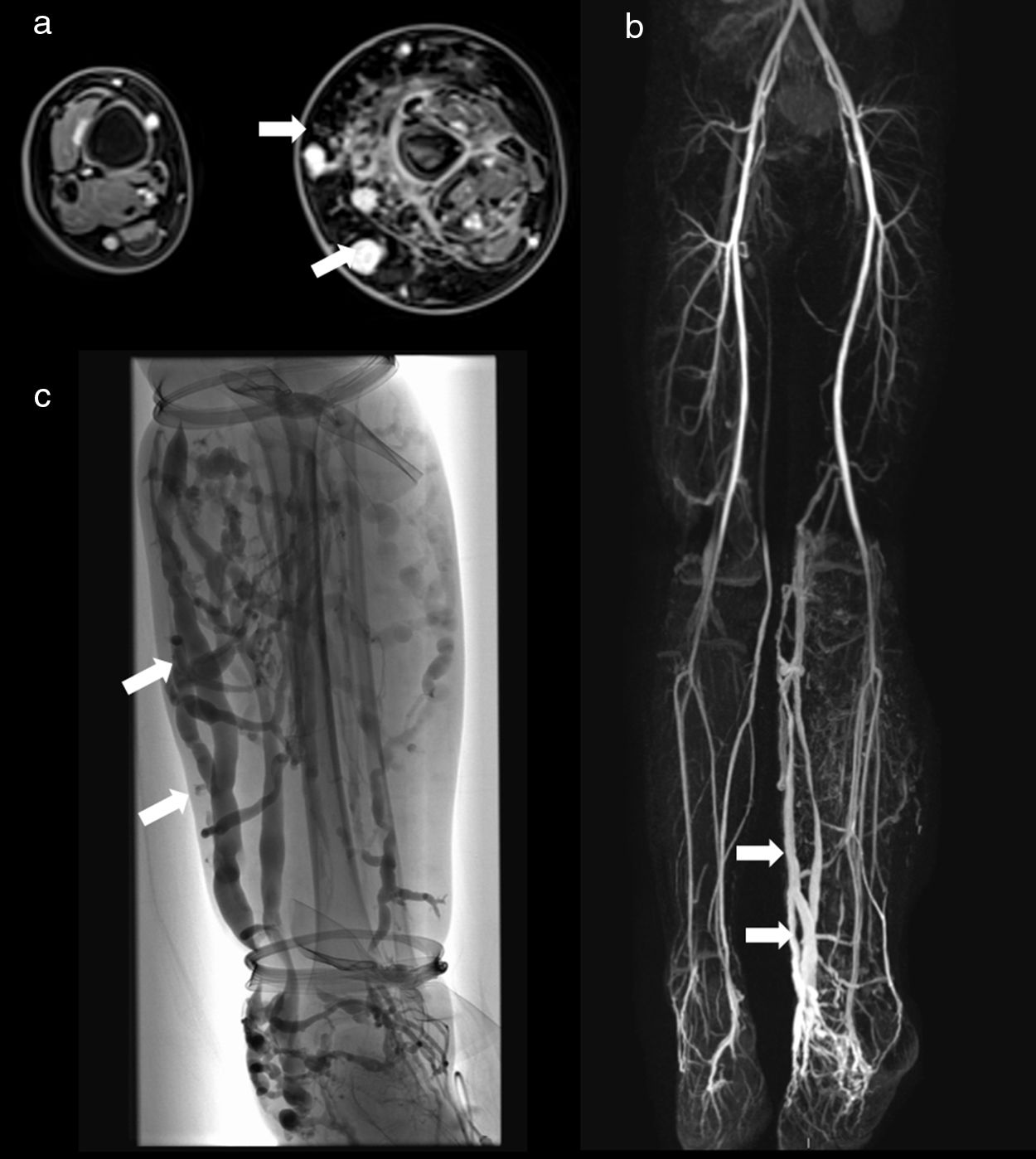
Klippel–Trenaunay syndrome is a condition of unknown etiology characterized by combined capillary, venous and lymphatic malformations of the extremities, usually the lower limbs, in association with bone and soft-tissue hypertrophy resulting from overgrowth2,8,13,16 (Fig. 10).
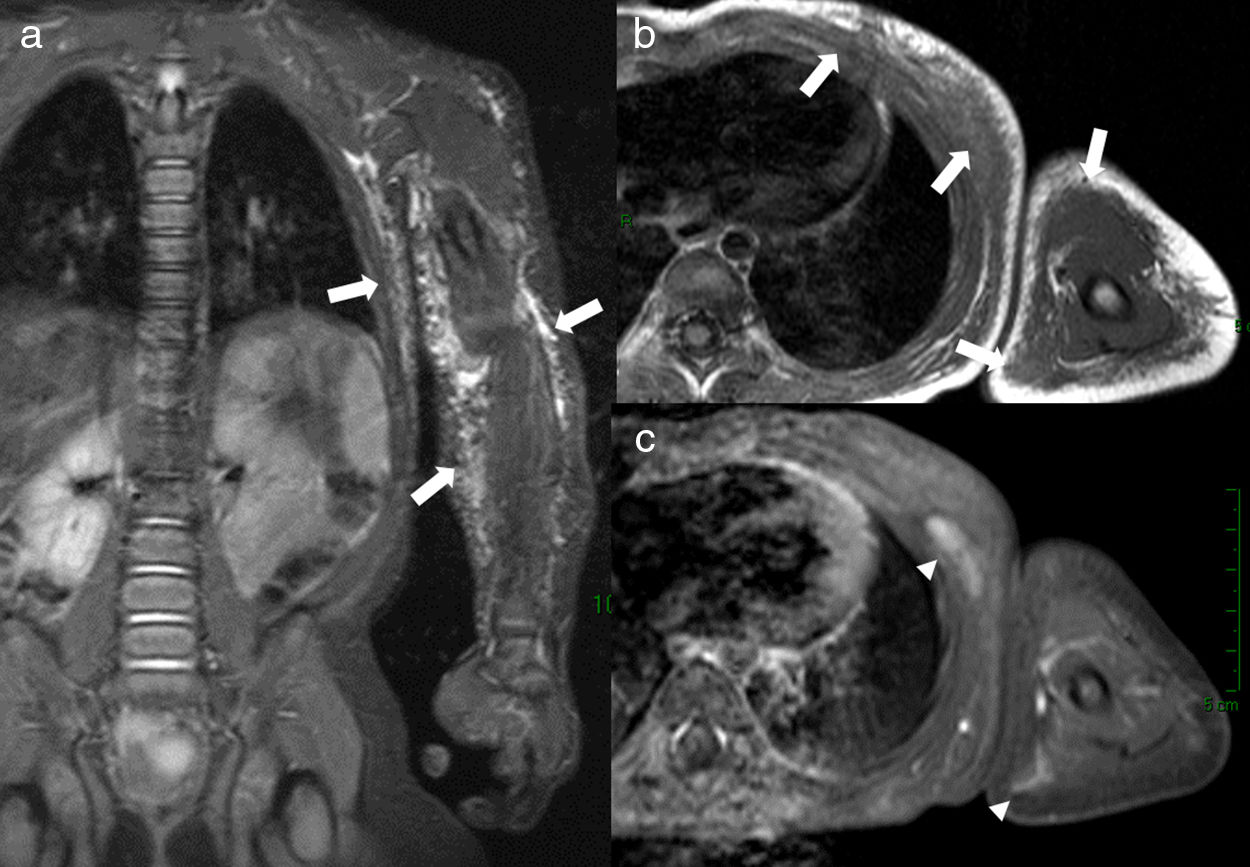
5-Year-old male with Klippel–Trenaunay syndrome and hemihypertrophy of the left lower extremity presented with extensive subcutaneous and intramuscular venous malformations of the left calf and distal thigh. Axial delayed post-contrast fat-suppressed 3D VIBE image (a) shows the enhancing venous malformations as well as the left-sided hemihypertrophy with significant fatty overgrowth. Varicose draining veins (arrows) are demonstrated on this image (a) as well as on the coronal venous phase MRA (MIP reconstruction) (b) and venogram (c).
Low-flow vascular malformations are rare but important entities. Recognizing whether a vascular malformation is low-flow or high-flow is the most important step in terms of patient management. Certain distinct imaging findings are also characteristic for certain histopathologic types. Low-flow vascular malformations can also be the hallmark of certain rare vascular syndromes. It is important for radiologists to be familiar with these lesions and their imaging findings and the associated syndromes.
Conflicts of interestThe authors declare that they have no conflicts of interest. Klaus D. Hagspiel and Patrick T. Norton receive financial support from Siemens Medical Solutions, Malvern, PA, USA.
Please cite this article as: Flors L, Hagspiel KD, Park AW, Norton PT, Leiva-Salinas C. Malformaciones vasculares y tumores de partes blandas. Parte 2: lesiones de bajo flujo. Radiología. 2019;61:124–133.