Nontraumatic emergencies of the head and neck represent a challenge in the field of neuroradiology for two reasons. As explained in the first part of this update, these entities affect an area where the thorax joins the cranial cavity and can thus compromise both structures; second, they are uncommon, so they are not well known.
Maintaining the same approach as in the first part, focusing on the clinical presentations in the emergency department rather than on the anatomic regions affected, we will study the entities that present with two patterns: those that present with a combination of cervical numbness, dysphagia, and dyspnoea and those that present with acute sensory deficits. In the latter group, we will specifically focus on visual deficits, because this is the most common symptom that calls for urgent imaging studies.
Las urgencias no traumáticas de cabeza y cuello son un reto en el campo neurorradiológico por los motivos referidos en la primera parte: su área de afectación, en la encrucijada del tórax y la cavidad craneal, y su baja incidencia en la urgencia, lo que supone que sean poco conocidas.
Manteniendo el mismo enfoque que en la actualización previa, a partir de las formas clínicas de presentación en el ámbito de la urgencia, en lugar de la división por regiones anatómicas estudiaremos las entidades que se presentan con patrones que combinan tumefacción cervical, disfagia y disnea, y los déficits agudos de los sentidos. Dentro de este último grupo, el síntoma al que haremos referencia específica será el déficit visual, puesto que es el que de forma más frecuente requiere estudio radiológico urgente.
Nontraumatic head and neck emergencies take over a small portion of the routine radiological day; however, they are very important. As Brucker et al. claim,1 this anatomic region contains few structures that can be dispensable. After analyzing the images and taking the clinical data into consideration, the role of the radiologist should be defining the location and spread of the processes, identifying all those radiological signs indicative that the patient might be vitally compromised, or that might condition his/her treatment, and establishing differential diagnosis.
As we said in Part 1, in most papers, emergencies are classified according to the region affected by the pathological process, but since the patient presents to the hospital with clinical symptoms or signs, an approach from this point of view is interesting. In this review, we will be dealing with how radiological management is accomplished, and with the clinical characteristics of those entities that present with combined clinical manifestations of cervical tumefaction, dysphagia, and diarrhoea, and we will also be dealing with those patients who present to the hospital with acute sensory deficits.
However, this paper will not deal with all the possible emergency entities that have such clinical manifestations. This is why we chose the most common ones, tried to establish differential diagnoses, set the record straight on some concepts and, same as we did in Part 1, emphasized the clinical manifestations that are less common but with characteristic radiological images that will help the radiologist achieve a correct diagnosis yet despite the rarity of such clinical manifestations.
Nosological division and imaging modalitiesThere are four clinical situations in nontraumatic head and neck emergencies: cervical tumefaction cervical, dysphagia, dyspnoea, and sensory deficit. The causes responsible for these clinical presentations may be of inflammatory-infectious, tumour or vascular origin.2 In this Part 2 we will be looking into those clinical manifestations that combine facial tumefaction, dysphagia, and dyspnoea, as well as sensory deficits.
The combination of cervical tumefaction, dysphagia, and dyspnoea is not rare. It has been reported in oral cavity-mouth floor infections, Ludwig's angina, necrotizing fasciitis, in collections of retropharyngeal abscesses, and spontaneous emphysemas. These situations require performing one cervical computed tomography (CT) scan including the carina and even the whole thoracic cavity. The anatomic continuity of retropharyngeal spaces (retropharyngeal space and danger space) up to the lower mediastinum allow the spread of cervical processes towards the thorax. The use of one single X-ray might be applicable in cases of young individuals with sudden onsets of this clinical combination and associated cervical crepitus to be able to rule out a clinical manifestation as uncommon as spontaneous emphysema.
In sensory deficits only the visual deficit is included. Taste and smelling deficits will not be studied here because they are not as invalidating as the visual deficit, and for the patient they are not as alarming as to go to the emergency room. Sudden hearing loss usually occurs within a 12 hour-span3 and implies the loss of ≥30dB as confirmed in at least three frequency tests conducted during the first 72h. It has a powerful impact in the patient's life, above all in association with tinnitus and vertigo, occurring in 90 and 20–60% of cases, respectively.4,5 Its study in the acute phase requires performing one otoscopy and one audiometry in order to rule out any underlying causes and confirm its diagnosis, and all this is the responsibility of the ENT unit. Although included in the diagnostic armamentarium of this entity, the magnetic resonance imaging (MRI) is never performed in emergent situations.
Cervical tumefaction and dysphagia-dyspnoeaAbscesses of the oral cavity and the mouth floorMost head and neck infections have an odontogenic origin.6 The dental inflammatory disease may have an endodontal or periodontal origin. The origin of endodontal infections may be found in one dental cavity that progressively destroys the dentine, then the dental pulp, and ultimately the dental nerve canal. This is how the infection reaches the dental apex while making up one granuloma or apical abscess. In the periodontal disease, the infection starts as gingivitis that progresses along the periodontal ligament while making up one periodontal abscess. From the radiological standpoint, apical or periodontal abscesses look like radiolucent cavities surrounding the tooth. The abscess may rupture the maxillary or mandibular cortical bones and depending on the dental piece affected, progress following variable location and spread.
Infections that originate in the maxillary bone7 may spread towards the mouth, the masticator space or the parapharyngeal space, depending on whether the pathological pieces are located in the pre-maxillary region, or the second or third molars, respectively. In infections of mandibular origin, the inflammatory processes of the anterior dental pieces are found in the sublingual space, and when infections affect the second and third molars, the inflammatory processes will be found in the submandibular space, since the roots of these molars are located underneath the mylohyoid line.
Other than for dental abscess identification, CT scans also confirm the presence of fluid collections with peripheral enhancement. The location and spread of these fluid collections should appear in the radiological report since this is essential to guide surgical drainage. Also, the affected dental piece should be identified (Fig. 1). The nomenclature used to define the affected tooth speaks of its name (incisive, canine, premolar, and molar) and location at the quadrant of the mouth (upper: right or left, lower: right or left). Following one numerical pattern, the most widely used method in Spain is that of the World Dental Federation (WDF) that numbers teeth as 11–18, 21–28, 31–38 and 41–48, the corresponding pieces to the right and left maxillary dental arches, and the left and right mandibular dental arches, respectively, starting from the first incisive until the last molar in each of the quadrants. Nomenclature from the World Dental Federation (WDF) numbers dental pieces from 1 to 32, starting from the right upper arch third molar and continuing successively with the remaining quadrants following the WDF system.8,9
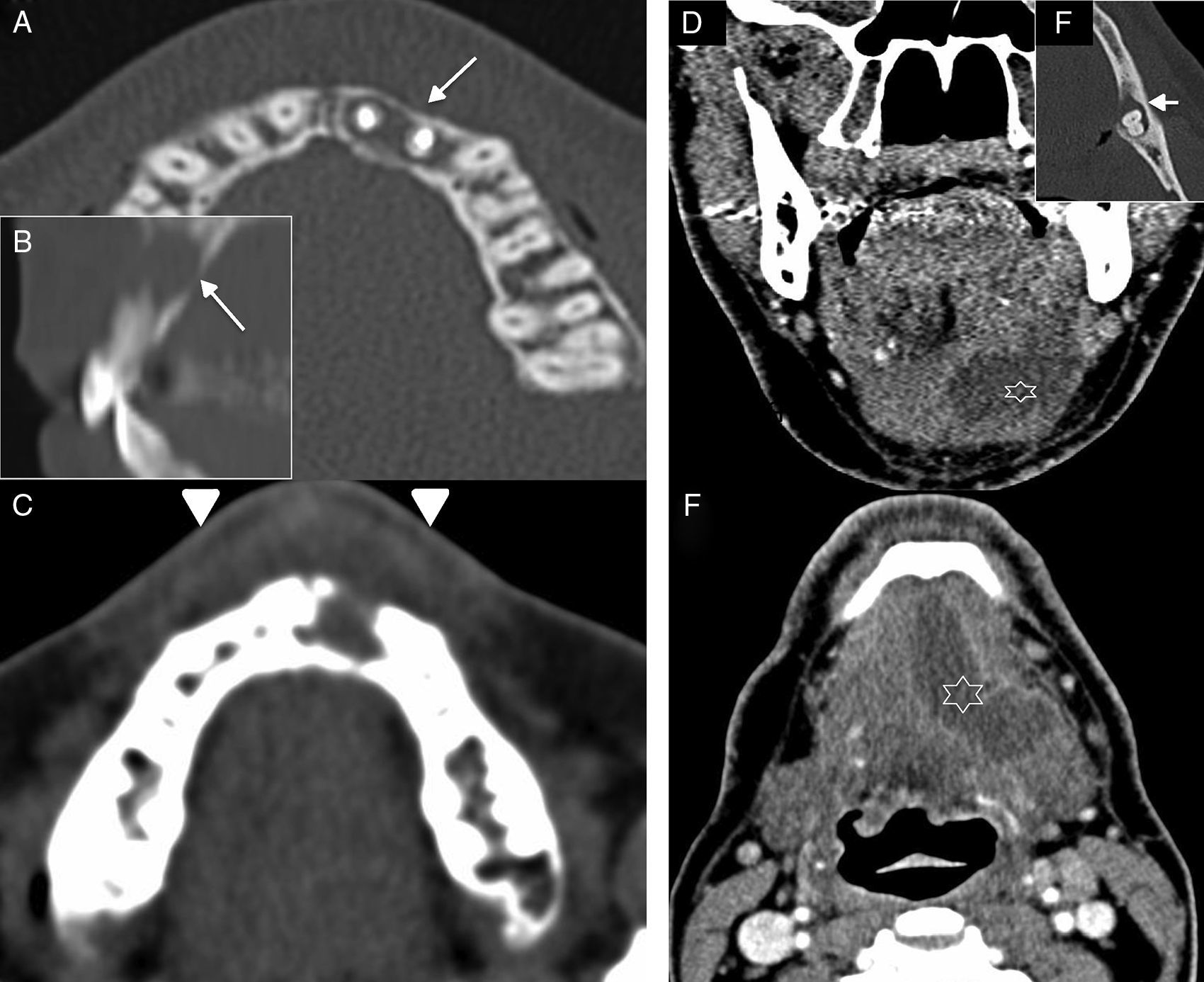
Abscesses of the oral cavity. Abscess in the oral vestibule space. (A) Computed tomography (CT) scan, axial cut reconstructed in bone window. (B) CT scan, sagittal reconstruction in bone window. (C) CT scan, axial cut reconstructed in soft tissues. Female with clinical manifestations of neuralgia and anterior facial tumefaction. The CT scan shows periodontal affectation in the premaxillary region (arrows in A and B), dental pieces #21 and #22–showing metallic material due to prior endodoncy–in the form of periapical abscess with phlegmonous affectation of the oral space and the vestibule (short arrows in C). Submandibular abscess. (D) CT scan, coronal cut with intravenous (IV) contrast. (E) CT, oblique axial reconstruction in bone window. (F) CT scan, axial with IV contrast. Sixty-two year old-male with left submandibular pain. In the CT scan there was one collection with peripheral enhancement (abscess) in the left mouth floor (asterisk in D and F). The infectious focus was located in dental piece #38 (arrow in E) showing one periapical abscess with dehiscence of the lingual cortex.
It is a specific and serious type of cellulitis occurring in the mouth floor and spreading bilaterally towards the soft tissues of the oral cavity and deep spaces of the neck, and at the same time it implicates the muscles located between the mouth floor and the larynx10 (Fig. 2). Clinically, it presents as pain and tumefaction at the mouth floor and can associate chest pain. Before the era of antibiotics, the spread of this process towards the mediastinum was far more common and, therefore, responsible for the denomination given to this entity. It may affect the airways and require tracheostomy more commonly than other cervical abscesses.11 The CT scan is used to assess the spread of inflammatory process, the presence of blood collections, or the formation of abscesses that can occur in some cases.12,13
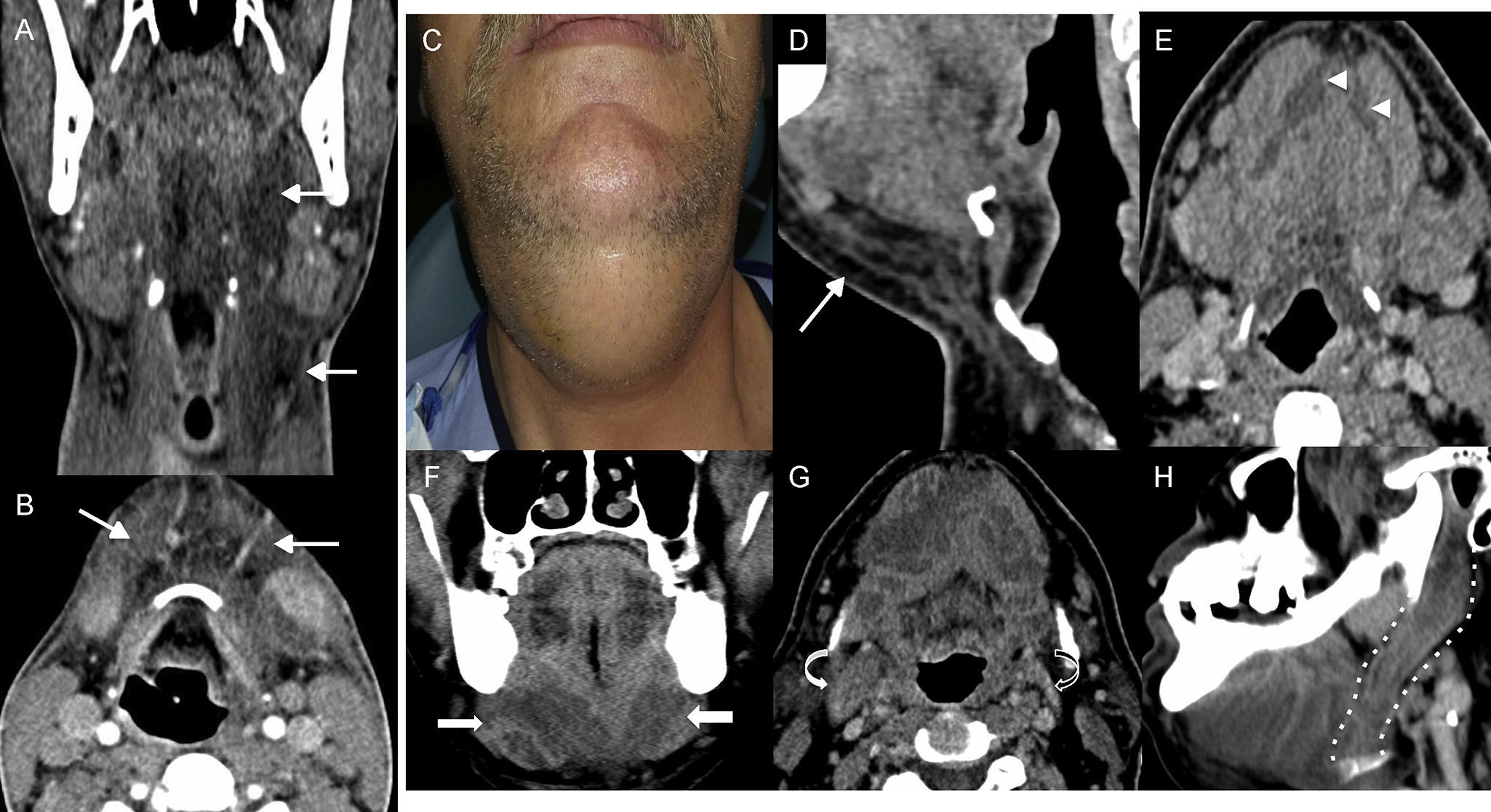
Ludwig's angina. (A) Computed tomography (CT) scan, coronal cut with intravenous contrast. (B) CT scan, axial cut with IV contrast. Eighteen-year old patient who presented with odynophagy and (dis) thermal sensation of three day duration. During the medical examination, he had difficulties opening his oral cavity and showed over-elevation of his tongue. The image confirmed an increase in the fat density of submandibular and parapharyngeal spaces (arrows in A and B). (D–H) CT scan with IV contrast in a different patient (C) who presented to the hospital with cervical tumefaction. (D) Sagittal cut. (E and G) Axial cuts. (F) Coronal cut. (H) Oblique sagittal reconstruction. At the beginning (C–E), the CT scan showed an increase of fat density and trabeculation in the submandibular space (arrow) associated with small collections in the mouth floor (arrowheads). The patient is admitted to the hospital with a diagnosis of Ludwig's angina and IV antibiotic therapy. The patient worsens and another CT scan is performed (F-H) showing fluid collections (long arrows) in the mouth floor with clear oversize with respect to the initial study. Affectation spread along the digastric muscle posterior stomach (curved arrow in G and dotted line in H), which was oversized (compare with the left side one, black curved arrow). Drainage showed seropurulent content.
Necrotizing fasciitis is one aggressive polymicrobial infection of the superficial and deep soft tissues of the neck usually occurring in patients with comorbid situations like diabetes or immunosuppression. Infection can occur in the skin, the mucosa or the teeth, progress rapidly and reach the mediastinum and compromise the life of the patient.14 Clinically, patients show symptoms of cervical tumefaction and dysphagia-dyspnoea associated with high fever. There are certain lab indicators of risk for developing necrotizing fasciitis based on routine lab tests.15 Such indicators are used to distinguish this entity from other less aggressive infections of soft tissues in order to be able to early implement the treatment of choice: emergent surgical debridement.16
In the CT scan, the radiological signs that help us establish its diagnosis are the evidence of cellulitis, fasciitis, myositis, multiple collections, reactive adenopathies, and, on some occasions, septic thrombosis (Fig. 3). The presence of gas (in the absence of recent surgery or radiotherapy) is a highly suggestive finding of this entity, though this does not occur in all cases.17
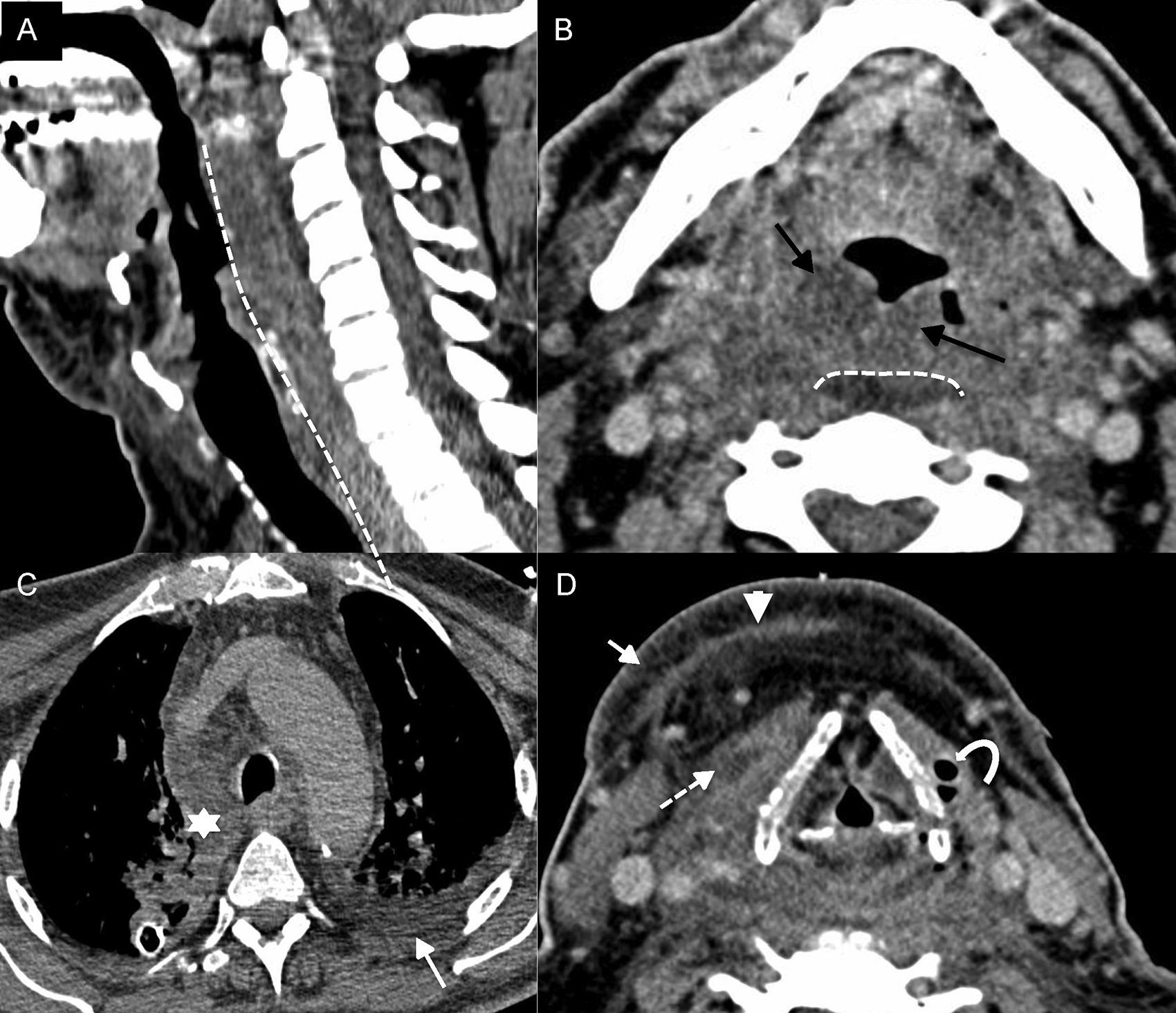
Necrotizing fasciitis. (A) Sagittal computed tomography (CT) with IV contrast. (B–D) Axial CT scan with IV contrast at different cervical levels. Seventy-four year old-patient who presents to the ER with clinical manifestations of dyspnoea, odynophagy, and fever. The patient is then referred to the ENT who confirms saturation levels at 87% and triage and retractions. The image shows thickening of the skin and the subcutaneous cellular tissue–celulitis (arrow in D), thickening and enhancement of superficial and deep fascias–fascitis (arrowhead in D), thickening and enhancement with fluid collections of prelaryngeal muscles–miositis (discontinuous arrow in D), swelling of the pharyngeal mucosal space (black arrows in B), and swelling of the whole retropharyngeal space (dashed line in A and B) spreading towards the mediastinum (asterisk in C). Also, there was presence of gas (curved arrow in D) and right pleural effusion (arrow in C).
The spontaneous cervical emphysema is defined as the presence of gas in cervical and mediastinal spaces that is produced spontaneously in young adults and that is of self-limiting an benign character.18 Clinically, it appears as an intense sudden retrosternal pain associated with dyspnoea of sudden onset. There is no relation whatsoever with iatrogenia, trauma or infection. The underlying physiopathological mechanism is pressure increase in alveoli (caused by multiple etiologies: cough, Valsalva, etc.) that causes its rupture. The air progresses in a centripetal motion towards the mediastinum since there is less pressure there. From the radiological standpoint, there are air bubbles of random distribution both in the different cervical spaces and the mediastinum (Fig. 4A and B). We should distinguish here between Boerhave's syndrome and esophageal rupture secondary to vomit – one clinical manifestation associated with greater morbimortality that requires surgical management.
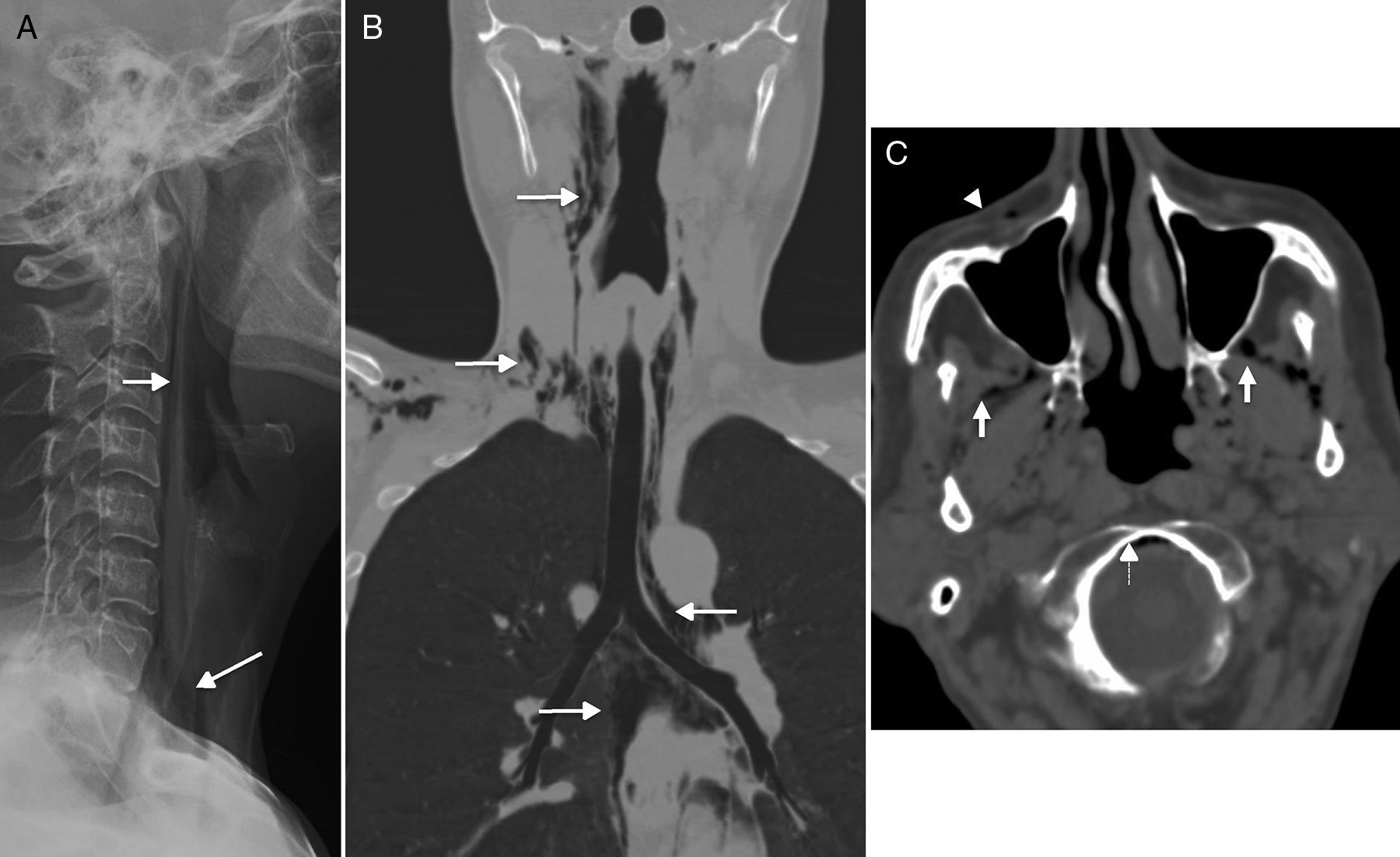
Spontaneous cervical emphysema. (A) Simple lateral X-ray. (B) Computed tomography (CT) scan with coronal reconstruction. (C) Axial CT scan. (A and B) Spontaneous neumomediastinum in one young patient who presents to the ER with clinical manifestations of retrosternal pain, sensation of dyspnoea, and odynophagy after choking. There is presence of cervical emphysema (arrows) spreading towards the mediastinum–a very recognizable finding in the simple X-ray. The CT scan was performed in order to distinguish it from Boerhave's syndrome. (C) CT scan of the brain of one patient with suspicion of stroke who is a carrier of one peripheral route. In the image there was presence of air in the masticator space (white arrows), in the anterior fascial vein (arrowhead), in the epidural space, in anterior portion of the foramen magnum (discontinuous arrows).
The spontaneous emphysema should not be confused with the presence of small quantities of air in the cervical spaces that can be incidentally observed in CT scan (Fig. 4C). It has been reported in 0.034% of CT scans of the brain performed in the emergency room to patients with one peripheral route considered the air access point.19 The location of the air bubbles happens at random; it is more common in the orbital veins, the masticator space, or the cavernous sinuses. Patients with this finding do not have a history of trauma or other relevant data.
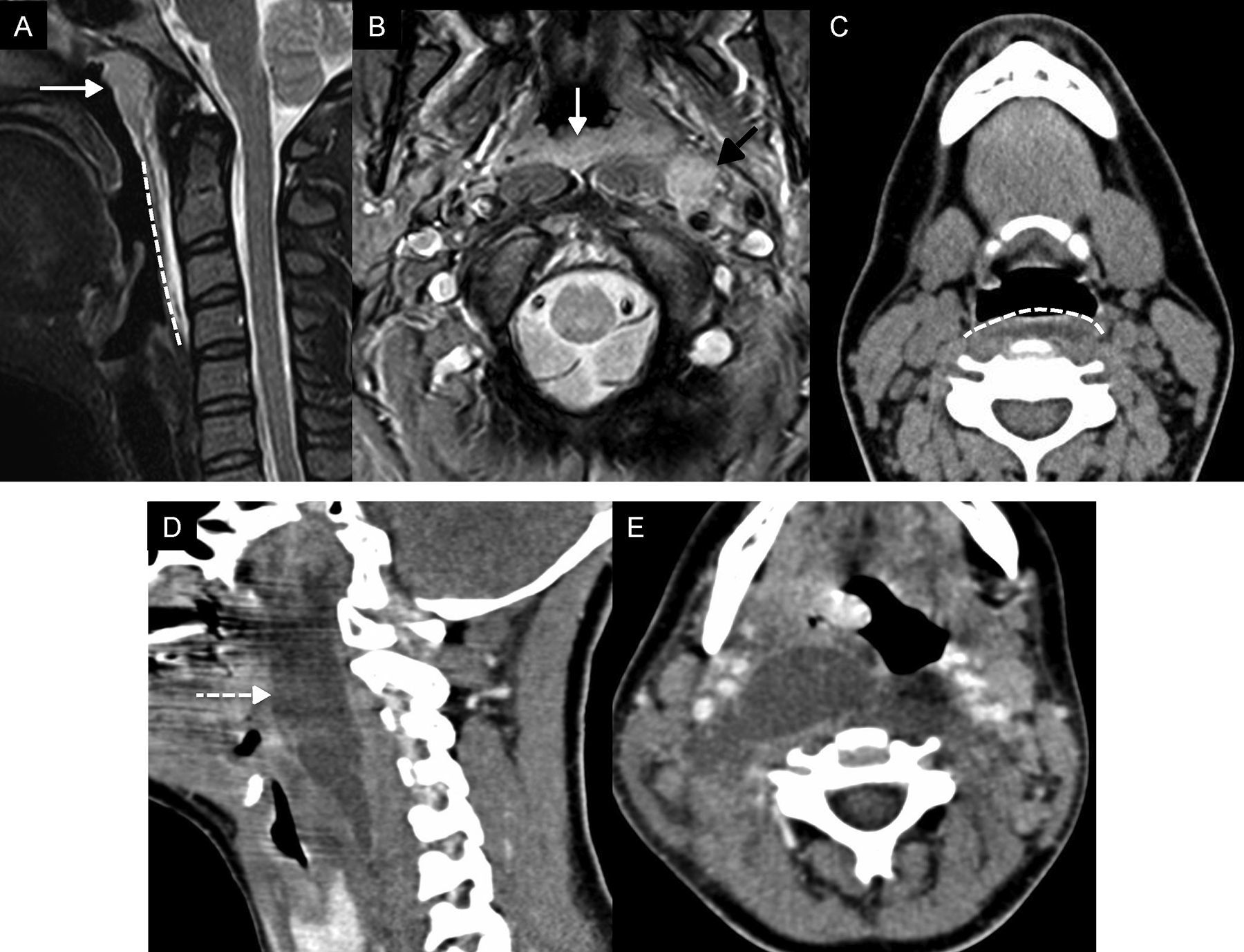
Retropharyngeal and pre-vertebral infectionThe retropharynx or posterior space to the pharynx is a virtual space that spreads from the base of the skull towards the upper mediastinum. There is another virtual space, posterior to the retropharyngeal one, called danger space that connects to the posterior mediastinum and descends towards the lower part of the thoracic cavity. The distinction between these spaces at cervical level is not possible and it is its caudal spread that defines if the inflammatory process occurs in one or the other (spread beyond T3 would be indicative of danger space affectation). The implication of any of these spaces is usually secondary to an infection originated in the upper aerodigestive tract, whose pattern of lymphatic drainage is in the nodes of the retropharyngeal space. The infectious process promotes adenitis and inflammatory changes in the adjacent tissue. Adenitis manifests itself as an oversized lymphatic node. The inflammatory affectation of the adjacent soft parts or cellulitis appears radiologically as a symmetrical increase of the retropharyngeal space that is occupied by a layer of fluid that is usually no thicker than a few millimetres (Fig. 5A–C). Poorly treated adenitis usually suppurates, ruptures and originates abscesses. In this situation, there is a greater fluid collection that happens to be asymmetric and with variable parietal enhancement (Fig. 5D and E).17 Suppurated adenitis represents a relatively common complication of secondary formation of retropharyngeal abscesses in children.
Infection of the retropharyngeal space. (A) Magnetic resonance imaging (MRI), sagittal cut T2-weighted imaging with fat saturation. (B) MRI, axial T2-weighted imaging. C) Computed tomography (CT) scan, axial cut. (D) CT scan, sagittal reconstruction after the administration of IV contrast. (E) CT scan, axial cut after the administration of IV contrast. (A–C) Female with cervical pain, fever, and odynophagia. In the MRI we saw hypertrophy of the mucosal space of pharyngeal tonsils–amigdalitis (white arrows in A and B), one left retropharyngeal adenitis (black arrow in B), and retropharyngeal swelling (celulitis) (dashed line in A and C). Swelling grows bilaterally in the retropharyngeal space and barely distends to space of a few millimetres. (D and E) Fifteen year old-female diagnosed with mononucleosis who presents with high fever, significant odynophagia, and trismus. The CT scan confirmed the presence of one asymmetric fluid collection with peripheral enhancement in the retropharyngeal space, suggestive of an abscess (dashed line arrow), that was confirmed after aspiration and drainage.
The pre-vertebral space is that space defined by the deep cervical fascia where pre-vertebral musculature can be found. The infectious process of the spine, spondylodiscitis, may reach this space and are usually responsible for its affectation.13,20 Spondylodiscitis occurs due to direct inoculation (traumatic or surgical), spread of an adjacent infection or due to the hematogenous dissemination of pathogen germs. The most common causal agent is Staphylococcus aureus and it is usually located in the thoracic or lumbar regions. The MRI is the most sensitive imaging modality for its study since it accurately defines spinal disc affectation and all possible pre-vertebral or epidural collections (Fig. 6).
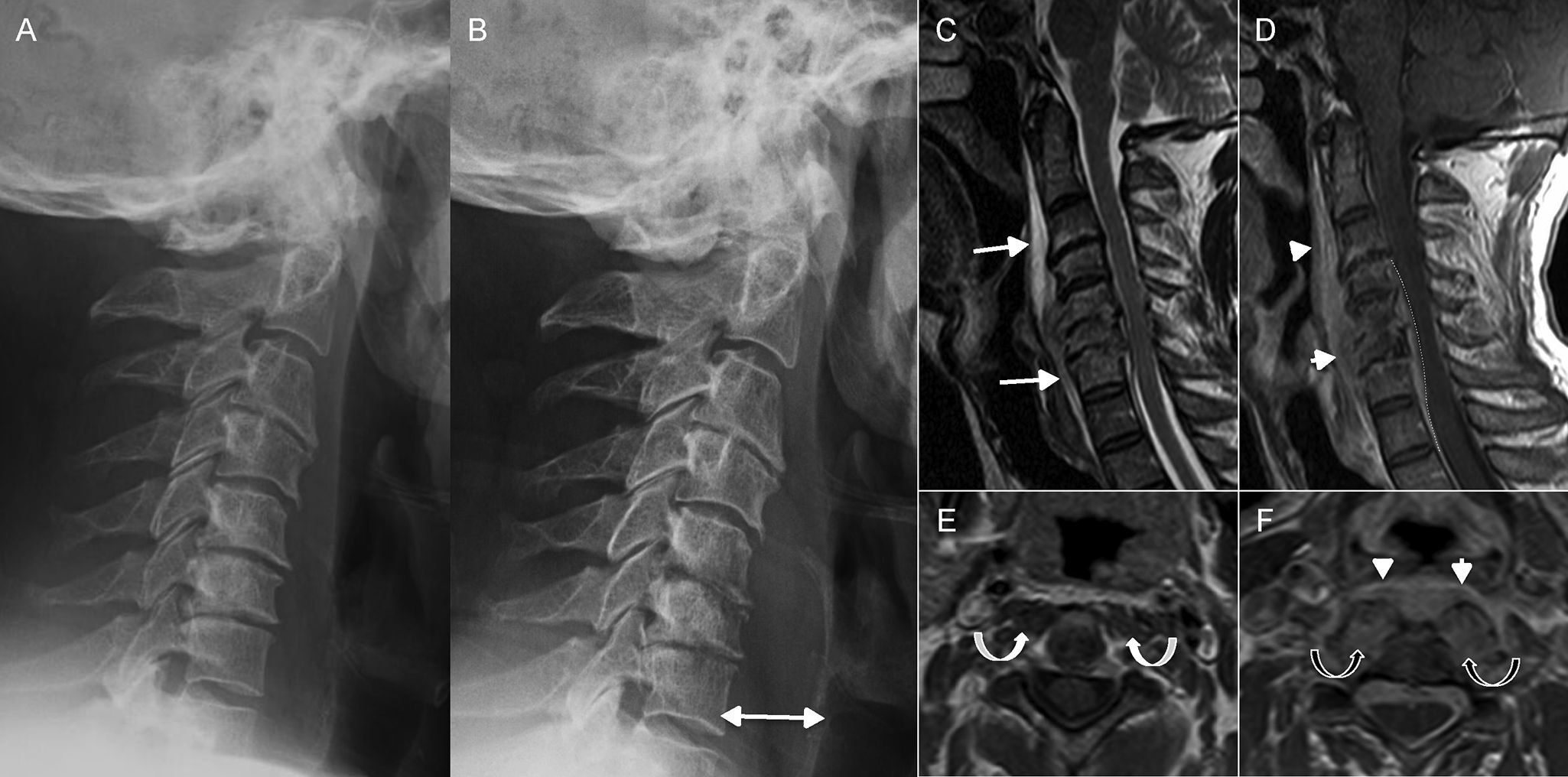
Spondylodiscitis. (A and B) Lateral X-ray of the spine. (C) Magnetic resonance imaging (MRI), T2-weighted sagittal cut. (D) MRI, T1-weighted sagittal cut enhanced with gadolinium. (E and F) MRI, T1-weighted axial cuts enhanced with gadolinium at C2 level (E) and C6 level (F). Patient presents with neck pain and undergoes ones lateral X-ray (A). The X-ray shows reduction of intervertebral spaces and mild erosion of C5–C6 and C6–C7 discs that is interpreted as degenerative changes. Twelve (12) days later the patient presents to the hospital with more intense pain. Another X-ray is performed (B) that confirms clear progression with loss of height in vertebral bodies C5 and C6 and greater enhancement of pre-vertebral space (double arrow in B). Since spondylodiscitis was suspected, one MRI is performed that confirms such condition as well as the existence of one pre-vertebral collection (long arrows in C) with intense enhancement after the administration of gadolinium (arrowheads in D and F). There was also one collection in the epidural space (dashed line in D). In the location of greater affectation, that is segment C5–C6, there was an oversized pre-vertebral musculature with increased uptake (black curved arrows in F) that was not present in upper levels like C2 (white curved arrows in E).
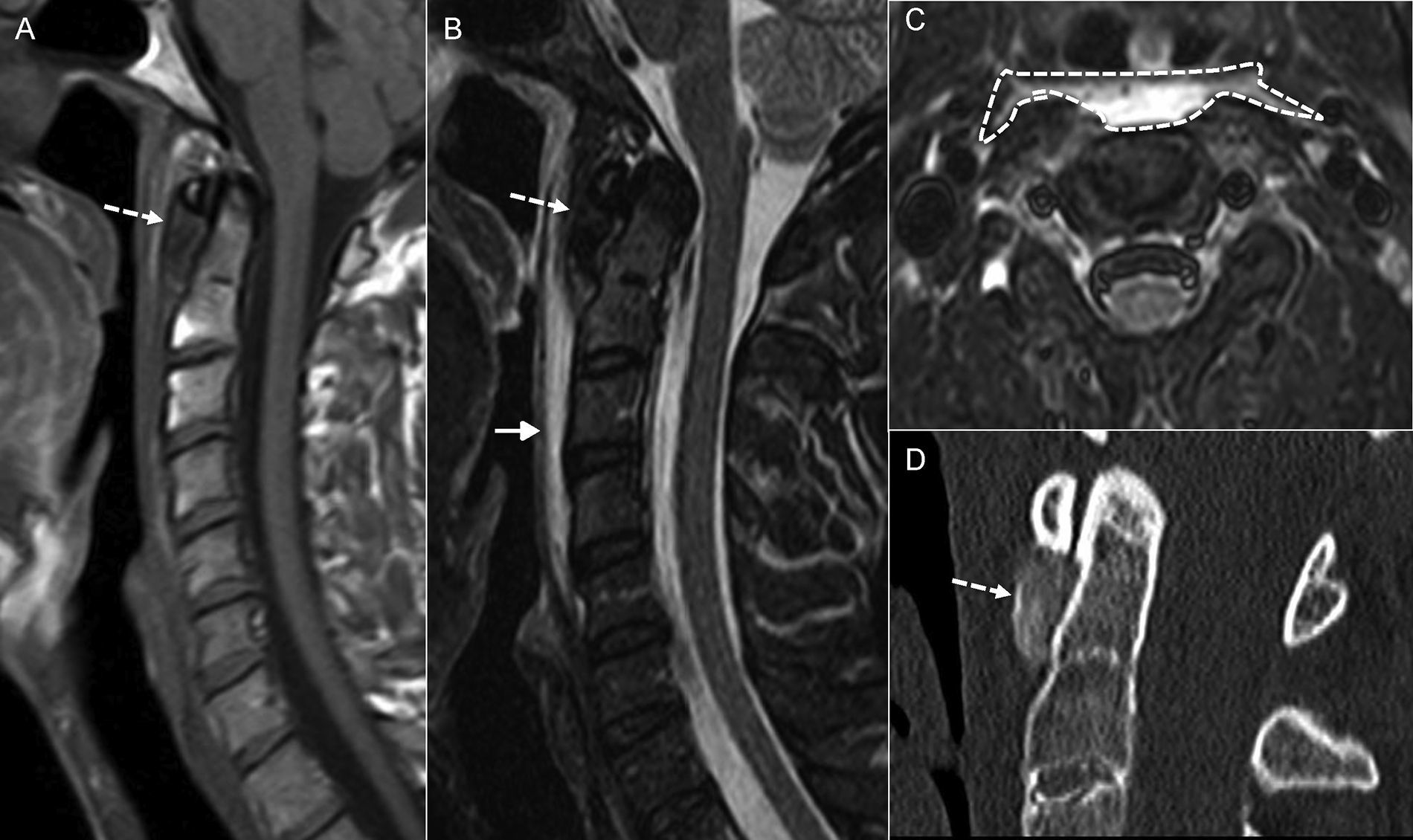
There exists a rare entity, probably because it is misdiagnosed, called calcific tendonitis of the longus colli muscle (Fig. 7) that can also implicate the pre-vertebral space too. It is caused by the deposit of calcium hydroxyapatite in the tendon of the long muscle of the neck (longus colli muscle). This anomalous deposit originates one inflammatory response to foreign bodies. The diagnosis of this clinical manifestation is achieved after observing, in one simple X-ray, the pathognomonic signs, and after observing in one CT scan, the presence of dense image calcifications (not bony) in the pre-vertebral-retropharyngeal space at C1–C2 level, associated with soft tissue swelling, that spreads from cervical level of C1 to C4.21,22
Calcific tendonitis of the longus colli. (A) Magnetic resonance imaging (MRI), sagittal cut T1-weighted imaging. (B) MRI, sagittal cut T2-weighted imaging. (C) MRI, axial cut T2-weighted imaging with fat saturation. (D) Computed tomography (CT) scan, sagittal reconstruction in bone window. Patient presents with neck pain. One MRI was performed that confirmed pre-vertebral space swelling (arrow in B and dashed silhouette in C). Also, there was significant hyposignal anterior to the odontoid apophysis (dashed arrow in A and B) that in the CT scan was confirmed as a deposit of calcium (dashed arrow in D).
The acute loss of eyesight is studied by taking two factors into consideration: for how long has the loss been happening, and what is the segment responsible for the visual system.
The duration of the visual loss may be transient or persistent. The transient duration is defined as a visual loss lasting less than 25h and usually due to one vascular occlusion affecting the eyeball or the visual cortex, or being secondary to epileptic seizure or migraine. Persistent visual losses last more than 24h and are usually due to transient ischaemias. Whichever the duration of the visual loss is, it should be assessed by one ophthalmologist, who, if necessary, will be conducting imaging modalities for diagnostic purposes. The transient visual loss or unilateral amaurosis fugax is suspicious of a carotid origin as the cause for the arterial occlusion. In this case, we should conduct one Doppler ultrasound, one angio-CT scan, or one angio-MRI. The loss of bilateral vision, above all in the elderly, is associated with vertebrobasilar symptoms and requires performing one CT scan and/or one MRI for the assessment of the encephalic parenchyma and the vascular structures of posterior circulation.
If we study the visual segment responsible for the visual loss, we should remember that the luminous stimulus should run across the different components of the eyeball, continue along the optic nerve, then the intracranial visual pathways, to eventually reach the occipital cortex. Therefore, this condition may be divided into three different segments: the eyeball (except for the retina), the retina and neural visual patterns.
The alterations in the eyeball and the retina are assessed by ophthalmologists, and such alterations do not usually require any additional images. It is the issues in the neural patterns that may require additional radiological studies.23
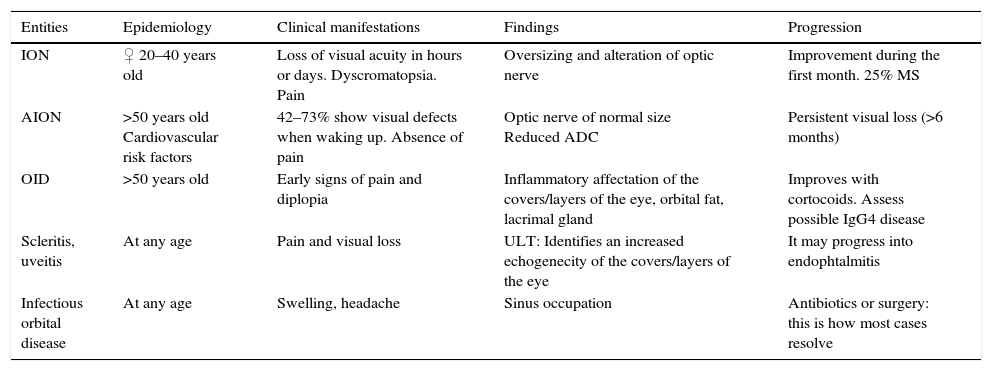
Setting the amaurosis fugax aside (except for the image of ischaemic optic neuritis to be able to distinguish it from inflammatory optic neuritis), we wanted to use this review to talk about all those clinical manifestations of the most common neural visual patterns in our daily radiological routine which, though rare, can be interpreted to then maybe establish the correct diagnosis (Table 1).
Differential diagnosis of the entities responsible for visual deficit.
| Entities | Epidemiology | Clinical manifestations | Findings | Progression |
|---|---|---|---|---|
| ION | ♀ 20–40 years old | Loss of visual acuity in hours or days. Dyscromatopsia. Pain | Oversizing and alteration of optic nerve | Improvement during the first month. 25% MS |
| AION | >50 years old Cardiovascular risk factors | 42–73% show visual defects when waking up. Absence of pain | Optic nerve of normal size Reduced ADC | Persistent visual loss (>6 months) |
| OID | >50 years old | Early signs of pain and diplopia | Inflammatory affectation of the covers/layers of the eye, orbital fat, lacrimal gland | Improves with cortocoids. Assess possible IgG4 disease |
| Scleritis, uveitis | At any age | Pain and visual loss | ULT: Identifies an increased echogenecity of the covers/layers of the eye | It may progress into endophtalmitis |
| Infectious orbital disease | At any age | Swelling, headache | Sinus occupation | Antibiotics or surgery: this is how most cases resolve |
ADC: apparent diffusion coefficient; ULT: ultrasound; OID: orbital idiopathic inflammatory disease; MS: multiple sclerosis; ION: inflammatory optic neuritis; AION: acute ischaemic optic neuritis.
The clinical manifestations of inflammatory optic neuritis (ION) and acute ischaemic optic neuritis (AION) are well established. The ION is the most common cause of optic nerve disease in young adults, while the AION is the most rare aetiology in the elderly.
ION is characterized by a progressive loss of visual acuity, and can occur in a matter of hours or days, and the following are very characteristic presentations: dyscromatopsia, reduced sensitivity to contrast, and pain with eye movements during the first 5 days. Visual acuity improves in most cases during the first month. Patients are usually young women in their twenties or forties, and in many times it is associated with multiple sclerosis.24–26
AION is characterized by a sudden loss of visual acuity or by a campimetric defect, usually painless with eye movements. Forty two per cent of patients notice some visual defect when they wake up.27 The loss of visual acuity is maintained though time, though it may improve during the first 6 months. These patients are usually above 50 years old and associate cardiovascular risk factors. AIONs are divided into anterior, those affecting the optic nerve papilla, posterior or retrobulbar. They may be of arteritic or nonarteritic origin.
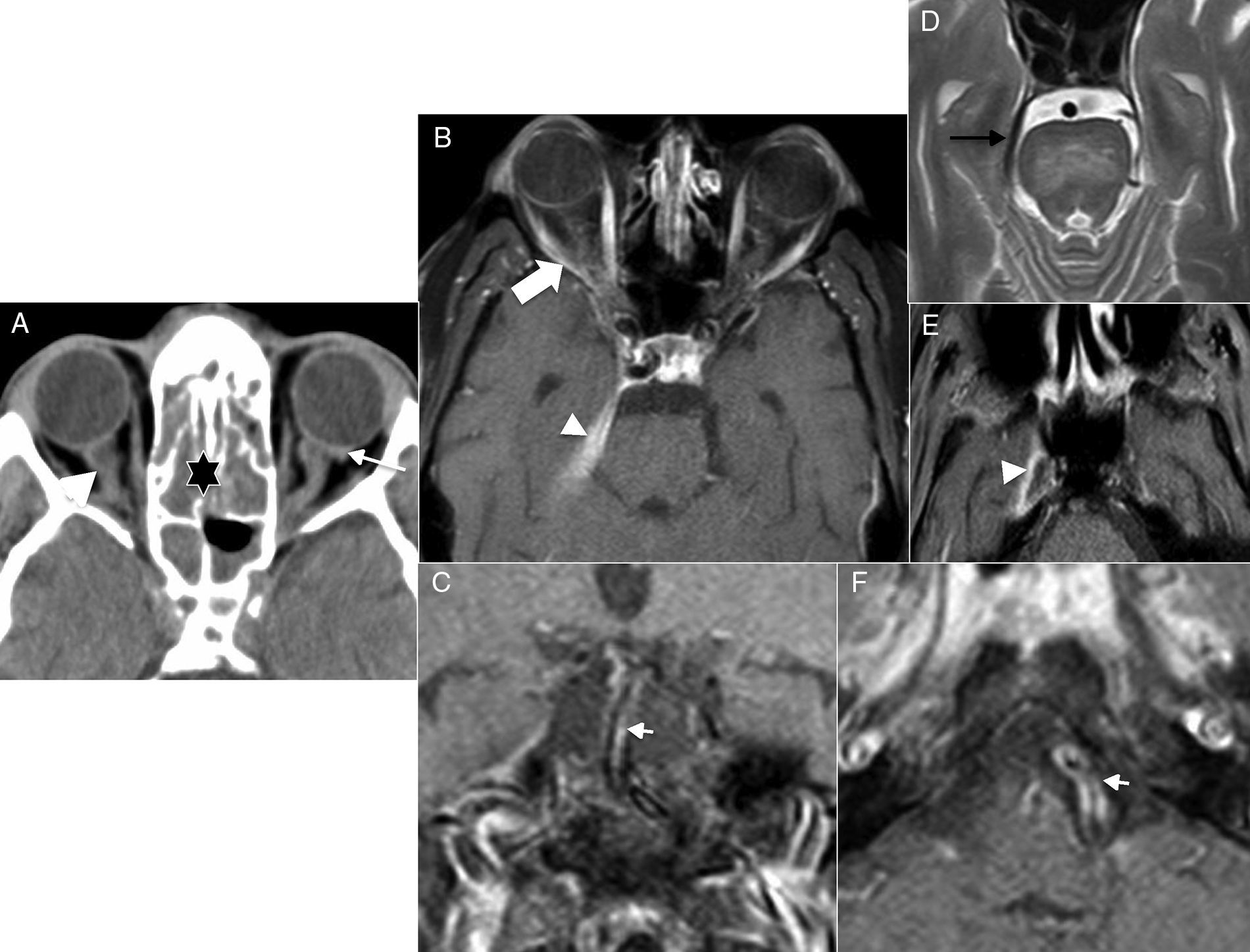
However, even though there are different clinical manifestations between both entities, there are times that these characteristics overlap, and then additional imaging modalities are necessary to help us achieve the correct diagnosis.28 In the ION an enlarged optic nerve may be seen in the emergent/urgent CT scan of the brain,29 although there is no doubt that the MRI is the most sensitive imaging modality to help us distinguish between these two entities. Added to the possible enlargement of the optic nerve, this modality may also show contrast uptake in the T1-weighted image with gadolinium,30 and hypersignal and poor definition in the T2-weighted image of the optic nerve in the ION (Fig. 8A and B). In the AION, the optic nerve shows normal thickness, yet it may show hypersignal diffusion at optic nerve papilla level (in the anterior variants) more commonly than in the cases of ION31 (Fig. 8C–E).
Optic neuritis. (A) Computed tomography (CT) scan of the brain, axial cut. (B) Magnetic resonance imaging (MRI), coronal cut, T2-weighted imaging with fat saturation. Twenty-three year old-female who presents to the hospital with visual loss and pain in her right eye. The CT scan confirms an increased calibre of the optic nerve (arrow in A). The MRI better defines oversized optic nerves showing hypersignal and poor definition of bulbar covers (arrow in B). Acute ischaemic optic neuritis. (C) CT scan of the brain, axial cut. (D) MRI, axial cut T2-weighted imaging with fat saturation. (E) MRI, axial cut diffusion-weighted imaging. Sixty-two year old patient presents to the ER with visual loss in left eye. The CT scan of the brain did not show any significant alterations at encephalic parenchyma or orbital levels. In the MRI, the optic nerve showed normal thickness and definition. The diffusion-weighted imaging, however, showed hypersignal in the left optic nerve papilla (arrow in E).
In the arteritic variants of AION, we should rule out giant cell arteritis (GCA). This disease is the most common systemic vasculitis. It may cause anterior or posterior affectation of the optic nerve. The visual loss usually happens in one eye and is transient, yet in 15–20% of the cases it can be permanent or bilateral; the latter clinical manifestations are more common if diagnosis is not achieved or early treatment with corticoids is administered. This entity is suspected in elderly patients, with visual loss in one eye that characteristically associate claudication of the jaw, rheumatic polymyalgia, and elevated values of C-protein and speed of sedimentation. From the pathogenic standpoint, this condition affects the aorta, the supra-aortic vessels, and the epicranial superficial arteries like the superficial temporal artery and the occipital artery. The biopsy with histopathological analysis confirming the presence of one granulomatose and lymphocytic inflammatory affectation of the arterial wall is the best diagnostic test. However, there are studies that show that the high-resolution MRI with contrast can be a promising noninvasive diagnostic technique.32 With this modality we can see thickening and enhancement after the administration of contrast at parietal-periadventitial level in the superficial and occipital temporal arteries.33 Affectation of intracranial arteries has also been reported, such as affectation of the internal carotid artery and vertebral arteries, but compared to the high sensitivity and specificity of extracranial vessels, sensitivity to intracranial affectation is low.34
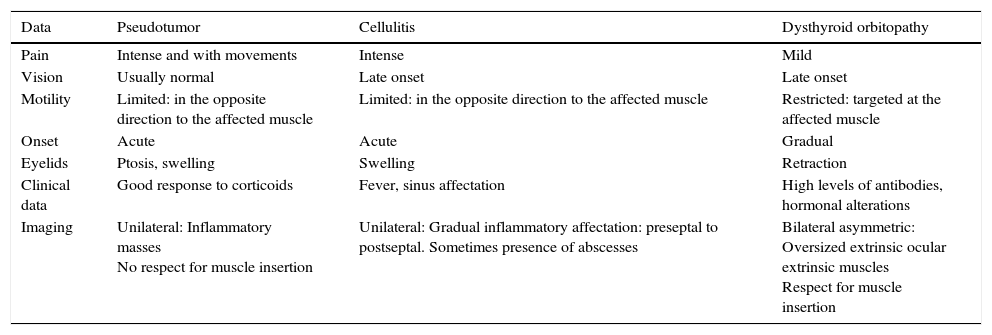
Orbital idiopathic inflammatory diseaseLike we mentioned in Part 1, under this epigraph we find conditions causing acute orbital inflammation usually associated with pain with no infectious aetiology. Hence, in this nosological group we find entities such as Wegener's granulomatosis, GCA, inflammatory pseudotumor, and IgG4-related disease. They appear as inflammatory or oversized soft tissues masses and poor definition of the eye structures, which poses differential diagnosis with other conditions such as orbital cellulitis or dysthyroid orbitopathy (Table 2).
Clinical-radiological differential diagnosis of the most common orbital inflammatory entities.
| Data | Pseudotumor | Cellulitis | Dysthyroid orbitopathy |
|---|---|---|---|
| Pain | Intense and with movements | Intense | Mild |
| Vision | Usually normal | Late onset | Late onset |
| Motility | Limited: in the opposite direction to the affected muscle | Limited: in the opposite direction to the affected muscle | Restricted: targeted at the affected muscle |
| Onset | Acute | Acute | Gradual |
| Eyelids | Ptosis, swelling | Swelling | Retraction |
| Clinical data | Good response to corticoids | Fever, sinus affectation | High levels of antibodies, hormonal alterations |
| Imaging | Unilateral: Inflammatory masses No respect for muscle insertion | Unilateral: Gradual inflammatory affectation: preseptal to postseptal. Sometimes presence of abscesses | Bilateral asymmetric: Oversized extrinsic ocular extrinsic muscles Respect for muscle insertion |
IgG4-related disease is one relatively recent entity denomination. It is characterized by elevated concentrations of serum IgG4 and tissue tumefaction or infiltration due to IgG4 positive plasma cells. In the histological study we can see several IgG4 positive plasma cells, fibrosis, and obliterative flebitis. It is an autoimmune disease that usually progresses during long periods of time, affecting one or several organs, and usually responding to corticoids. In 2010, Umehara et al.35 established the diagnostic criteria of this entity: serum IgG4 levels >135mg/dL, over 40% IgG4 positive plasma cells, and presence of over 10 cells per high-magnification field in the biopsy sample. In the field of neuroradiology, at orbital level it is expressed as an oversized lacrimal gland or inflammatory pseudotumor, or as hyperthrophic pachymeningitis. As an inflammatory pseudotumor it can give rise to coronal or extracoronal masses, but we should not forget that most inflammatory pseudotumors are not part of the clinical spectrum of IgG4. The oversized calibre of trigeminal nerve branches, especially the infraorbital nerve, is one piece of information that is typical of this entity.36,37 There are studies that claim that inflammatory pseudotumors as somehow associated with the IgG4-related disease of the trigeminal nerve in the form of soft tissue masses that are homogeneously enhanced and affect the base of the skull along the trigeminal V2 and V3 divisions.
The diagnostic criteria of the different entities responsible for orbital idiopathic inflammatory disease (OID) are established based on clinical, analytical and anatomopathological data. In images, inflammatory injuries with more or less characteristic locations depending on the entities, but without any clear signs as to be able to establish the exact etiological diagnosis. That is, as radiologists we cannot define this entity based on images only, but we will need to know the clinical parameters as well. Also, some times the diagnostic clinical criteria for these entities are not fully met. In these cases, in view of the radiological findings, for its management we could use a more general term such as orbital idiopathic inflammatory disease (Fig. 9).
Orbital idiopathic inflammatory disease due to giant cell arteritis. (A) Computed tomography (CT) scan of the brain, axial cut. (B, E and F) Magnetic resonance imaging (MRI), axial cut T1-weighted imaging with fat saturation after the administration of gadolinium. (C) MRI, coronal cut T1-weighted imaging with fat saturation after the administration of gadolinium. (D) MRI, axial cut T2-weighted imaging. Sixty eight year old patient (A) who presents to the hospital with visual loss in left eye and diagnosed with acute ischaemic optic neuritis. In the CT scan there was presence of thickening in both optic nerves (short arrow in A) and bulbar covers (long arrow in A). Yet despite the fact that nor the clinical data or the biopsies were not conclusive, giant cell arteritis was diagnosed and with this suspicion in mind it was treated with corticoids. Four (4) months later the patient presents to the hospital again (B–F) with new clinical manifestations in the right eye. One PET (positron emission tomography) scan is performed (not shown) that confirms Meckel's cave and right orbital vertex uptake. The MRI confirms orbital vertex affectation (thick arrow in B) and shows thickening and hyper-uptake of Meckel's cave dura mater (arrowhead in E) and right petroclinoid ligament (arrowhead in B), the latter being hypointense in the T2-weighted imaging (black arrow in D). Also, there was thickening and hyper-uptake of the basilar and vertebral arterial wall (arrows in C and F).
The infectious orbital disease usually manifests itself as septal and retroseptal cellulitis whose origin is usually pyogenic sinusal affectation or, more rarely, fungal affectation. It can associate loss of visual acuity when the infection spreads and damages the optic nerve, or when the inflammatory component compromises the orbital vertex.
Fungal sinusitis is one relatively rare sinus infectious disease. It may be categorized into invasive and noninvasive.38,39 The invasive variant is defined by the presence of fungi outside the sinus air cavity and occurs in immunosuppressed individuals, while clinically it presents itself acute or chronically. In acute cases, the individual presents to the hospital with a fever, rhinorrhea, and mucosal ulceration. In the chronic cases, symptom onset is insidious and is characterized by the progressive invasion of paranasal sinus structures, like the orbital vertex, which clinically translates into reduced visual acuity and eye motility.
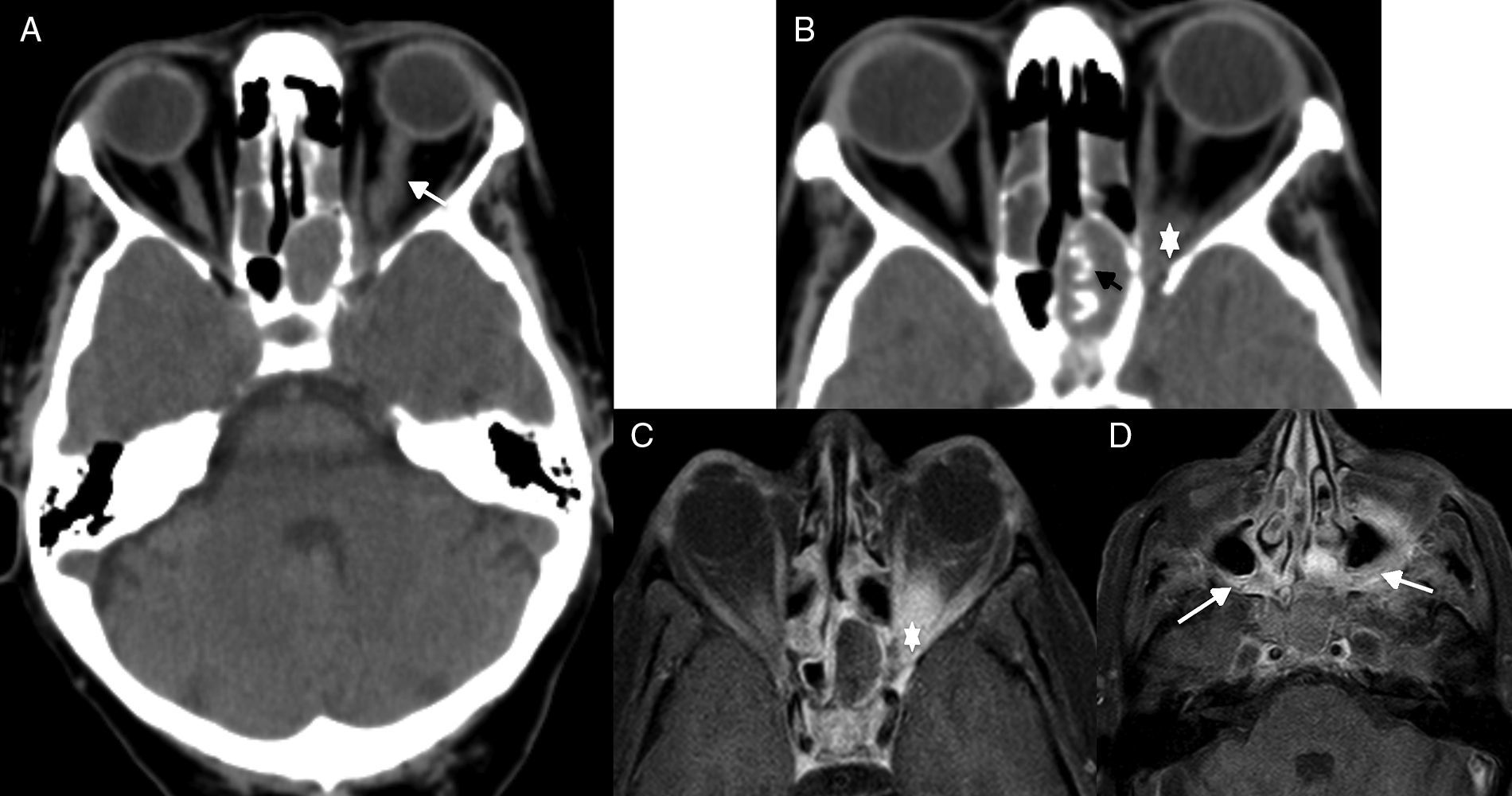
In the CT scan we can see sinus occupation, presence of dense tortuous calcifications,40 and poor definition of orbital vertex structures secondary to the presence of inflammatory-fungal tissue. The MRI is a better imaging modality to define orbital affectation and the scope of the spread process (Fig. 10).
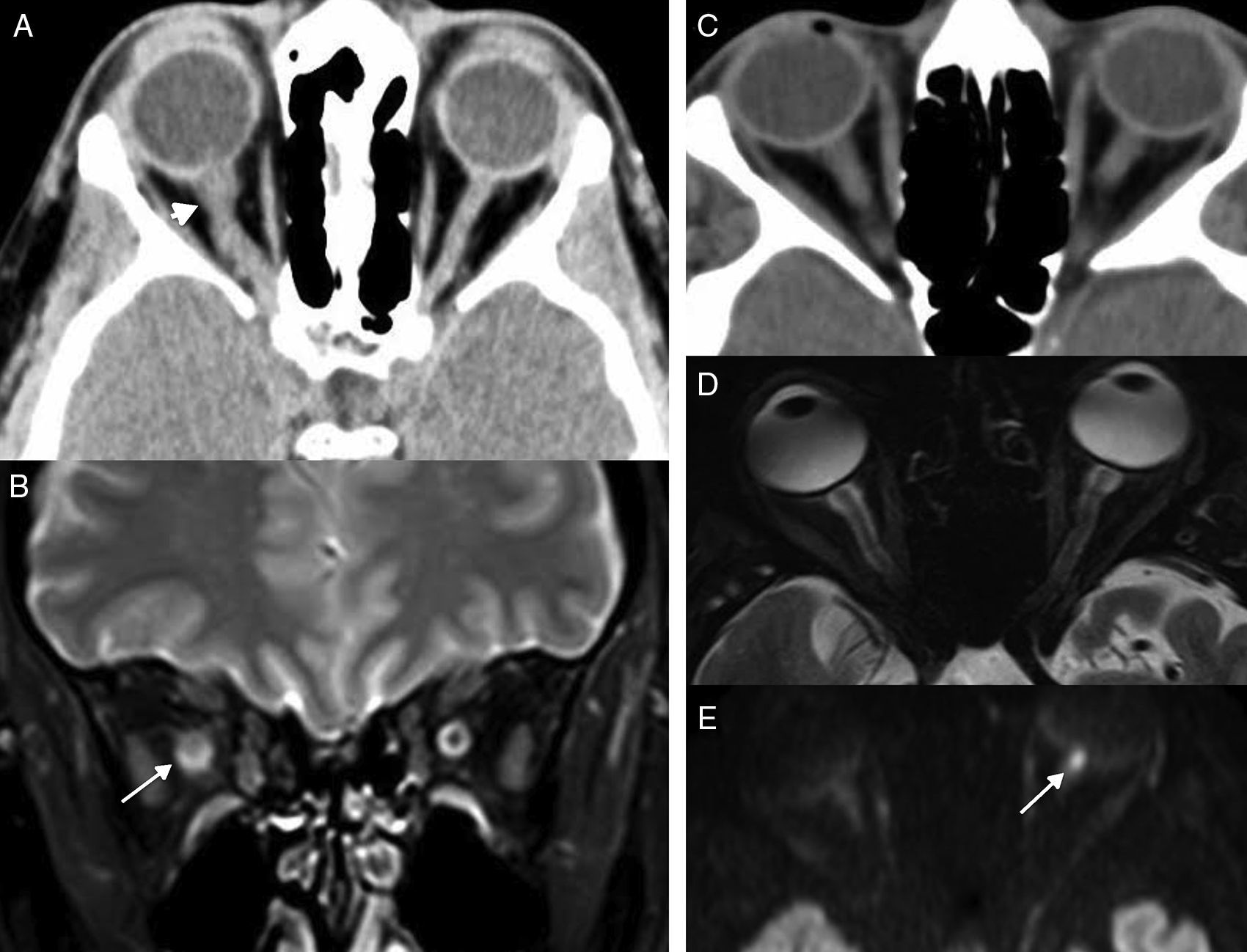
Orbital apex syndrome due to fungal sinusitis. (A) Computed tomography (CT) scan of the brain, axial cut. (B) CT scan of paranasal sinus structures, axial cut. (C and D) Magnetic resonance imaging (MRI), axial cut T1-weighted imaging with fat saturation after the administration of gadolinium. Patient presents to the ER with visual loss in left eye. One CT scan of the brain is performed (A) that confirms presence of an oversized optic nerve (arrow in A), complete opacification of the sphenoid sinus that showed thickened walls, very thick intrasinus calcifications (black arrow in B), and soft tissue increase in the apex (asterisk in B). Due to suspicion of invasive fungal sinusitis, one MRI is performed (C and D), that confirms soft tissue increase in the orbital apex (asterisk in C) and that the swelling has spread towards adjacent soft tissues (white arrows in D) in both pterygopalatine fossae.
In patients with radiological data of chronic rhinosinusitis and obliteration of the perisinus fat planes, this diagnosis should be suspected.
ConclusionNontraumatic head and neck emergencies that clinically appear as a combination of cervical tumefaction, dysphagia, dyspnoea, and acute sensory deficit are a small percentage of all the head and neck emergencies that we see in imaging modalities. The role of the radiologist should be determining the location and spread of the process, identifying findings in order to be able to assess the severity of the clinical manifestations, like the presence of gas in fasciitis, and eventually differentiating inflammation (non-drainable) from abscesses (drainable). However, we should not forget that we are clinical radiologists and that in order to be able to achieve successful diagnoses we should interpret the images we see in a correct and structured way, always within the clinical context of the patient, and not in isolation. Only then we will be able to achieve the correct diagnosis of the different process, even when dealing with uncommon cases, or rare clinical and radiological manifestations.
Ethical responsibilitiesProtection of people and animalsThe authors declare that no experiments with human beings or animals have been performed while conducting this investigation.
Data confidentialityThe authors confirm that they have followed their centres protocols on the publication of data from patients.
Right to privacy and informed consentThe authors confirm that in this article there are no data from patients.
Authors- 1.
Manager of the integrity of the study: BBA.
- 2.
Study Idea: BBA.
- 3.
Study Design: BBA, MTG, LEG, YCI.
- 4.
Data Mining: BBA, MTG, LEG, YCI.
- 5.
Data Analysis and Interpretation: BBA, MTG, LEG, YCI.
- 6.
Statistical Analysis: BBA.
- 7.
Reference: BBA, MTG, LEG, YCI.
- 8.
Writing: BBA, MTG, LEG, YCI.
- 9.
Critical review of the manuscript with intellectually relevant remarks: BBA, MTG, LEG, YCI.
- 10.
Approval of final version: BBA, MTG, LEG, YCI.
The authors declare no conflict of interests associated with this article whatsoever.
Please cite this article as: Brea Álvarez B, Esteban García L, Tuñón Gómez M, Cepeda Ibarra Y. Urgencias no traumáticas de cabeza y cuello: aproximación desde la clínica. Parte 2. Radiología. 2017;59:182–195.