The association between obstructive sleep apnea (OSA) and glucose metabolism remains controversial. This study investigates the relationship between OSA and incident type 2 diabetes (DM) and prediabetes (preDM), as well as the effect of long-term CPAP (continuous positive airway pressure) treatment.
MethodsFollow-up study in a retrospective clinical cohort of patients with OSA and randomly selected controls. Data on incident DM and preDM as well as CPAP were obtained from hospital records. The relationship between baseline OSA and incident DM was examined using COX regression models.
Results356 patients, 169 with OSA and 187 controls were followed for a median of 98 months; 47 patients (13.2%) developed DM and 43 (12.1%) developed pre-DM. The 5-year cumulative incidence of DM was 10.7% (6.5–13.9%). 87% of subjects with preDM in the baseline sample progressed to incident DM. It is shown that body mass index (BMI), nocturnal hypoxia and apnea hypopnea index (AHI) are risk factors for the development of DM and that CPAP reduces this risk.
ConclusionsPatients with OSA have a higher risk of developing DM. The risk factors involved are BMI, nocturnal hypoxia and AHI. Regular long-term CPAP use was associated with a decreased risk.
La asociación entre la apnea obstructiva del sueño (AOS) y el metabolismo de la glucosa sigue siendo controvertida. Este estudio investiga la relación entre la AOS y la diabetes tipo 2 (DM) y prediabetes (preDM) incidentes, así como el efecto del tratamiento con CPAP (presión positiva continua en la vía aérea) a largo plazo.
MétodosEstudio de seguimiento en cohorte retrospectiva clínica de pacientes con AOS y controles seleccionados de manera aleatoria. Los datos sobre DM incidente y preDM, así como sobre la CPAP se obtuvieron de los registros hospitalarios. La relación entre AOS basal y la DM incidente se examinó utilizando modelos de regresión de COX.
ResultadosDe un total de 356 pacientes, 169 con AOS y 187 controles fueron seguidos por una mediana de 98 meses; 47 enfermos (13,2%) desarrollaron DM y 43 (12,1%) preDM. La incidencia acumulada a los cinco años de DM fue de 10,7% (6,5–13,9%). De los sujetos con preDM en la muestra basal, 87% evolucionaron a DM incidente. Se demuestra que el índice de masa corporal (IMC), la hipoxia nocturna y el índice de apnea hipopnea (IAH) son factores de riesgo para el desarrollo de DM, y que la CPAP los disminuye.
ConclusionesLos pacientes con AOS tienen mayor riesgo de desarrollar DM. Los factores de riesgo implicados son el IMC, la hipoxia nocturna y el IAH. El uso regular de CPAP a largo plazo se asoció con una disminución del riesgo.
Obstructive sleep apnoea (OSA) is a disorder characterised by episodes of total or partial occlusion of the upper airway, which presents with hypoxaemia, sleep fragmentation and excessive daytime sleepiness (EDS).1 The standard treatment for OSA is continuous positive airway pressure (CPAP) which prevents airway obstruction and reduces the clinical impact of the disease.2
Several studies have shown that OSA is associated with type 2 diabetes mellitus (DM)3 with obesity being a common factor for the development of both conditions.4 The association between OSA and DM increases the risk of cardiovascular disease, partly due to the coexistence of vascular risk factors such as hypertension (HT) and/or dyslipidaemia.5,6
Several meta-analyses show that the presence of OSA is associated with an increased risk of developing DM,7–9 although there is debate as to whether it increases the risk of incident prediabetes (preDM).10 Also, there is no conclusive evidence on the parameters associated with the risk of developing DM or pre-DM in people with OSA.8 Furthermore, CPAP has not been consistently shown to improve glycaemic control or reduce insulin resistance. While some studies do not demonstrate an association,11,12 other do.13,14 Further research is needed to clearly establish whether CPAP could have a clinically relevant and independent effect on metabolic disorders.15 This study aims to analyse the connection between OSA and DM in a clinical cohort of patients and to evaluate the effect of long-term CPAP therapy.
MethodologyDesignFollow-up study of a retrospective cohort of patients. The study included patients referred to the Hospital Clínico Universitario de Santiago for suspected OSA who underwent respiratory polygraphy (RP) to confirm/rule out this disease. The methodology used to select the sample, as well as the variables collected, has been described in a previous study.16 The study was approved by the hospital's Ethics Committee (registration code: 2019/219).
Medical history and biochemical analysisDemographic variables such as age, gender and body mass index (BMI) were recorded, as well as personal history, including the presence of HT, smoking and alcohol habits and pre-DM. Comorbidity was assessed using the Charlson index. Symptoms obtained from the medical records such as snoring, witnessed respiratory pauses, morning headache and EDS were assessed.
Laboratory variables such as glucose, urea, creatinine, glycosylated haemoglobin (HbA1c), total cholesterol, low-density lipoprotein (LDL), high-density lipoprotein (HDL), triglycerides and thyroid stimulating hormone (TSH) were recorded (data closest to the date of the RP were chosen).
Respiratory polygraphy and associated variablesRecording was performed using the Embletta® polygraph system with Embla® software (Embla Systems Kanata,Canada) RemLogic™ which monitors nasal flow, oxygen saturation (SaO2), chest movements, actigraphy, snoring sound and position. Apnoea was defined as an interruption of airflow of at least 10 s and hypopnoea as a decrease in airflow of at least 10 s combined with a desaturation ≥3% compared to pre-event baseline value during sleep. The frequency of obstructive episodes was reported with the apnoea hypopnoea index (AHI). OSA was considered to be the presence of an AHI ≥ 15/h, predominantly obstructive, or the observation of an AHI ≥ 5/h accompanied by one or more of the following: EDS, unrefreshing sleep, excessive tiredness and/or impaired sleep-related quality of life not justifiable by other causes.16,17 If the AHI in the RP was <5, the test was considered negative. The severity of OSA was expressed according to the AHI: mild for values between 5 and 14, moderate between 15 and 30 and severe over 30. In addition to the AHI, the total number of obstructive apnoeas (OA) during the recording, the total number of hypopnoeas, the mean and minimum SaO2, the hourly O-desaturation index2 (ODI) and the cumulative time with SaO2 below 90% (CT90) were taken into account.
Continuous positive airway pressure therapyCPAP therapy was prescribed based on standard criteria and guidelines for people with moderate or severe OSA and for patients with mild OSA, EDS or other significant symptoms or risks.17
A qualified sleep technician instructed the CPAP therapists on device management and the physician provided routine care. CPAP usage data was obtained from the electronic health management system and downloaded at each clinic visit to be collected, as well as leakage and residual AHI.
Compliance was considered to be an average use of 4 h/night in more than 70% of these during the entire follow-up period. Subjects who refused CPAP use at the time of OSA diagnosis, those with less than usual management or who discontinued CPAP use during the course of monitoring in the sleep clinic, and those who dropped out of observation were classified as "untreated".
Diabetes or pre-diabetes. Diagnostic criteriaDM was defined as the presence of fasting plasma glucose levels ≥126 mg/dL and/or HbA1c ≥ 6.5, and preDM as fasting plasma glucose levels between 100 and 125 mg/dL and/or HbA1c of 5.7–6.4.18
Data analysisData are presented as mean (standard deviation [SD]) for normal distribution variables and median (range) for non-normal. Between-group comparisons were performed with Student's t-test and Mann Whitney U-test, as appropriate. Categorical parameters were cross-checked with an X-test2. The AHI was treated as a continuous, categorical variable.
We used multivariable Cox regression models to investigate the relationships between predictors related to OSA and DM incidence and expressed the results as hazard ratios.
Statistical analyses were conducted using Statistical Package for the Social Sciences (SPSS) for Windows (version 11.5.1; SPSS, Chicago, IL, USA).
ResultsOf the 416 patients selected between March 2011 and August 2013, 60 patients diagnosed with DM were withdrawn. Thus, 169 of the case group and 187 controls underwent follow-up to June 2020.
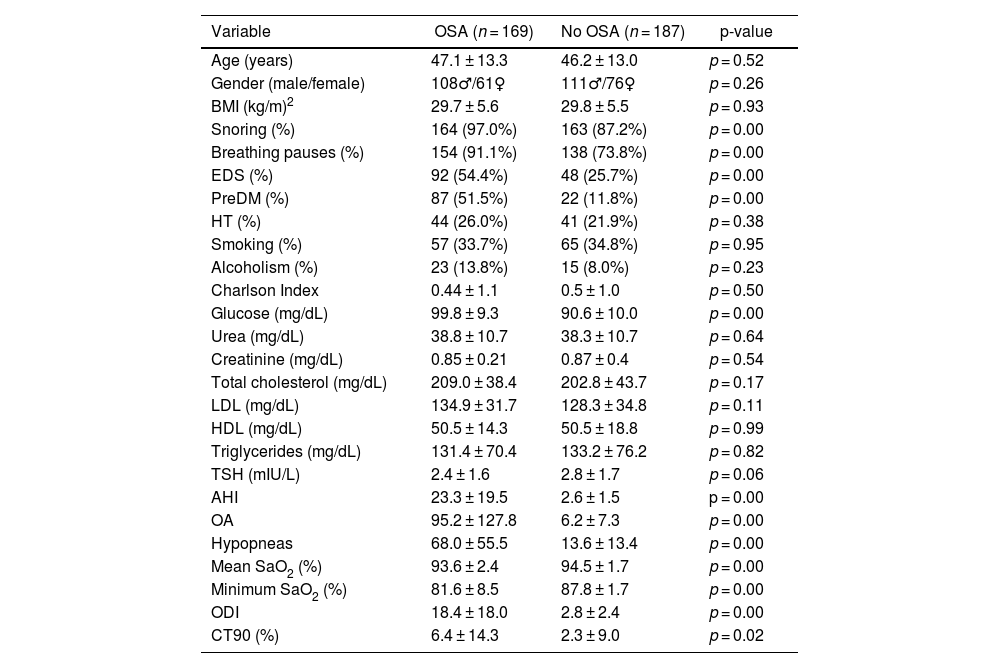
Demographic characteristics, reported symptoms, comorbidities, laboratory and polygraphic data of patients with and without OSA are shown in Table 1. No differences were found between the groups with respect to age, gender and BMI. As expected, cases had significantly higher percentages of snoring, respiratory pauses, EDS and higher preDM and glucose levels. AHI, ODI, OA, hypopneas and CT90 had significantly higher values in the study group than in the control group. Mean SaO2 (%) and minimum SaO2 (%) were lower in the study group.
Patient characteristics.
| Variable | OSA (n = 169) | No OSA (n = 187) | p-value |
|---|---|---|---|
| Age (years) | 47.1 ± 13.3 | 46.2 ± 13.0 | p = 0.52 |
| Gender (male/female) | 108♂/61♀ | 111♂/76♀ | p = 0.26 |
| BMI (kg/m)2 | 29.7 ± 5.6 | 29.8 ± 5.5 | p = 0.93 |
| Snoring (%) | 164 (97.0%) | 163 (87.2%) | p = 0.00 |
| Breathing pauses (%) | 154 (91.1%) | 138 (73.8%) | p = 0.00 |
| EDS (%) | 92 (54.4%) | 48 (25.7%) | p = 0.00 |
| PreDM (%) | 87 (51.5%) | 22 (11.8%) | p = 0.00 |
| HT (%) | 44 (26.0%) | 41 (21.9%) | p = 0.38 |
| Smoking (%) | 57 (33.7%) | 65 (34.8%) | p = 0.95 |
| Alcoholism (%) | 23 (13.8%) | 15 (8.0%) | p = 0.23 |
| Charlson Index | 0.44 ± 1.1 | 0.5 ± 1.0 | p = 0.50 |
| Glucose (mg/dL) | 99.8 ± 9.3 | 90.6 ± 10.0 | p = 0.00 |
| Urea (mg/dL) | 38.8 ± 10.7 | 38.3 ± 10.7 | p = 0.64 |
| Creatinine (mg/dL) | 0.85 ± 0.21 | 0.87 ± 0.4 | p = 0.54 |
| Total cholesterol (mg/dL) | 209.0 ± 38.4 | 202.8 ± 43.7 | p = 0.17 |
| LDL (mg/dL) | 134.9 ± 31.7 | 128.3 ± 34.8 | p = 0.11 |
| HDL (mg/dL) | 50.5 ± 14.3 | 50.5 ± 18.8 | p = 0.99 |
| Triglycerides (mg/dL) | 131.4 ± 70.4 | 133.2 ± 76.2 | p = 0.82 |
| TSH (mIU/L) | 2.4 ± 1.6 | 2.8 ± 1.7 | p = 0.06 |
| AHI | 23.3 ± 19.5 | 2.6 ± 1.5 | p = 0.00 |
| OA | 95.2 ± 127.8 | 6.2 ± 7.3 | p = 0.00 |
| Hypopneas | 68.0 ± 55.5 | 13.6 ± 13.4 | p = 0.00 |
| Mean SaO2 (%) | 93.6 ± 2.4 | 94.5 ± 1.7 | p = 0.00 |
| Minimum SaO2 (%) | 81.6 ± 8.5 | 87.8 ± 1.7 | p = 0.00 |
| ODI | 18.4 ± 18.0 | 2.8 ± 2.4 | p = 0.00 |
| CT90 (%) | 6.4 ± 14.3 | 2.3 ± 9.0 | p = 0.02 |
♂: male, ♀: female; AHI: apnoea hypopnoea index; BMI: body mass index; CT90: recording time with SaO2 below 90%; DM: diabetes mellitus; EDS: excessive daytime sleepiness; HDL: high-density lipoprotein; HT: hypertension; LDL: low density lipoprotein; OA: obstructive apnoeas; ODI: oxygen desaturation index; OSA: obstructive sleep apnoea; TSH: thyroid stimulating hormone.
At five years follow-up, 38 of the 356 patients had developed DM, giving a cumulative DM incidence of 10.7 (95% confidence interval [CI] [6.5–13.9]). During a complete follow-up, with a median of 98 months (interquartile range [IQR] 86–103), 47 users (13.2%) experienced DM and 43 pre-DM (12.1%). Of the 47 who developed DM, 42 had OSA (89.4%) and five (10.6%) did not. Of the 43 who had pre-DM, 35 had OSA (81.4%) and eight (18.6%) did not. Eighteen patients died (58.7 ± 10.6 years, BMI 31.1 ± 9.5 kg/m3, 13 males and five females), eight from neoplasm, five from respiratory infection, two from traumatic brain injury, two from stroke and one from heart failure. Of these, 10 had OSA (five on CPAP treatment) and eight were controls. Of note was the presence of nocturnal desaturation with mean saturations below 90% and/or CT90 > 30% in nine patients (50%).
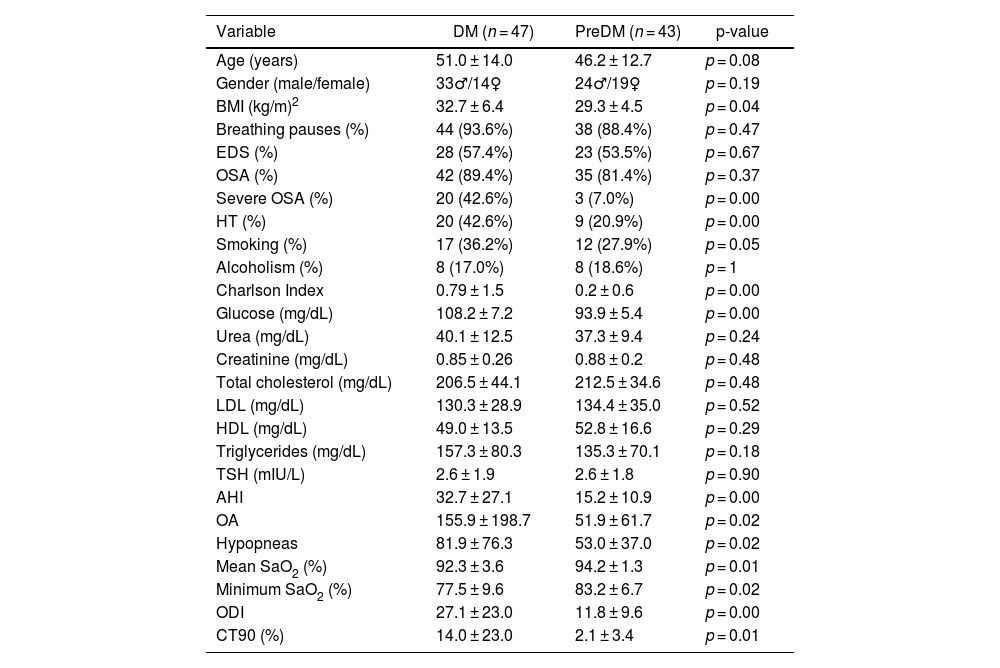
The demographic characteristics, reported symptoms, comorbidities, laboratory and polygraphic data of the users who developed DM or preDM are shown in Table 2. BMI was significantly higher in the DM group, with no differences with respect to age and gender. No differences were observed in the clinical symptomatology of OSA, such as snoring, respiratory pauses or EDS, nor in the percentage of OSA. Severe OSA was significantly more common in the group of patients with DM, as well as the presence of AHT and smoking. Existing comorbidity, according to the Charlson index, was higher in those with DM than in the pre-DM group. Glucose, as expected, was significantly higher in the DM group than in the pre-DM group. Also, AHI, OA, mean SaO2, ODI and CT90 were significantly higher in the DM group. Mean SaO2 (%) and minimum SaO2 (%) were lower in the DM group.
Characteristics of patients who develop DM or pre-DM during follow-up.
| Variable | DM (n = 47) | PreDM (n = 43) | p-value |
|---|---|---|---|
| Age (years) | 51.0 ± 14.0 | 46.2 ± 12.7 | p = 0.08 |
| Gender (male/female) | 33♂/14♀ | 24♂/19♀ | p = 0.19 |
| BMI (kg/m)2 | 32.7 ± 6.4 | 29.3 ± 4.5 | p = 0.04 |
| Breathing pauses (%) | 44 (93.6%) | 38 (88.4%) | p = 0.47 |
| EDS (%) | 28 (57.4%) | 23 (53.5%) | p = 0.67 |
| OSA (%) | 42 (89.4%) | 35 (81.4%) | p = 0.37 |
| Severe OSA (%) | 20 (42.6%) | 3 (7.0%) | p = 0.00 |
| HT (%) | 20 (42.6%) | 9 (20.9%) | p = 0.00 |
| Smoking (%) | 17 (36.2%) | 12 (27.9%) | p = 0.05 |
| Alcoholism (%) | 8 (17.0%) | 8 (18.6%) | p = 1 |
| Charlson Index | 0.79 ± 1.5 | 0.2 ± 0.6 | p = 0.00 |
| Glucose (mg/dL) | 108.2 ± 7.2 | 93.9 ± 5.4 | p = 0.00 |
| Urea (mg/dL) | 40.1 ± 12.5 | 37.3 ± 9.4 | p = 0.24 |
| Creatinine (mg/dL) | 0.85 ± 0.26 | 0.88 ± 0.2 | p = 0.48 |
| Total cholesterol (mg/dL) | 206.5 ± 44.1 | 212.5 ± 34.6 | p = 0.48 |
| LDL (mg/dL) | 130.3 ± 28.9 | 134.4 ± 35.0 | p = 0.52 |
| HDL (mg/dL) | 49.0 ± 13.5 | 52.8 ± 16.6 | p = 0.29 |
| Triglycerides (mg/dL) | 157.3 ± 80.3 | 135.3 ± 70.1 | p = 0.18 |
| TSH (mIU/L) | 2.6 ± 1.9 | 2.6 ± 1.8 | p = 0.90 |
| AHI | 32.7 ± 27.1 | 15.2 ± 10.9 | p = 0.00 |
| OA | 155.9 ± 198.7 | 51.9 ± 61.7 | p = 0.02 |
| Hypopneas | 81.9 ± 76.3 | 53.0 ± 37.0 | p = 0.02 |
| Mean SaO2 (%) | 92.3 ± 3.6 | 94.2 ± 1.3 | p = 0.01 |
| Minimum SaO2 (%) | 77.5 ± 9.6 | 83.2 ± 6.7 | p = 0.02 |
| ODI | 27.1 ± 23.0 | 11.8 ± 9.6 | p = 0.00 |
| CT90 (%) | 14.0 ± 23.0 | 2.1 ± 3.4 | p = 0.01 |
♂: male, ♀: female; AHI: apnoea hypopnoea index; BMI: body mass index; CT90: recording time with SaO2 below 90%; DM: diabetes mellitus; EDS: excessive daytime sleepiness; HDL: high-density lipoprotein; HT: hypertension; LDL: low density lipoprotein; OA: obstructive apnoeas; ODI: oxygen desaturation index; OSA: obstructive sleep apnoea; TSH: thyroid stimulating hormone.
Among the 169 patients with OSA, 45 (26.6%) started CPAP treatment. The mean pressure used was 7.8 ± 1.1 cm H2O and mean usage 6.0 ± 2.3 h. Of the 45 patients who complied with CPAP treatment, 15 developed DM (33.3%).
Table 3 relates the level of OSA severity to the prevalence of DM and preDM, showing an increase in the percentage of patients with DM as the level of OSA severity increases in the initial follow-up sample. Of the users with moderate-severe OSA, 34 (38.2%) developed DM, compared to eight (10%) with mild OSA and five (2.7%) without OSA. No association was found between OSA severity level and incident pre-DM. Of those with moderate-severe OSA, 18 (20.2%) developed pre-DM, compared to 17 (21%) in the mild OSA group.
Cumulative incidence of diabetes and pre-diabetes in relation to OSA severity level.a
| No OSA (n = 187) | Mild OSA (n = 80) | Moderate-severe OSA (n = 89) | |
|---|---|---|---|
| DM | 5 (2.7%) | 8 (10.0%) | 34 (38.2%) |
| PreDM | 8 (4.3%) | 17 (21.2%) | 18 (20.2%) |
OSA: obstructive sleep apnoea; DM: diabetes mellitus; preDM: prediabetes.
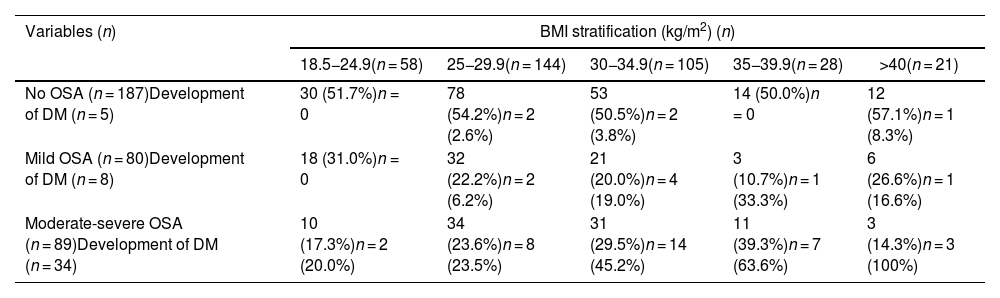
Table 4 relates different BMI levels to the severity of OSA (based on AHI levels) and to the development of DM. Subjects without OSA show no change in the prevalence of DM with respect to the different BMI strata. The prevalence of DM in mild OSA increases especially from a BMI of 30 kg/m2. As can be seen, it is with moderate-severe OSA that the prevalence of DM is very high, increasing progressively in comparison with BMI. In fact, 63% of patients with moderate-severe OSA and BMI between 35−39.9 kg/m2 and all patients with moderate-severe OSA and BMI over 40 kg/m2, suffer from DM.
Stratification of BMI, presence or absence of OSA and development of DM.
| Variables (n) | BMI stratification (kg/m2) (n) | ||||
|---|---|---|---|---|---|
| 18.5−24.9(n = 58) | 25−29.9(n = 144) | 30−34.9(n = 105) | 35−39.9(n = 28) | >40(n = 21) | |
| No OSA (n = 187)Development of DM (n = 5) | 30 (51.7%)n = 0 | 78 (54.2%)n = 2 (2.6%) | 53 (50.5%)n = 2 (3.8%) | 14 (50.0%)n = 0 | 12 (57.1%)n = 1 (8.3%) |
| Mild OSA (n = 80)Development of DM (n = 8) | 18 (31.0%)n = 0 | 32 (22.2%)n = 2 (6.2%) | 21 (20.0%)n = 4 (19.0%) | 3 (10.7%)n = 1 (33.3%) | 6 (26.6%)n = 1 (16.6%) |
| Moderate-severe OSA (n = 89)Development of DM (n = 34) | 10 (17.3%)n = 2 (20.0%) | 34 (23.6%)n = 8 (23.5%) | 31 (29.5%)n = 14 (45.2%) | 11 (39.3%)n = 7 (63.6%) | 3 (14.3%)n = 3 (100%) |
BMI: body mass index; DM: diabetes mellitus; OSA: obstructive sleep apnoea.
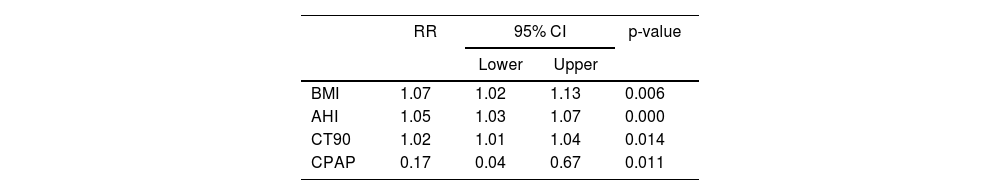
Table 5 shows that the risk factors for developing DM were BMI, AHI, CT90 and CPAP treatment. While the first three variables increased the likelihood of DM, CPAP treatment decreased it. Applying CPAP therapy reduced the risk of DM by 83% compared to those who did not receive it.
Model fit and effect of predictors expressed as RR and 95% CI for DM.
| RR | 95% CI | p-value | ||
|---|---|---|---|---|
| Lower | Upper | |||
| BMI | 1.07 | 1.02 | 1.13 | 0.006 |
| AHI | 1.05 | 1.03 | 1.07 | 0.000 |
| CT90 | 1.02 | 1.01 | 1.04 | 0.014 |
| CPAP | 0.17 | 0.04 | 0.67 | 0.011 |
AHI: apnoea hypopnoea index; BMI: body mass index; CI: confidence interval; CPAP: continuous positive airway pressure; CT90: recording time with SaO2 below 90%; DM: diabetes mellitus; RR: relative risk.
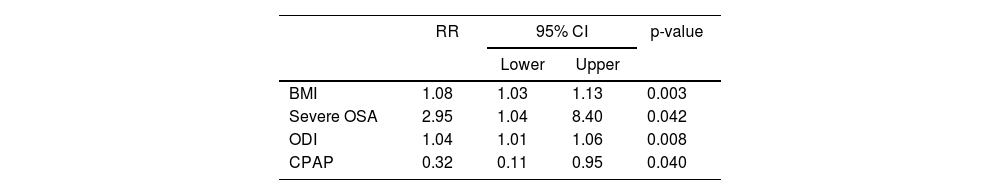
Table 6 shows a second model where severe OSA is included as a risk factor, with a relative value of 2.95 (95% CI [1.4–8.4]). BMI and ODI are also added. CPAP treatment reduces the risk of developing DM by 68% compared to those who did not receive CPAP.
Model fit and effect of predictors expressed as RR and 95% CI for DM.
| RR | 95% CI | p-value | ||
|---|---|---|---|---|
| Lower | Upper | |||
| BMI | 1.08 | 1.03 | 1.13 | 0.003 |
| Severe OSA | 2.95 | 1.04 | 8.40 | 0.042 |
| ODI | 1.04 | 1.01 | 1.06 | 0.008 |
| CPAP | 0.32 | 0.11 | 0.95 | 0.040 |
Severe OSA: AHI ≥ 30.
AHI, apnoea hypopnoea index; BMI, body mass index; CI, confidence interval; CPAP, continuous positive airway pressure; DM, diabetes mellitus; OSA, obstructive sleep apnoea; ODI, oxygen desaturation index; RR, relative risk.
Our results show that patients with OSA are more likely to have DM and preDM and that BMI, AHI, CT90 and ODI are risk factors for incident DM. These findings are consistent with several recent prospective studies8,19,20 and meta-analyses.9,21
OSA leads to several physiological disorders, such as intermittent hypoxia, sleep fragmentation and increased autonomic tone. These disorders have been associated with insulin resistance and DM in animal and human studies. Intermittent hypoxia plays a key role in the pathogenesis of OSA and experimental research indicates that it leads to attenuation of glucose-induced insulin secretion from pancreatic β cells and increased insulin resistance in peripheral cells and tissues. The combination of sleep disruption and a hyperactivated sympathetic system also increases the release of catecholamines by the hypothalamic-pituitary-adrenal axis, partly due to elevated levels of norepinephrine which mediates the secretion of corticotropin-releasing factor. Increased secretion of this component causes an increase in glucocorticoid levels, which promote gluconeogenesis in the liver and reduce glucose uptake and utilisation in skeletal muscle and white adipose tissue by antagonising the insulin response. This results in hyperglycaemia and insulin resistance.22,23
Our study, with a median follow-up of 98 months, found that 13.2% of patients developed DM, with a five-year cumulative incidence of 10.7% (6.5–13.9%), similar to other series. In a Canadian study, with a median follow-up of 67 months, 11.7% users developed DM with a 5-year cumulative incidence of 9.1%.20 In another study of 1206 patients with a median follow-up of 7.3 years, 12.6% developed DM.8 Another series with a longer median follow-up than ours (13 years) found a higher percentage of incident DM (19.6%).24 A recent study with five years of follow-up showed that 14.5% of users developed incident DM.19 Finally, in a Korean study of 1216 subjects, the cumulative incidence was 7.9% at four years and 14% at eight years.25
Several series have shown an increased risk of developing DM in patients with severe OSA. Kendzerska et al. found that patients with severe OSA were 30% more likely to develop DM than those without OSA.20 These increased in other studies to 71%.24 In our study, 38% of users with moderate-severe OSA had DM compared to 10% with mild OSA and 2.7% without OSA. The relative risk of developing DM in people with severe OSA was 2.95 (95% CI [1.04–8.40]); p = 0.042], similar to those reported by Xu et al. who found risk ratios of 2.01 (95% CI [1.06–3.81]) in moderate OSA and 2.62 (95% CI [1.40–4.93]) in severe OSA.8 The study by Siddiquee et al. found that participants with moderate-severe OSA had a 1.5-fold increased risk of developing DM at the end of the eight-year follow-up, after adjusting for possible covariates.25
Although several recent studies link OSA to incident DM,3,9,21 there is, however, little information regarding the association between OSA and incident preDM. In our series, 12.1% went on to develop pre-DM after a median follow-up of 98 months and, in contrast to DM, we found no connection between OSA severity and incident pre-DM. However, we did find that preDM is a situation that many people suffer from and subsequently go on to develop DM, with OSA being a factor that may be involved. In fact, 41 of the 47 patients with incident DM (87.2%) had preDM in the initial sample. Of these 41, 38 had OSA. This progression from pre-DM to DM has also been observed in other studies.9,26 This emphasises the importance of preDM and identifies the links with OSA. Of the 38 patients with OSA, 11 (30%) were already on CPAP treatment but still progressed to DM. It is therefore possible that they could benefit from oral antidiabetic treatment. Only one of the patients was on metformin therapy. Studies on this topic are scarce. Tang et al., in 36 patients and a 24-month follow-up, found that the combination of dapagliflozin and metformin optimised glycaemic control in users with OSA and DM, as well as improving OSA-associated symptomatology. In another study, metformin improved glycaemic control but did not help in the management of OSA.27,28 Also noteworthy is the greater presence of comorbidity according to the Charlson index and cardiovascular disease, such as hypertension, in the incident DM group compared to the incident pre-DM group. Therefore, treatment of OSA is an essential factor in preventing metabolic and cardiovascular pathology. Among the treatments, CPAP is the one that has shown a clear benefit in these patients.2
Regular use of CPAP, which was achieved in approximately one third of subjects with moderate-severe OSA, was associated with a reduction in incident MD.8 In our study, 26% completed CPAP treatment during the entire follow-up, according to the indications of our guidelines.17 This therapy was associated with a reduced risk of DM in our patients.
Several studies have reported beneficial metabolic effects of CPAP in patients with high adherence to therapy and in the very short term.29–31 Recently, in a long-term, five-year follow-up study, CPAP was found to reduce the development of DM in people with OSA.32 However, in a study by Loffler et al. with 888 participants and a median follow-up of 4.3 years, there was no evidence that CPAP therapy affected glycaemic control in those with DM or preDM compared to standard treatment.12 Multiple factors may contribute to the heterogeneity of results, including different levels of CPAP compliance or different CPAP therapy duration.
There are some limitations to be considered in the interpretation of our findings. The number of patients treated with CPAP is not large and the sample size for this analysis may be small. Moreover, generalisation of the results should be done with caution as this is a single-centre study. Finally, assessment of OSA severity based on AHI alone does not reflect the heterogeneity of the disease, although we also included CT90 as a reflection of hypoxaemia, obesity as measured by BMI, and comorbidities.
Ultimately, our study shows that the presence of OSA is a risk factor for incident DM, which increases in patients with severe OSA and decreases after CPAP treatment in moderate and severe cases. These findings suggest that healthcare providers working in the field of diabetes and OSA should screen users who already have one condition for the presence of the other condition. Early intervention may prevent cardiovascular events through early diagnosis of OSA in people with DM or preDM and reduce morbidity in this population.
Ethical considerationsInformed consent according to the opinion of the Santiago-Lugo Research Ethics Committee. Registration Code: 2019/219.
FundingThis study has not received any funding.
Conflict of interestThe authors declare that they have no conflicts of interest.
Romina Abelleira. Conception and design of the study. Author. Data collection. Data analysis and interpretation. Critical revision of the article. Final approval of the manuscript.
Carlos Zamarrón. Data collection. Conception and design of the study. Author. Analysis and interpretation of the data. Critical revision of the article. Final approval of the manuscript.
Vanessa Riveiro. Data collection. Data analysis and interpretation. Critical revision of the article. Final approval of the manuscript.
Ana Casal. Data collection. Data analysis and interpretation. Critical revision of the article. Final approval of the manuscript.
María Elena Toubes. Data collection. Data analysis and interpretation. Critical revision of the article. Final approval of the manuscript.
Carlos Rábade. Data collection. Data analysis and interpretation. Critical revision of the article. Final approval of the manuscript.
Jorge Ricoy. Data collection. Data analysis and interpretation. Critical revision of the article. Final approval of the manuscript.
Adriana Lama. Data collection. Data analysis and interpretation. Critical revision of the article. Final approval of the manuscript.
Nuria Rodríguez-Núñez. Data collection. Data analysis and interpretation. Critical revision of the article. Final approval of the manuscript.
Lucía Ferreiro. Data collection. Data analysis and interpretation. Critical revision of the article. Final approval of the manuscript.
Juan Rodríguez Ozores. Data collection. Data analysis and interpretation. Critical revision of the article. Final approval of the manuscript.
Luis Valdés. Conception and design of the study. Author. Analysis and interpretation of the data. Critical revision of the article. Final approval of the manuscript.