Vascular malformations and tumors, also known as “vascular anomalies”, comprise an extensive variety of lesions involving all parts of the body. Knowledge of their classification and imaging characteristics is paramount. Whereas hemangiomas are benign vascular tumors, characterized by cellular proliferation and hyperplasia; vascular malformations are not real tumors and characteristically exhibit normal endothelial turnover. Vascular malformations are classified according to the predominant vascular channel as arterial, capillary, venous, lymphatic, or mixed. Ultrasound and MRI are the main imaging modalities used in the diagnosis and classification of the vascular anomalies. In this series of two articles we review the classification of vascular anomalies, describe the role of imaging, summarize their distinctive histopathogenic, clinical and imaging features, and discuss the treatment options. On the first article we discuss the high-flow lesions, whereas the slow-flow lesions will be reviewed on the second. Complex syndromes with associated vascular tumors and malformations will be also presented.
Las malformaciones vasculares y los tumores, también conocidos como «anomalías vasculares», comprenden una extensa variedad de lesiones en diferentes partes del cuerpo. Conocer su clasificación y características en las imágenes es de vital importancia. Si bien los hemangiomas son tumores vasculares benignos caracterizados por proliferación celular e hiperplasia, las malformaciones vasculares no son verdaderos tumores y muestran, típicamente, una renovación endotelial normal. Las malformaciones vasculares se clasifican, según el vaso predominante, en arteriales, capilares, venosas, linfáticas o mixtas. La ecografía y la resonancia magnética son las técnicas de imagen que se usan en el diagnóstico y la clasificación de las anomalías vasculares. En esta serie de dos artículos revisaremos la clasificación de las anomalías vasculares, describiremos el papel que desempeñan las pruebas de imagen, resumiremos sus características histopatogénicas, clínicas y de imagen, y comentaremos las posibles opciones terapéuticas. En este primer artículo hablamos de las lesiones de alto flujo, y en el segundo lo haremos de las de bajo flujo. También trataremos los síndromes complejos asociados tanto a los tumores vasculares como a las malformaciones.
Vascular malformations and tumors, also known as “vascular anomalies”, comprise an extensive variety of lesions involving all parts of the body, and are the most common pediatric soft-tissue tumors.1 Knowledge of the classification and clinical and imaging characteristics of this group of lesions is paramount when managing these patients.
Due to a lack of a complete understanding of the origin and histopathology of such lesions, this field has been traditionally obscured by the use of an unclear nomenclature. For example, the term “cavernous hemangioma” has been used interchangeably to describe both the benign pediatric vascular tumor (infantile hemangioma) and the adult non-neoplastic low-flow vascular malformation (venous malformation). This may result in confusion, misdiagnosis and improper treatment; as the therapeutic options are strongly determined by an accurate classification.
Although their diagnosis is usually clinical, imaging plays an important role in clinical uncertain cases. Besides, imaging is crucial in the evaluation of their extent and relationship with adjacent tissues, as well as their response to treatment.
The objective of this series of two articles is to review the current classification of vascular anomalies, to describe the role of imaging in their diagnosis, to summarize their distinctive histopathogenic, clinical and imaging features, and to discuss the treatment options. On the first article we will discuss the high-flow lesions, whereas the slow-flow lesions will be reviewed on the second. Complex syndromes with associated vascular tumors and malformations will be also presented on each article.
Classification of vascular anomaliesOur understanding of vascular anomalies was extremely facilitated by the work of Mulliken and Glowacki2 who established the most widely accepted classification for vascular lesions based on cellular turnover, histology, natural history and physical findings. It basically divides vascular anomalies into two categories: hemangiomas and vascular malformations.
Hemangiomas are benign vascular tumors, characterized by the presence of cellular proliferation and hyperplasia, which initially exhibit a rapid proliferative phase, followed by an involuting phase. Vascular malformations are not real tumors; they are believed to be the result of an inborn error of vascular morphogenesis that occurs between the 4th and 10th weeks of intrauterine life.3 Vascular malformations characteristically exhibit normal endothelial turnover, and grow proportionally with the child growth without regression. Vascular malformations are subclassified based on the predominant vascular channel as arterial, capillary, venous, lymphatic, or mixed.
A radiological classification of vascular malformation was proposed later. It aimed to help in treatment planning and classified these lesions according to their flow dynamics into high-flow and low-flow malformations.4 Malformations with an arterial component are considered high-flow, whereas if the arterial component is absent they are considered low-flow.
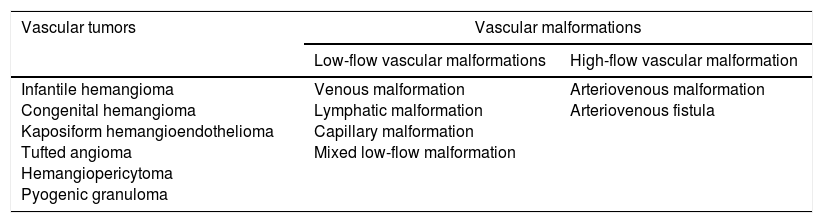
Combining the radiological categorization based on flow dynamics and the original description of Mulliken and Glowacki, the International Society for the Study of Vascular Anomalies (ISSVA)5 proposed two broad categories of vascular anomalies: vascular tumors (infantile hemangioma as their hallmark) and vascular malformations (Table 1).
Classification of vascular anomalies based on the International Society for the Study of Vascular Anomalies.
| Vascular tumors | Vascular malformations | |
|---|---|---|
| Low-flow vascular malformations | High-flow vascular malformation | |
| Infantile hemangioma Congenital hemangioma Kaposiform hemangioendothelioma Tufted angioma Hemangiopericytoma Pyogenic granuloma | Venous malformation Lymphatic malformation Capillary malformation Mixed low-flow malformation | Arteriovenous malformation Arteriovenous fistula |
The clinical presentation and physical exam of vascular anomalies are frequently sufficient to establish the correct diagnosis. Doppler ultrasound (US) and MRI are useful in clinically uncertain cases.
Due to its wide availability, lack of ionizing radiation and relatively low cost, US is usually performed first.6 Traditionally it has been considered the imaging modality of choice,7 but it has limitations such as the limited field of view, restricted penetration and operator dependency.8 Different-frequency transducers should be used depending on the depth of the lesion. Both gray-scale and Doppler are useful in differentiating the characteristics of the lesions.
MRI is nowadays the most important tool in the evaluation of vascular anomalies. It permits an accurate diagnosis and classification, and excellent definition of the extension of the lesion and involvement of adjacent structures.9 The MRI protocol should include T1-weighted imaging for basic anatomic assessment, T2-weighted imaging with fat suppression or STIR (short tau inversion recovery) for extent evaluation, and contrast-enhanced (CE) MR angiography (MRA) to evaluate the vascular characteristics of the lesion. In addition, Gradient-echo (GRE) T2*-weighted imaging permits to evaluate the presence of hemosiderin and calcification (characteristically showing low signal) and high-flow vessels (which show high signal intensity). CE MRA should be acquired in an arterial and several venous phases. In addition to the traditional CE MRA, time-resolved CE MRA is strongly recommended if available. Traditional CE MRA acquires one high spatial-resolution 3D dataset every approximately 15–20s, whereas dynamic time-resolve MRA obtains one 3D dataset every 1–2s proving a set of rapidly continuous images, similar to conventional digital subtraction angiography (DSA). Time-resolved CE MRA allows excellent depiction of the hemodynamics of the vascular malformation, detection of arterial component, and arterial and venous differentiation, frequently impossible with traditional CE MRA and important for treatment planning. Detailed description of the MRI protocol developed at our institution has been published before.9
High flow lesionsAs previously explained, vascular anomalies with an arterial component are classified as high-flow, whereas those without arterial component are low-flow. Vascular tumors (except for infantile hemangioma in the involuting phase), arteriovenous malformations and arteriovenous fistulae are considered high-flow vascular anomalies.
Vascular tumorsVascular tumors originate from angioblasts that give rise to blood vessels.10 They can be subcategorized based on the presence or absence of the endothelial cell glucose transporter (GLUT1) isoform protein, an erythrocyte-type glucose transporter. The placenta and infantile hemangiomas are the only human tissues that express this protein.10 The rest of vascular tumors do not. In fact, it has been hypothesized that hemangiomas may originate from embolized placental implants.10
There is a wide spectrum of vascular tumors. We review the most common types below.
Infantile hemangiomaThe infantile hemangioma is a mesenchymal tumor. By histopathology, it shows multiple arterial vascular channels lined by a single layer of plump endothelial cells with abundant mast cells supported by fibrous tissue.11 Vascular endothelial growth factor (VEGF) and other cell surface proteins that reflect the rapid cellular turnover of the tumor are found in the tissue.10 As mentioned earlier, infantile hemangiomas are characteristically GLUT 1 positive,10,11 permitting the distinction with the rest of vascular anomalies, and, therefore, allowing a definite diagnosis and proper therapy.
Clinical presentationIt is the most common tumor of infancy and childhood1,10,11 occurring in approximately 3–10% of infants.12,13 They are not clinically evident at birth and appear during the first weeks of life, usually becoming fully visible by 3 months.1 Infantile hemangiomas are more common in white Caucasian infants,12,14 specially girls (4:1), and premature infants with low weight.8,11,13,15 Preeclampsia, placenta previa and multiple gestations are potential maternal risk factors.12 They are found in the head and neck in 60% of cases, while 25% are found in the trunk, and 15% in the extremities.16–18
They follow a biphasic pattern of evolution: proliferation and involution. During the proliferative phase, they manifest as a slightly raised, bright red, strawberry-like, subcutaneous mass with bruit, pulsatility and warmth8 which are characteristics of a high-flow lesion. When the lesion is located in the deep dermis, subcutis, or muscle, it may have a bluish hue and be confused for a venous malformation.11 Depending on their size and location, they can present as highly disfiguring masses or be life-threatening when causing compression of vital structures such as the airways. Although very rare, high output heart failure can be occurred.
Usually after 1 year, lesions tend to regress (involuting phase) and become grayish dark red.5,13 Progressive perivascular deposition of fibrofatty tissue and thinning of the endothelial lining8,16 are seen at histology. Complete involution is achieved in 50% of cases by the age of 5 years, 70% by the age of 7 years and virtually all by the age of 8–12.17
When a patient presents with five or more cutaneous hemangiomas, abdominal ultrasound should we performed to rule out hemangiomas involving solid organs. If a patient presents with an orbital hemangioma, MRI should be performed to fully assessed the extent of the lesion and evaluate a potential occult intraorbital hemangioma.
Imaging featuresThe appearance on gray-scale US varies, but a solid soft tissue mass is identified with characteristic high vascularity on color Doppler. Arterial and venous waveforms can be seen on spectral Doppler ultrasound.18 The arterial flow is typically of low resistance with relatively high velocities.19 During the involuting phase, they show decreased vascularity19 and increased vascular resistance.
MRI findings depend on the phase of the lesion. During the proliferative phase, they present as well-defined masses, hypointense on T1 and hyperintense on T2 weighted images (Fig. 1), often with presence of internal flow voids on spin-echo (SE) imaging reflecting high-flow vessels9 (Fig. 1). High-flow vessels appear hyperintense on GRE, as mentioned earlier, permitting the distinction with phlebolitis or other calcifications, which are hypointense on all imaging sequences.9 Early homogenous contrast enhancement is characteristic during the proliferative phase1 (Fig. 1), and large feeding arteries are usually depicted with time-resolved MRA.11 During the involuting phase, they appear as heterogeneous masses with progressive deposition of internal fat (hyperintense foci on T1), decreased flow voids when compared to the proliferative phase,12 and more heterogeneous contrast enhancement.1 Finally, after complete involution, a residual scar is seen which appears hypointense on both T1 and T2-weighted imaging.12 Noticeably, perilesional edema should never be seen7,20; if present, other soft tissue tumors (e.g. metastases from neuroblastoma or rhabdomyosarcoma among many others) should be suspected and biopsy is needed.
1-Month-old infant with proliferating infantile hemangioma in the left supraclavicular region. Coronal T1-WMR image (a) shows a well-defined lobulated hypointense mass. The mass is hyperintense on STIR image (b). Signal voids within the lesion, reflecting fast flow vessels (arrows), are also seen on these images (a, b). No perilesional edema is identified. Arterial phase 3D MRA (c) image shows the characteristic early enhancement of the lesion without arterio-venous shunting.
Infantile hemangiomas tend to spontaneously involute and no treatment is necessary in most of the cases. When symptomatic, or when the lesion is located in an anatomical region where it may lead to functional or significant esthetic impairment, medical treatment is preferred. Propanolol is the first line therapy; it reduces the expression of VEGF and other proangiogenic factors while also inducing apoptosis of vascular endothelial cells17; excellent results have been reported.7,21 When propranolol is contraindicated, oral prednisolone can be attempted, with complete involution rate of 30% and stop progression in 40% of cases.17 Embolization and surgery are reserved for unresponsive cases.7 Embolization can be performed for tumoral growth control, preoperatively to reduce or minimize bleeding, and for consumption coagulopathy.
Congenital hemangiomaCongenital hemangiomas are much less common than infantile hemangiomas. They are subcategorized into: rapidly involuting congenital hemangiomas (RICHs) and non-involuting congenital hemangiomas (NICHs). The GLUT-1 marker is negative.
Clinical presentationUnlike infantile hemangiomas, congenital hemangiomas are fully present at birth,1,8 and can be seen in utero.12 They typically present as solitary lesions, most commonly in the head and neck or the extremities,17 without gender predilection.12
RICHs present as raised gray-blue lesions with peripheral pale halo and central depression, ulceration or scar and typically involute much faster than the infantile hemangiomas, usually within during the first 12–24 months of life.1,7,12 On the contrary, NICHs continue to grow in proportion with the child without regression, and usually present as pink-to-purple raised lesions with prominent telangiectasia and blue pallor either peripherally or centrally.17
Imaging featuresDue to important overlap, imaging features alone do not allow differentiating congenital from infantile hemangioma, and clinical history is paramount (Figs. 2 and 3). Large and irregular feeding arteries in disorganized patterns, arterial aneurysms, direct arteriovenous shunting and thrombosis are more frequently seen on congenital hemagiomas.7,22 Unlike NICHs, RICHs may present a central, nonenhancing, hypoechoic, and T2 hyperintense component which represents necrosis.11
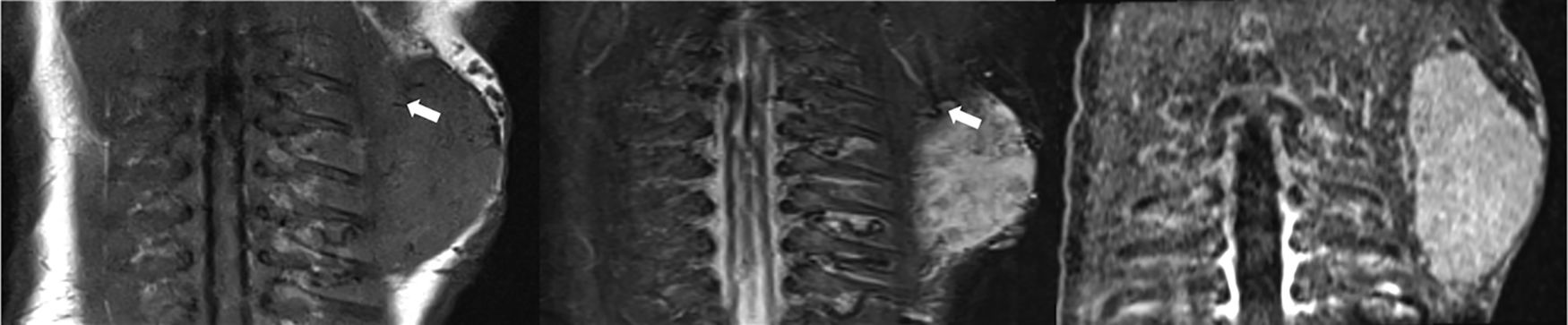
1-Day-old infant with congenital hemangioma in the left scapular area. A well defined soft tissue mass is seen on MR images which is hypointense on Coronal T1-WMR image (a) and hyperintense on STIR image (b). Signal voids within the lesion, reflecting fast flow vessels (arrows), are also seen on these images (a, b). No perilesional edema is identified. Arterial phase contrast enhanced MRA (c) image shows the characteristic early enhancement of the lesion.
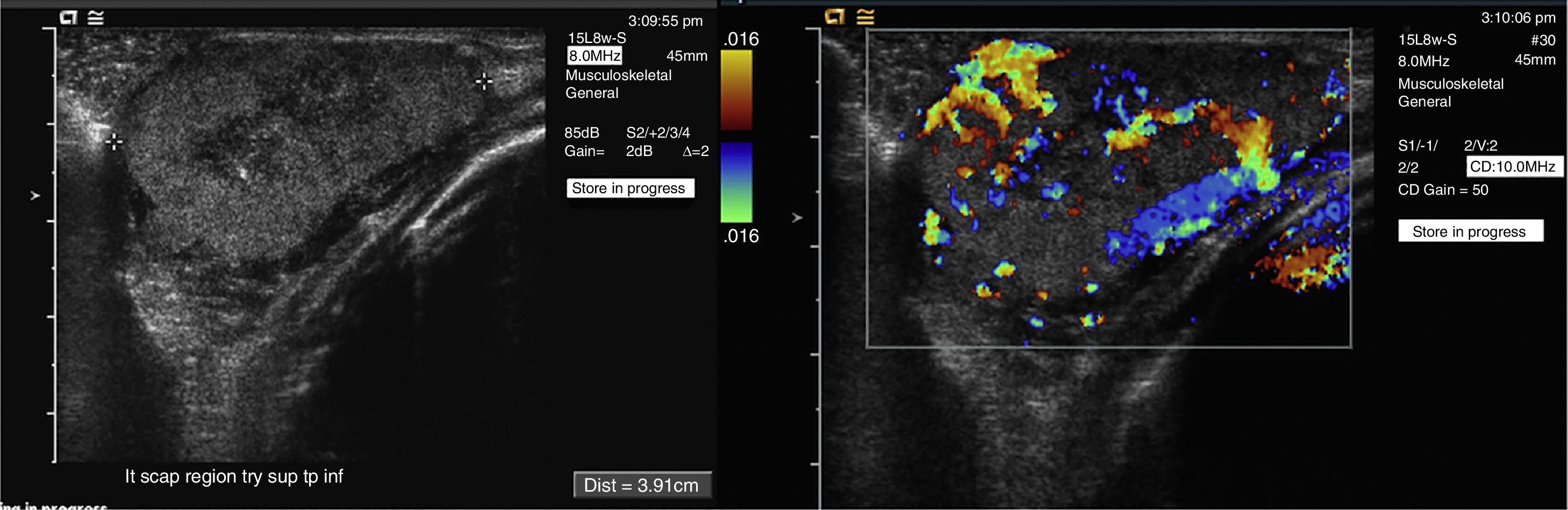
1-Day-old infant with congenital hemangioma in the left scapular area (same patient Fig. 2). Gray scale ultrasound image (a) shows a well-defined, solid soft-tissue mass. High vascularity is demonstrated with color Doppler (b).
NICH require treatment and surgical resection is the treatment of choice because embolization is usually not effective.7
Kaposiform hemangioendotheliomaKaposiform hemangioendothelioma is a rare locally aggressive GLUT-1 negative tumor, usually present at birth. Histologically, Kaposiform hemangioendothelioma consists of irregular and nodular areas of compressed vessels, resembling capillary vascular malformation and Kaposi sarcoma. As opposed to Kaposi sarcoma, there is a lack of periodic-acid-Schiff-positive globules and comparing to capillary hemangioma, there is no evidence of spindle cell fascicles.23
Clinical presentationPatients may present Kasabach–Merritt syndrome, a potentially life threatening thrombocytopenia, anemia and coagulopathy. The tumor has a very low malignant potential but, regional nodal metastases can be seen rarely.1
They typically occur in the first decade of life, and most commonly involve the peritoneal or retroperitoneal space, upper and lower extremities, and head and neck region. As seen in infantile hemangiomas, this tumor can present rapid initial proliferation followed by regression. Continuous persistence or recurrence also has been described.11 The irregular borders of the lesion, and the signs of skin infiltration14 reflect the aggressiveness of the tumor.
Imaging featuresKaposiform hemangioendothelioma appearance varies depending on the tumor size. Large lesions tend to show an aggressive destructive tendency with internal flow voids and hemorrhagic foci on MRI and intratumoral vessels with arterial spectrum on Doppler.
Small lesions are non-specific, but may grow abruptly and show aggressive features.
Distinctive features of aggressiveness on MR include: ill-defined margins commonly due to perilesional edema, involvement of multiple tissue planes with frequent skin thickening and subcutaneous fat stranding, early contrast enhancement, smaller feeding arteries and draining veins, hemosiderin deposits, and destructive changes.9,11
TreatmentA number of therapeutic options have been published. The treatment of choice for localized cutaneous disease has been complete surgical excision, occasionally combined with radiation. Early diagnosis is very important since it may enable total resection. In many cases, surgical excision is technically inappropriate because of the local tissue invasion and the associated KMP For life-threatening cases with associated Kasabach–Merritt syndrome, multi-modality approach including surgical resection, steroids, interferon, vincristine or radiation therapy, has been attempted,24 but resulted in variable effectiveness.
High-flow vascular malformationsHigh-flow vascular formations refer to lesions with an arterial component including arteriovenous malformations (AVMs) and arteriovenous fistulae (AVFs). Both types of lesions are characterized by abnormal connection between the arteries and the veins with flow diverting the capillary bed.
Arteriovenous malformationWhile AVFs consist of a single direct vascular connection between an artery and a vein, AVMs are formed by a various combination of single or multiple feeding arteries and draining veins separated by a nidus of dysplastic vascular channels. The arteries are usually larger, tortuous, with frequent destruction of their internal elastic lamina. As a result of shunting of arterial pressure blood into the low pressure venous system, the veins show muscular hypertrophy of the media, fibrosis of the intima, and lack of adventitia.12
Congenital AVMs occur most often sporadically and, less common, as part of a broader syndrome such as Parkes Weber syndrome, phosphatase and tensin homologhamartoma syndrome, Osler–Weber–Rendu syndrome, and Congenital Lipomatous Overgrowth, Vascular malformations, Epidermal nevi, and Skeletal deformities (CLOVES) syndrome.25
Clinical presentationAlthough AVMs are already present at birth,13 their diagnosis may be challenging and delayed until they become fully evident clinically in late childhood or adulthood, or are large enough to cause hemodynamic disturbance. Like other vascular malformations, they generally increase in size as the child grows. Growth can be triggered by physical factors such as thrombosis, infection or trauma3 or hormonal changes as occur during puberty or pregnancy.26
On physical examination, these anomalies usually present as red pulsatile warm masses with palpable thrill and possible audible bruits, reflecting their high blood flow. Skeletal overgrowth, limb hypertrophy,14 arterial steal phenomenon, and cutaneous ischemia with ulceration and hemorrhage may be seen in severe cases.13 A high-output cardiac state with subsequent cardiopulmonary overload is a rare occurrence with extremity AVMs secondary to the left-to-right shunting caused by the lesion, but can potentially develop in extensive, widespread cases.25 The Schobinger clinical staging system, introduced at the 1990 meeting of the International Workshop for the Study of Vascular Anomalies in Amsterdam, describes the clinical course of AVMs, especially when left untreated (Table 2).
Schobinger staging system of arteriovenous malformations. Based on: Eighth Meeting of the International Workshop on Vascular Malformations. Amsterdam, Netherlands, 1990. Adapted from Ref. [11].
| Stage | Description |
|---|---|
| I: Quiescence | Pink-blush stain with warmth and shunting on Doppler ultrasound |
| II: Expansion | Same as Stage I plus: Lesion enlargement, pulsation, bruit, thrill |
| III: Destruction | Same as Stage II plus: Ulceration, bleeding, persistent pain or tissue necrosis |
| IV: Decompensation | Same as Stage III plus: High-output cardiac failure |
Ultrasound is frequently used as an initial screening modality revealing an ill-defined area of heterogeneous echogenicity without a discrete mass and a hypervascular network of dilated, tortuous channels including multiple arterial feeders and venous drainers, which may become aneurysmal as a result of long-standing arterialization of the venous system.25 US can be used to grossly estimate the size of the lesion and determine its complexity by assessing the number of inflow and outflow vessels and its association with adjacent structures.19 Spectral Doppler ultrasound reveals high-flow, low-resistance vascular bed25 and arterialized venous waveform.
MRI is usually required for definitive diagnosis and extent evaluation. As opposed to hemangiomas, AVMs usually present as ill defined non-mass like lesions infiltrating multiple tissue planes. High-flow large feeding arteries and draining veins are seen as tangles of serpiginous signal voids on SE images (Figs. 4 and 5), high-signal intensity foci on GRE Images 9,27 and intense contrast enhancement (Figs. 4 and 5). Areas of T1 hypointensity within the bone marrow reflect intraosseous extension of the lesion.13 Additional intralesional areas of T1-shortening may represent hemorrhage or thrombosis.20 Contrast-enhanced MRA in the arterial and venous phases with multi-planar reconstruction are useful for detailed anatomic display of the feeding arteries and draining veins (Fig. 6). Time-resolved dynamic 3D MRA permits excellent assessment of the hemodynamics of the components of the AVMs, including the nidus. Early venous shunting is a characteristic 9,27 (Figs. 4–6). Accurate characterization of the lesion is further achieved with conventional arteriography which, in addition, can be used for assessment of the feasibility of endovascular treatment and subsequent therapy.
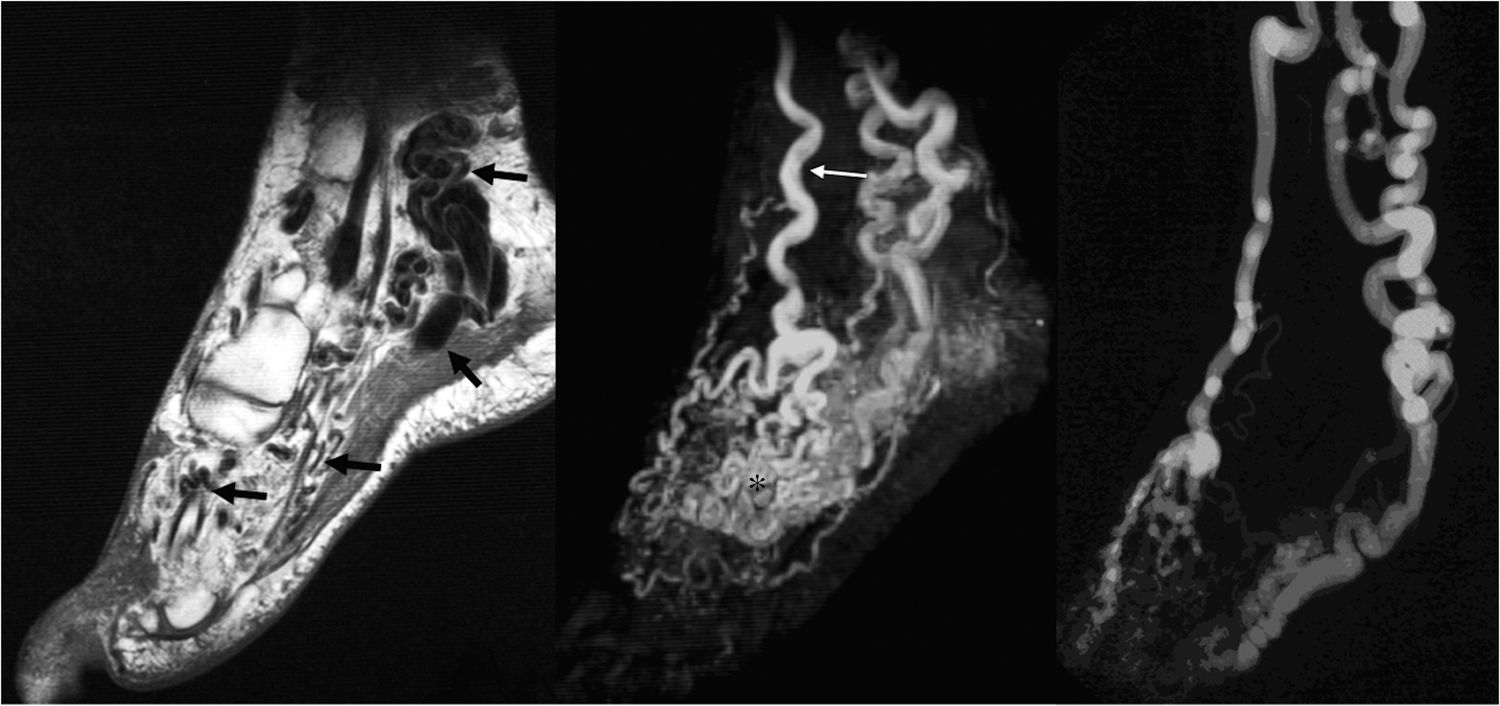
35-Year-old female with AVM within the left foot. Enlarged high-flow feeding arteries, draining veins, as well as the nidus of the AVM are seen as signal voids on FSE images (a) (arrows). Arterial phase MRA (b) depicts the arterial supply of the AVM, primarily by a tortuous and dilated dorsalis pedis artery (thin arrow), as well as early filling of the nidus (asterisk), and the draining veins (arrowheads). Excellent correlation with catheter angiography (c).
35-Year-old female with left facial arteriovenous malformation. Arteries, veins and nidus appear as signal voids (arrows) on fast spin echo coronal T1-weighted (a) and STIR (b) images. Arterial phase MRA (c) depicts arterial supply primarily by tortuous and dilated branches of the external carotid artery (arrow), nidus (asterisk) and venous drainage (arrowhead).
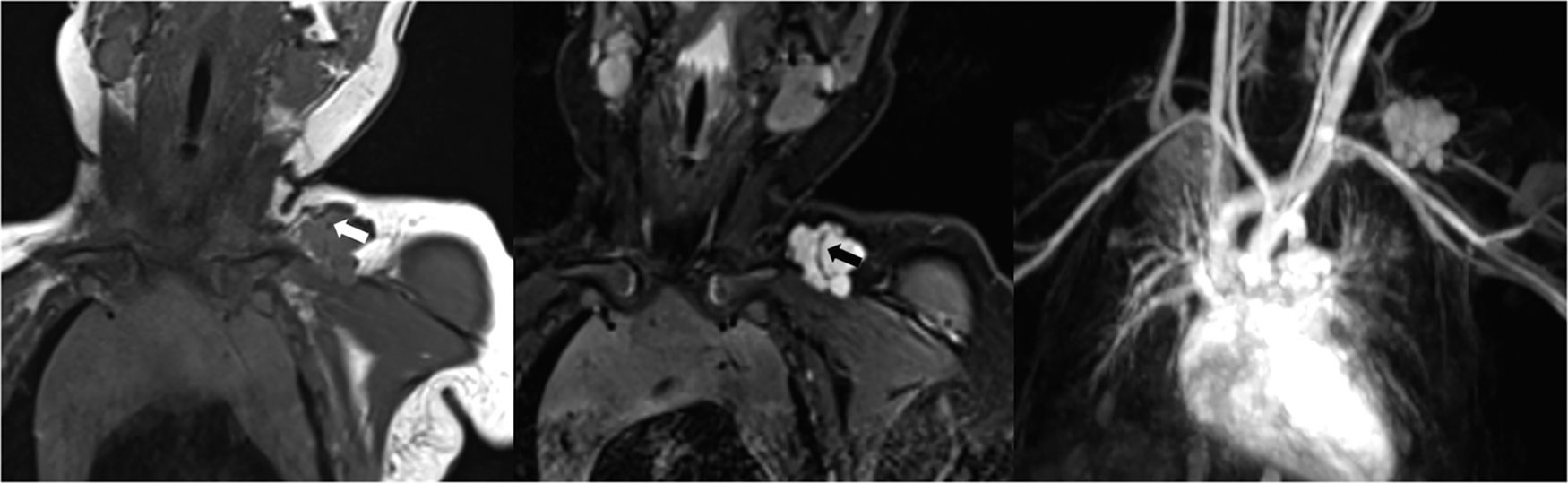
65-Year-old woman with AVM in the right side of the pelvis. Volume rendering reconstruction CE MRA shows that the AVM consists of branches from the hypertrophied right internal artery (arrow), a nidus and a large venous varix that drains into the right internal iliac vein (arrowhead).
As opposed to infantile hemangiomas that tend to regress without treatment, vascular malformations usually require appropriate treatment depending on size, location and severity of symptoms. Not infrequently, multiple sessions are needed due to residual or recurrent disease. Treatment will depend on the dynamic of the malformation and must be oriented to achieve a complete eradication of the nidus because any incomplete treatment may stimulate a more aggressive growth13 due to neovascularization and recruitment of collateral inflow to feed the nidus.25
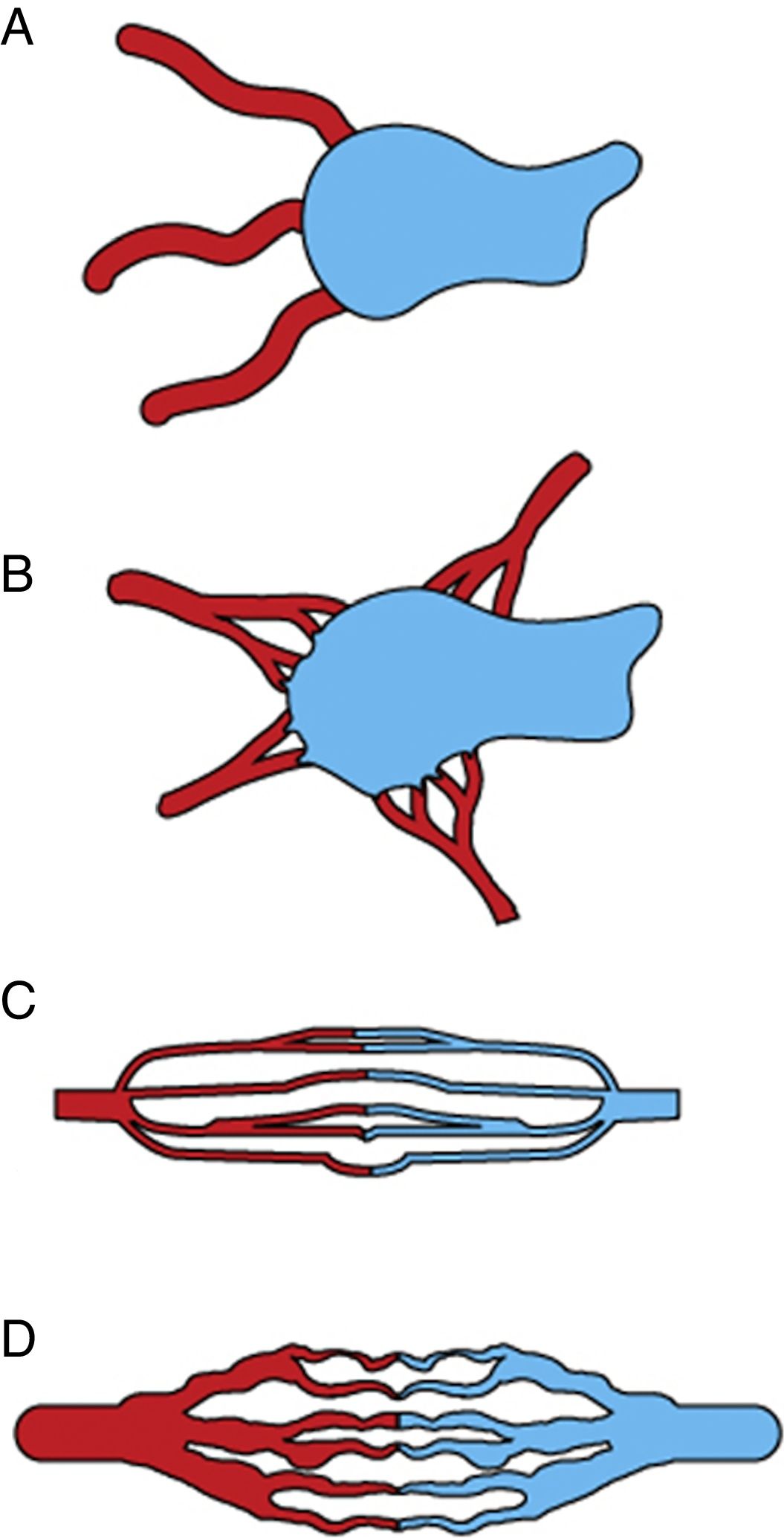
A baseline selective and superselective angiography is needed to precisely determine the flow characteristic of the AVM. We follow the angiographic classification proposed by Cho et al.28 for AVMs in the body and extremities. Based on the morphology of the nidus, AVMs are classified into 4 types (Fig. 7): type I, no more than 3 separate arteries shunt to a single draining vein; type II, multiple arterioles shunt to a single draining vein; type IIIa, multiple fine shunts between the arterioles and the venules which appear as fine striations or as a blush on angiography; type IIIb, multiple shunts between arterioles and venules which appear as a complex vascular network on angiography. Mixed types can also be found.
Schematic representation of the angiographic classification of arteriovenous malformations into 4 types adapted from Cho SK, et al. J Endovasc Ther. 2006;13:527–538. (A) Type I (arteriovenous fistulae) shows at most 3 separate arteries shunted to a single draining vein. (B) Type II (arteriolovenous fistulae) multiple arterioles shunting into a single draining vein. (C) Type IIIa (arteriolovenulous fistula with non-dilated fistula) multiple fine shunts between the arterioles and the venules; type IIIb (arteriolovenulous fistula with dilated fistula) multiple shunts between the arterioles and the venules forming a complex vascular network.
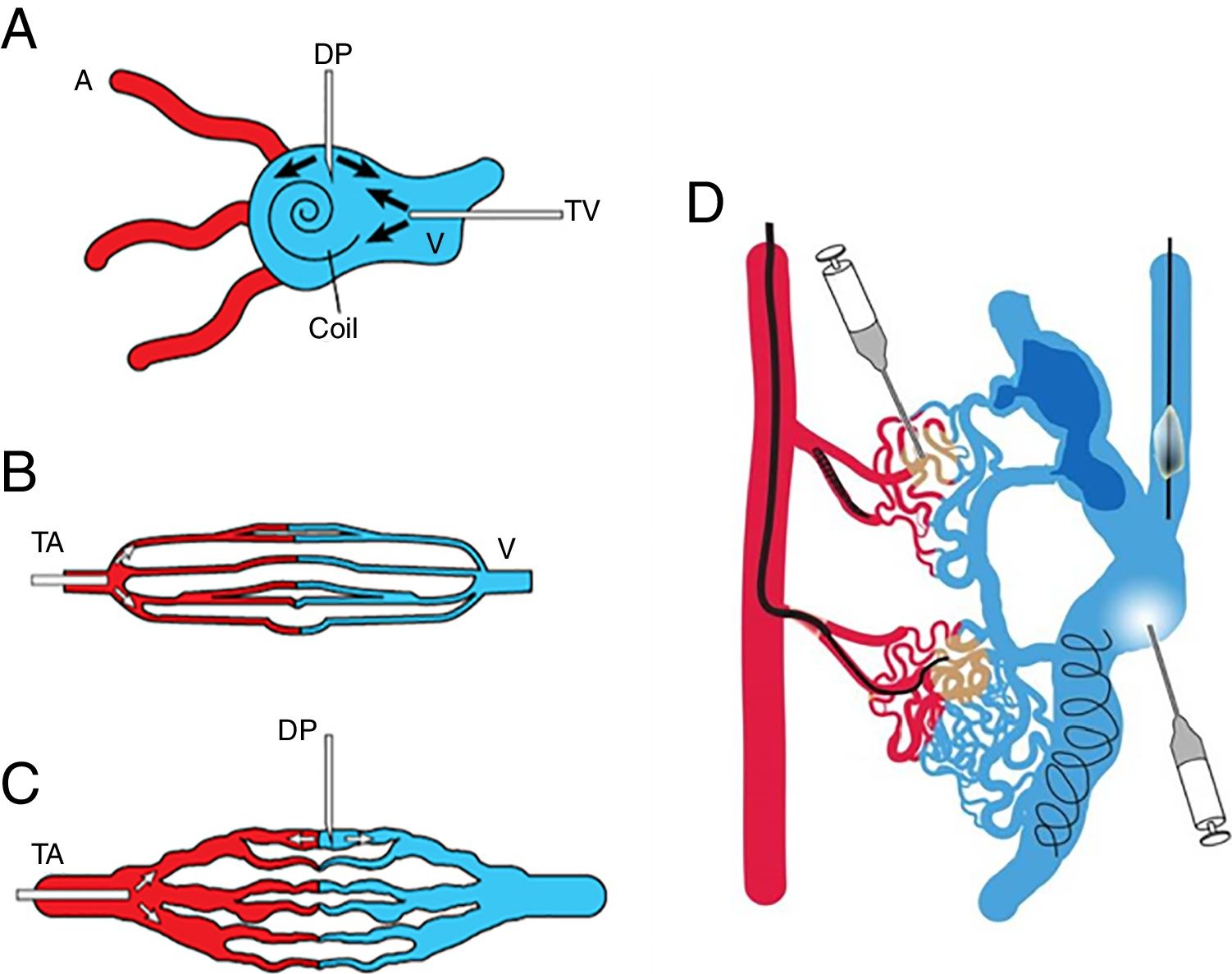
AVM nidus access can be performed via transarterial, transvenous, or direct puncture routes. Sometimes a combination of these approaches is necessary. Traditionally these lesions are managed with transarterial embolization, but direct puncture or transvenous approach may be needed especially in cases of: extreme arterial tortuosity, innumerable arterial feeders, important normal arterial branches arising in very close proximity to the malformation, or previous surgical ligation or embolization of the feeding artery. In general, we follow the treatment scheme proposed by Cho et al.28 which varies according to the angiographic type of malformation (Fig. 8). In type I and II AVMs it is important to attack the venous component of the nidus which can be access by direct puncture or transvenous access; coil embolization of the venous component of the nidus is often require prior to ethanol embolization in order to reduce the amount of ethanol and to stabilize the thrombosis within the large venous component. Type IIIa AVMs require transarterial approach because the nidus is too fine for direct puncture. Transarterial approach is preferred for Type IIIb AVMs, but this type is also amenable to direct puncture. Occlusive devices can also be used as adjunctive therapy to control the flow within aneurismal draining veins to minimize risk of nontarget embolization of liquid embolic agents during arterial approach or direct puncture.25 Transvenous embolization is contraindicated for type III AVMs because embolization material will not only do not successfully reach the shunt but will block the venous drainage with the potential risk for hemorrhage, rupture and aggressive growth. Mixed lesions usually require a combination of different approaches (Fig. 8d) although a single approach that allows treatment of all types simultaneously is preferred.28
Schematic representation of the access route for treatment according to the angiographic type of arteriovenous malformations. Adapted from Cho SK, et al. J Endovasc Ther. 2006;13:527–538. (A) Transvenous approach and direct puncture of the nidus for type I and II, (B) transarterial approach for type IIIa, (C) transarterial approach and direct puncture for type IIIb. (D) Mixed lesions may require a combined approach. A: artery; V: vein; TV: transvenous, DP: direct puncture; TA: transarterial.
As explained before, an AVF is a single abnormal connection between an artery and a vein without an intervening capillary. Pulmonary AVM and vein of Galen aneurysm malformation are some common misnomers regarding this anomaly since in reality these lesions are AVFs. Congenital AVFs, usually found in the head and neck, are different from the more common acquired AVFs which are mostly the consequence of an iatrogenic or traumatic penetrating injury.
Clinical presentationSimilar to AVMs, AVFs present as warm masses with thrill. High-out heart failure can also develop.
Imaging featuresDoppler ultrasound reveals low-resistance feeding arteries, draining veins with arterialized flow and turbulent flow at the point of communication.11 MRI shows the arterial and venous components as large signal voids on SE images or high-signal intensity foci on GRE images. Similarly to AVMs and as opposed to hemangiomas, AVFs present without a well-defined mass.1
TreatmentDirect arteriovenous fistulas can be cured by use of proximal occluding devices such as plugs and coils. Surgical resection may be sometimes needed.
Syndromes with high-flow vascular anomaliesKasabach–Merritt syndromeKasabach–Merritt syndrome described in 1940 by Kasabach and Merritt,29 is a condition characterized by the combination of pediatric hemangiomas and thrombocytopenia, hemolytic anemia and coagulopathy. It is a life threatening condition, with death occurring in 12–24% of patients, seen characteristically in patients with kaposiform hemangioendotheliomas or tufted angiomas.24 It is an entrapment coagulopathy that occurs when blood coagulation factors and platelets are trapped between vascular tumor cells.24
It should be noted that Kasabach–Merritt phenomena is a different entity that may occur in patients with venous or other types of vascular malformations, characterized by a being a consumptive coagulopathy that occurs when blood coagulation factors are consumed after hemorrhage.24
Patients with Kasabach–Merritt syndrome may require surgical resection, corticosteroid, interferon, chemotherapy, and radiation therapy, whereas replacement therapy with blood coagulation factors will be sufficient in case of Kasabach–Merritt phenomena.24
PHACE syndromePHACE syndrome is characterized by posterior fossa malformations, hemangiomas of the face and neck, arterial anomalies, cardiac defects and/or coarctation of the aorta, eye or endocrine anomalies.24 Posterior fossa anomalies include Dandy–Walker malformation and ventricular dilatation. A large segmental hemangioma, involving the face in 98% of cases is characteristic.30
Cardiac and aortic anomalies include aortic aneurysm, aortic dissection, atrial septal defect, and ventricular septal defect. Eye and endocrine anomalies include cataract, glaucoma, microphthalmos, and optic nerve hypoplasia.24 When ventral developmental defects (such as sterna clefting or supraumbilical raphe) are also present, the syndrome is referred to as PHACES.30
Patients with large infantile hemangiomas larger than 5cm in diameter in the face or head and neck, brain MR imaging or MRA is recommended to evaluate for PHACE syndrome.30
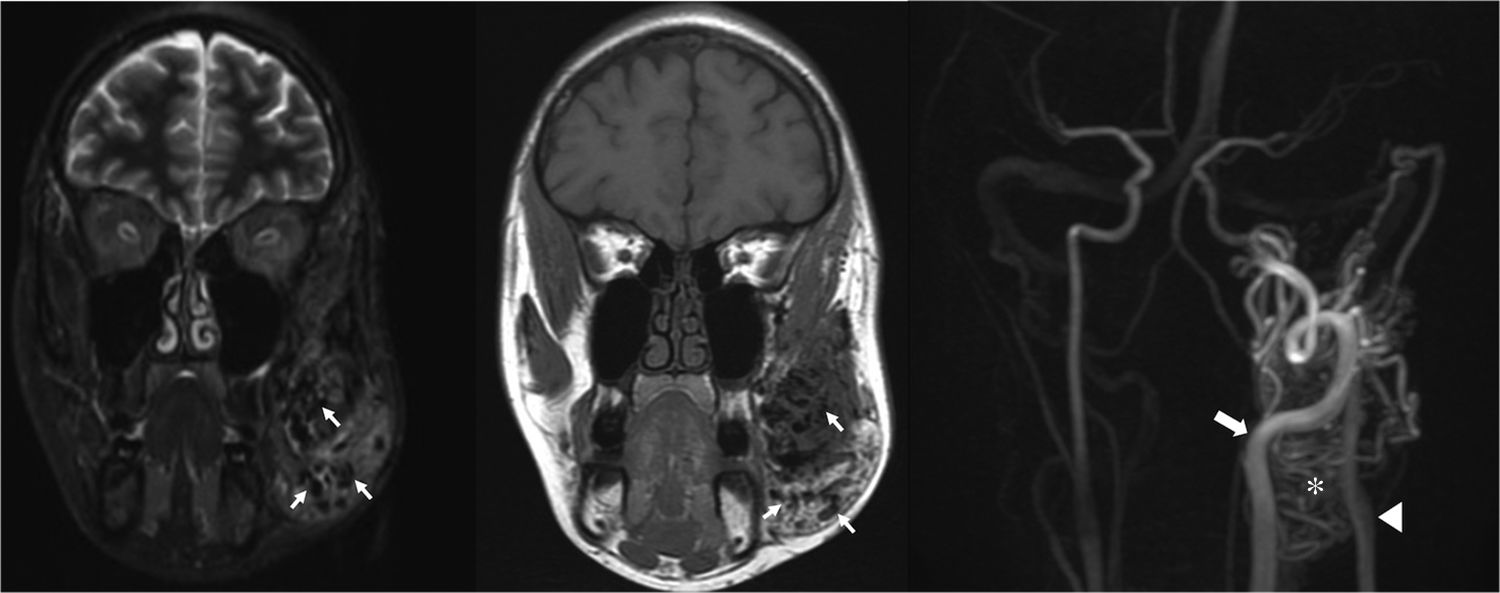
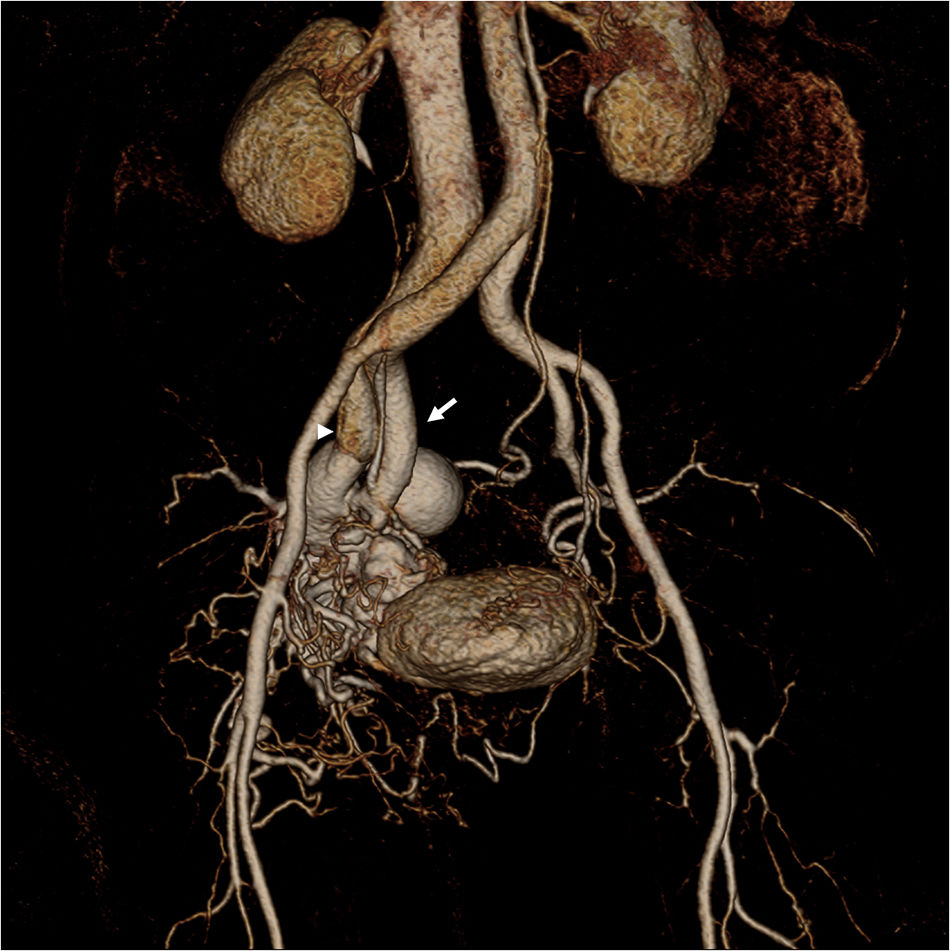
Parkes Weber syndromeParkes Weber syndrome, described in 1907 by the British dermatologist Parkes Weber,31 involves a cutaneous capillary malformation with limb hypertrophy in combination of AVMs-AVFs, and congenital varicose veins (Figs. 9 and 10).7 The existence of high-flow lesions is the hall mark of this syndrome which is easily confused with Klippel–Trénaunay syndrome, also characterized by limb hypertrophy.
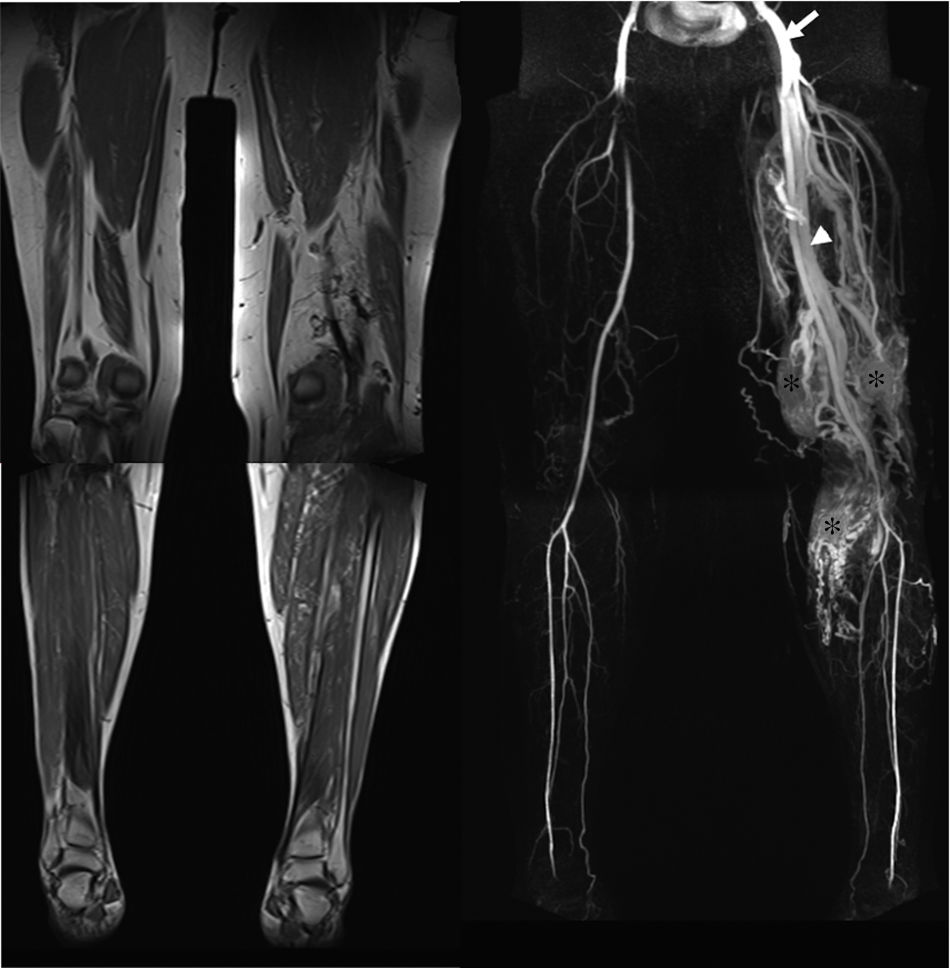
52-Year-old female with Parkes Weber syndrome. Cutaneous capillary malformations with marked left lower extremity hypertrophy were noticeable on clinical exam. T1-weighted MR image (a) reveals marked limb hypertrophy. Arterial phase Contrast-enhanced MRA (b) shows an enlarged femoral artery (arrow), numerous AVMs throughout the extremity (asterisk) and early venous shunting (arrowhead).
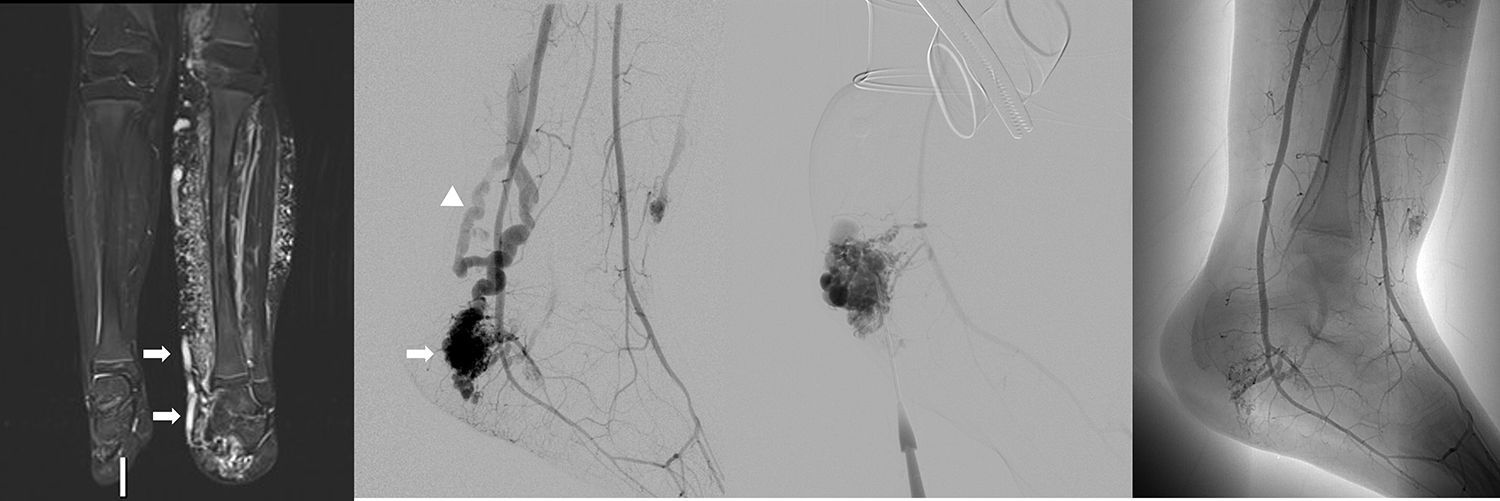
9-Year-old male with Parkes Weber syndrome. Coronal STIR MR image (a) reveals marked left limb hypertrophy, diffuse subcutaneous hyperintensity and dilated venous shunting (arrows) secondary to multiple AVMs. Left lower extremity arteriogram (b) reveals a large AVM overlying the medial malleolus of the left ankle (arrow) with dilated early venous shunting (arrowhead). Direct puncture of the venous outflow of the AVM in the left ankle (c) with alcohol sclerosis and decreased overall flow (d).
This malformation is evident at birth with enlargement and confluent erythematous staining of the involved limb. The lower limb is more frequently involved than the upper limb.32 The enlarged affected limb is warm, with possible bruit and/or thrill,11 confirming the diagnosis.32 Overgrowth in an affected extremity is subcutaneous, muscular, and bony.32 High-output cardiac failure, secondary to the AVMs, may occur.11,24 Diffuse AVMs or AVFs as well as fatty and bony overgrowth are evident on imaging (Fig. 10).11 Treatment is targeted to the underlying lesions (Fig. 10), which is usually challenging because of the presence of diffuse microfistulae.11
Rendu–Osler–WeberRendu–Osler–Weber syndrome, also known as hereditary hemorrhagic telangiectasia (HHT), is a multiorgan autosomal dominant disorder characterized by recurrent epistaxis, multiple mucocutaneous telangiectasis, and visceral arteriovenous malformations or fistulae24,33 (Fig. 11).
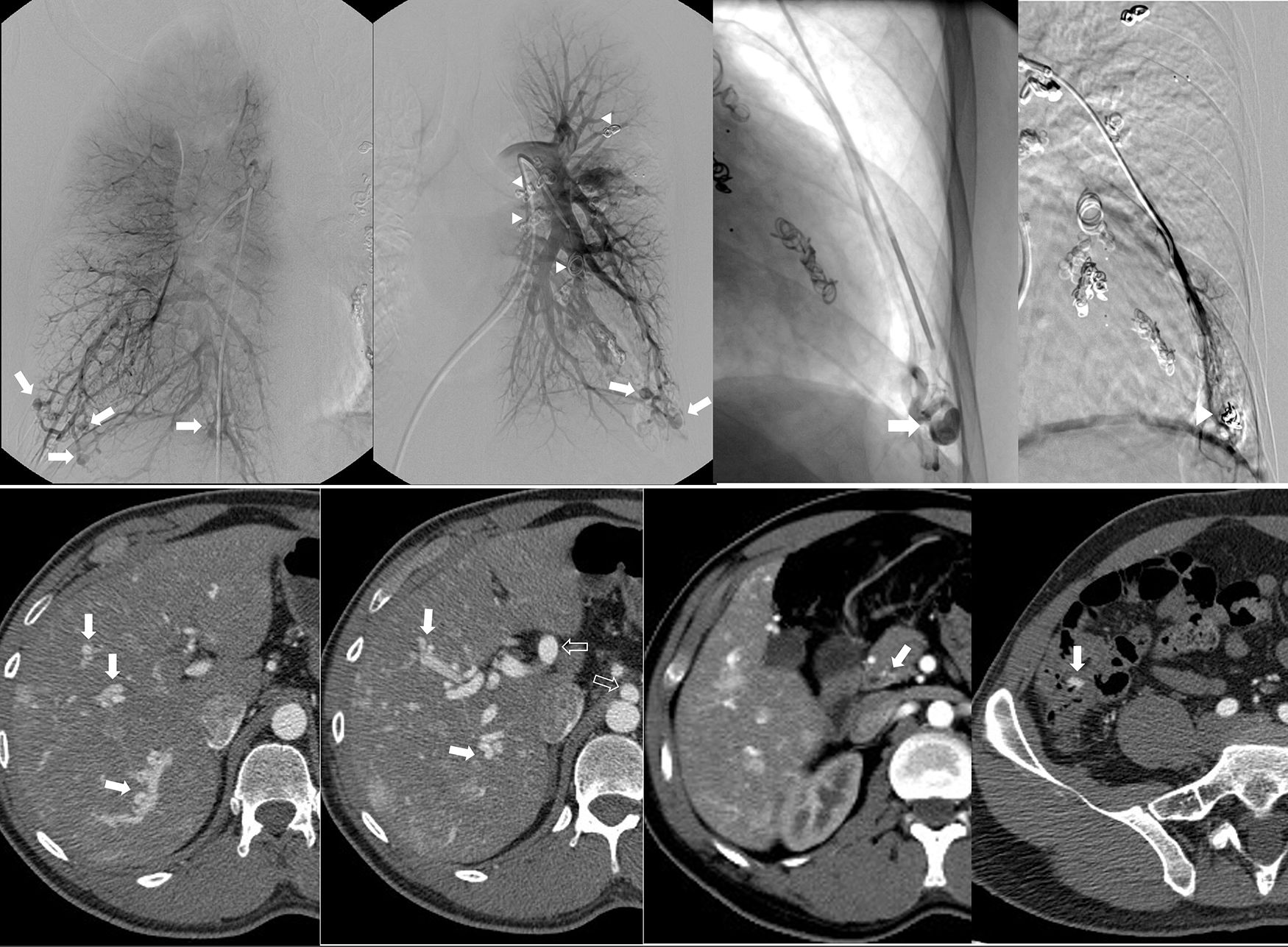
33-Year-old male with known diagnosis of Rendu–Osler–Weber syndrome. Pulmonary arteriogram (a–d) shows multiple bilateral arteriovenous malformations (AVMs) predominately involving the pulmonary bases (arrows) (a: right, b: left). Selective catheterization and arterial embolization of a left basilar AVM (arrow, c) with good results after treatment (arrowhead, d). Evidence of prior AVMs embolization also noted on b (arrowheads). Abdominal CT (e–h) reveals multiple AVMs seen throughout the hepatic parenchyma (arrows on e, f) with arterial portal shunting and associated hypertrophy of the celiac trunk and hepatic arteries (voided arrows on f). Multiple telangiectasias associated with enteric mucosa in the region of the pylorus, and ileocecal valve are also seen (arrows on g,h).
It is classified into five phenotypes according to different gene mutation. The first subtype, HHT1, caused by a mutation of the endoglin gene (ENG) is the most common and has the highest frequency of arteriovenous fistulae in the lungs.24
Although epistaxis is the most frequent clinical symptom, more severe manifestations of the disease may result from liver, brain, or gastrointestinal tract involvement.24,33
Embolotherapy, the primary treatment for pulmonary arteriovenous malformations, is generally indicated for lesions with feeding arteries 3mm in diameter or larger (Fig. 11).33
ConclusionVascular malformations and tumors are rare but important pathologies that may confer significant morbidity and often require aggressive treatment. Diagnosis and proper treatment has been somehow obscured in the past partly due to unclear nomenclature, inadequate classification, and diagnostic algorithm. Although clinical diagnosis is sufficient in some cases, imaging is commonly required with US and MRI, both having a complementary role in their evaluation. US is the first imaging modality due to availability, radiation free, and differentiation between high and low flow lesions. MRI provides confirmative diagnosis in equivocal cases, evaluates the extent of the lesion, relationship with the adjacent structures, and flow dynamics.
Conflicts of interestThe authors declare that they have no conflicts of interest. No funding. Klaus D. Hagspiel and Patrick T. Norton receive financial support from Siemens Medical Solutions, Malvern, PA, USA.
Please cite this article as: Flors L, Park AW, Norton PT, Hagspiel KD, Leiva-Salinas C. Malformaciones vasculares y tumores de partes blandes. Parte 1: clasificación, papel de las pruebas imagen y lesiones de alto flujo. Radiología. 2019;61:4–15.