The Spanish Antibiogram Committee (Comité Español del Antibiograma, COESANT) presents in this document a series of recommendations intending to unify how cumulative antibiogram reports must be made in Clinical Microbiology Spanish laboratories. This article is based on the information included in the Clinical Microbiology Procedure No. 51, «Preparation of cumulative reports on antimicrobial susceptibility» of the Spanish Society of Infectious Diseases and Clinical Microbiology (SEIMC), published in 2014. The recommendations also include the modifications in the definition of clinical interpretive categories recently published by the European Committee on Antimicrobial Susceptibility Testing (EUCAST) in 2019. Its final objective is to establish a homogeneous way of preparing these summaries to compare results from different centers or aggregate the information from these in order to carry out an adequate local or even national surveillance regarding the evolution of antimicrobial susceptibility.
El Comité Español del Antibiograma (COESANT) presenta en este documento una serie de recomendaciones cuya finalidad es unificar la forma en la que los Servicios y Unidades de Microbiología Clínica españoles realizan los informes de sensibilidad acumulada de las bacterias, aisladas en muestras clínicas, frente a los antimicrobianos. Las recomendaciones se fundamentan en las recogidas en el Procedimiento de Microbiología Clínica nº 51, «Preparación de informes acumulados de sensibilidad a los antimicrobianos» de la Sociedad Española de Enfermedades Infecciosas y Microbiología Clínica (SEIMC), publicado en 2014, y recoge las modificaciones en las definiciones de las interpretaciones de las categorías clínicas publicadas en el año 2019 por el European Committee on Antimicrobial Susceptibility Testing (EUCAST). Su objetivo final es establecer una forma homogénea de elaborar estos resúmenes para poder comparar resultados de diferentes centros o sumar su información y así realizar una adecuada vigilancia local o incluso nacional de la evolución de la sensibilidad a los antimicrobianos.
The joint analysis of data from the study of the antimicrobial susceptibility of bacteria isolated in microbiology laboratories is of great epidemiological and clinical value since it provides knowledge of temporal variations in susceptibility trends and is of great help in selection of empiric treatment in infected patients1–4. The need to understand the proportion of microorganisms that are susceptible to antimicrobials, together with the rise in multi-resistance in recent years, makes global surveillance that goes beyond the limits of a single centre essential. Examples of this are the Red de Vigilancia Microbiológica [Microbiological Surveillance Network] of the Comunitat Valenciana (RedMIVA)5, the Vigilancia de la Infección Nosocomial en Cataluña [Surveillance of Hospital-acquired Infection in Catalonia] programme (VINCat)6 or the recent creation of the Red de Laboratorios para la Vigilancia de los Microorganismos Resistentes [Network of Laboratories for the Surveillance of Resistant Microorganisms] (RedLabRA)7 set up as part of the Plan Nacional frente a la Resistencia a Antibióticos [National Plan against Antibiotic Resistance] (PRAN).
The general recommendations contained in Clinical Microbiology Procedure No. 51, “Preparation of cumulative reports on antimicrobial susceptibility” of the Sociedad Española de Enfermedades Infecciosas y Microbiología Clínica [Spanish Society of Infectious Diseases and Clinical Microbiology] (SEIMC)8–10, published in 2014 are still in force and are summarised in this article, with some modifications derived mainly from the new guidelines established by the European Committee on Antimicrobial Susceptibility Testing (EUCAST) in 2019 regarding the definitions of the clinical categories used in the interpretation of the susceptibility results (Table 1). Additionally, the key points to be taken into account when preparing the cumulative susceptibility report will be referred to, as will a series of relevant statistical aspects that should be considered.
Ten fundamental steps to be taken into account when preparing the cumulative susceptibility report.
| 1. Adhere to the EUCAST recommendations regarding the performance of antibiograms and their interpreted reading. |
| 2. If only one category is reported, combine S and I, since it provides clearer information regarding the choice of empiric treatment. Additionally report the percentage of presence of resistance mechanisms of epidemiological interest. |
| 3. Submit the report at least once a year. |
| 4. Include only results validated by the clinical microbiologist. |
| 5. Include only isolates from samples sent for clinical diagnosis of infections. |
| 6. Report susceptibility results at species level, including only those with ≥30 isolates, specifying the absolute number of isolates. |
| 7. Eliminate duplicates, including only the first isolate of a species for each patient. |
| 8. Include antimicrobials that are routinely reported in the clinical susceptibility report. |
| 9. In the case of antibiotic-microorganism combinations whose result depends on the original clinical picture, specify all assumptions. |
| 10. Precisely detail the calculation algorithm used to generate the accumulated report. |
- 1
Adhere to the EUCAST11,12 recommendations on the performance of antibiograms and their interpreted reading to guarantee standardisation and homogeneity in the methodology followed in the susceptibility study.
- 2
Although in susceptibility summaries it has been common practice to combine the “Resistant” and “Intermediate” clinical categories as non-susceptible, since the redefinition of clinical categories carried out in 2019 by the EUCAST13,14, this is no longer appropriate. The definition of the term “Intermediate” has been modified and is now considered “I” (Susceptible, increased exposure), with the only difference between “S” (Susceptible, standard dosing regimen) and “I” being the amount of drug that is required at the site of infection to achieve an adequate clinical response. It is recommended that the three categories be presented independently and, if necessary, combining S and I and making a note at the foot of the table in cases in which there are two dosages (Tables 2 and 3), since it provides clearer clinical information regarding the choice of empiric treatment. In some microorganisms it is useful to also report the percentage of presence of resistance mechanisms of special epidemiological interest. For instance, “Percentage of patients with extended-spectrum β-lactamase (ESBL)-producing Klebsiella pneumoniae”.
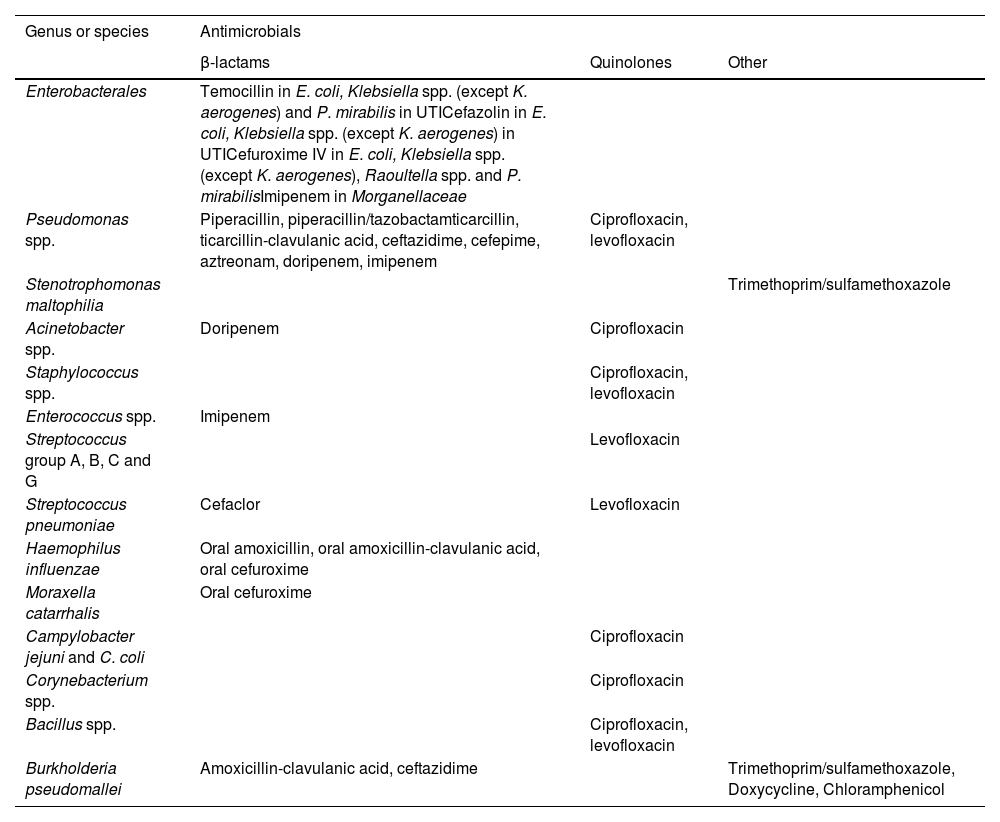
Table 2.Antimicrobials for which EUCAST has introduced breakpoints that classify wild-type microorganisms (organisms without phenotypically detected acquired resistance mechanisms for the agent) always as “Susceptible, increased exposure (I)" instead of “Susceptible, standard dosing regimen (S)”.23
Genus or species Antimicrobials β-lactams Quinolones Other Enterobacterales Temocillin in E. coli, Klebsiella spp. (except K. aerogenes) and P. mirabilis in UTICefazolin in E. coli, Klebsiella spp. (except K. aerogenes) in UTICefuroxime IV in E. coli, Klebsiella spp. (except K. aerogenes), Raoultella spp. and P. mirabilisImipenem in Morganellaceae Pseudomonas spp. Piperacillin, piperacillin/tazobactamticarcillin, ticarcillin-clavulanic acid, ceftazidime, cefepime, aztreonam, doripenem, imipenem Ciprofloxacin, levofloxacin Stenotrophomonas maltophilia Trimethoprim/sulfamethoxazole Acinetobacter spp. Doripenem Ciprofloxacin Staphylococcus spp. Ciprofloxacin, levofloxacin Enterococcus spp. Imipenem Streptococcus group A, B, C and G Levofloxacin Streptococcus pneumoniae Cefaclor Levofloxacin Haemophilus influenzae Oral amoxicillin, oral amoxicillin-clavulanic acid, oral cefuroxime Moraxella catarrhalis Oral cefuroxime Campylobacter jejuni and C. coli Ciprofloxacin Corynebacterium spp. Ciprofloxacin Bacillus spp. Ciprofloxacin, levofloxacin Burkholderia pseudomallei Amoxicillin-clavulanic acid, ceftazidime Trimethoprim/sulfamethoxazole, Doxycycline, Chloramphenicol UTI: Urinary tract infection.
Table 3.Antimicrobials for which there are two dosages and therefore the category of Sensitive to Increased Exposure (old intermediate).23
Genus or species Antimicrobials β-lactams Quinolones Other Enterobacterales Ticarcillin, ticarcillin-clavulanic acid, Cefepime and ceftazidimeCeftriaxone in an indication other than meningitisDoripenem, imipenem (enterobacterales except Morganellaceae), meropenem in an indication other than meningitisAztreonam Ciprofloxacin, levofloxacin, ofloxacin Trimethoprim/sulfamethoxazole Pseudomonas spp. Meropenem in an indication other than meningitis Acinetobacter spp. ImipenemMeropenem in an indication other than meningitis Levofloxacin Trimethoprim/sulfamethoxazole Staphylococcus spp. Ceftaroline in S. aureus and an indication other than pneumonia Clarithromycin, erythromycinroxithromycin Quinupristin/dalfopristinDoxycycline, tetracyclineTrimethoprim/sulfamethoxazole Enterococcus spp. Ampicillin, ampicillin/sulbactam, amoxicillin, and amoxicillin/clavulanic acid Quinupristin/dalfopristin in E. faecium Streptococcus group A, B, C and G Azithromycin, clarithromycin, erythromycinRoxithromycin, telithromycinTetracyclineTrimethoprim/sulfamethoxazole Streptococcus pneumoniae Benzylpenicillin, ampicillin, cefotaxime and ceftriaxone in indications other than meningitisAmoxicillin and amoxicillin-clavulanic acid, oralCefpodoxime and cefepimeCefuroxime, IV and oral Azithromycin, clarithromycin, erythromycinroxithromycin,telithromycinTetracycline, doxycyclineTrimethoprim/sulfamethoxazole S. viridans group Benzylpenicillin, ampicillin, amoxicillin Haemophilus influenzae Cefuroxime, IV DoxycyclineTrimethoprim/sulfamethoxazole Moraxella catarrhalis Cefixime, cefotaxime, ceftriaxone, cefuroxime, IV Azithromycin, clarithromycin, erythromycinroxithromycin, telithromycinDoxycyclineTrimethoprim/sulfamethoxazole Neisseria gonorrhoeae Benzylpenicillin Ciprofloxacin, ofloxacin Neisseria meningitidis Ampicillin and amoxicillin in an indication other than meningitis Helicobacter pylori Clarithromycin Corynebacterium spp. Rifampicin Aeromonas spp. Ceftazidime, cefepime, aztreonam, Ciprofloxacinlevofloxacin Trimethoprim/sulfamethoxazole Achromobacter xylosoxidans Meropenem UTI: urinary tract infection; IV: intravenous formulation.
Some antimicrobials such as teicoplanin, fosfomycin in intravenous formulation, fusidic acid and metronidazole have two doses in the EUCAST tables, but not an intermediate category (doses and/or breakpoints under review by EUCAST).
- 3
Submit the report at least once a year.
- 4
Include only results validated by the clinical microbiologist, not the raw data obtained from the antibiogram.
- 5
Include only isolates from samples sent for clinical diagnosis, excluding isolates from epidemiological surveillance cultures or environmental samples.
- 6
Report susceptibility results at species level, including only those with ≥30 isolates. The absolute number of isolates taken into account in the report must be indicated.
- 7
Eliminate duplicates following a strategy that fulfils clinical needs15. Only the first isolate of a determined species per patient and period of analysis should be included, regardless of the anatomical site of collection or antimicrobial susceptibility profile, as it helps to guide the selection of empiric treatments. This is a simple, more objective and reproducible method that averts biases introduced by the observer. However, in this way the level of resistance could be underestimated in species for which there is a risk of acquiring or developing them during treatment (Pseudomonas aeruginosa, which may exhibit significant heterogeneity in resistance phenotypes as a result of mutation, co-infection or colonisation with multiple clones). When this circumstance specifically is to be analysed specifically, it may be more useful to eliminate duplicates by excluding isolates with a false susceptibility change (erroneous S instead of R result; very major error) or false resistance change (R instead of S; major error) of an antibiotic among those previously defined as markers or key antibiotics in that bacterial species. The consideration of minor changes (S instead of I, I instead of S, I instead of R or R instead of I) is highly controversial because they may reflect phenotypic variants of expression or methodological problems. The criteria followed can therefore influence the final result, so it is important to detail in the report how duplicates were eliminated.
- 8
Antimicrobials that are routinely reported in the clinical susceptibility report should be included. Supplemental antibiotics included to detect resistance mechanisms in the context of the interpreted antibiogram reading should be excluded. For example, cefoxitin in Staphylococcus aureus, cefotaxime-clavulanic acid in Enterobacterales or norfloxacin in Streptococcus pneumoniae.
- 9
In the case of antibiotic-microorganism combinations whose result depends on the original clinical symptoms, it is recommended to specify all the assumptions (Escherichia coli and amoxicillin-clavulanic acid in urinary infection and Streptococcus pneumoniae and penicillin in meningitis or non-meningitis).
- 10
Precisely detail the calculation algorithm used to generate the accumulated report16,17 to facilitate the comparability of reports in different periods or from different centres. In other words, specify if categories have been added and indicate the method followed to eliminate duplicates when it is not the recommended ones.
- 1
Although there is no standardised model, it may be useful to use the table and/or figure format, in which microorganisms and antimicrobials are included, indicating the number of isolates included in each species and the corresponding susceptibility percentages, considering these as the sum of S+I, in order to reflect the percentages of microorganisms treatable with the antimicrobial studied instead of the detection of resistance mechanisms
- 2
Different criteria can be used to list the organisms in the report: alphabetically, by taxonomic group or by prevalence. The PNT-IASA-1 of procedure no. 51 of the SEIMC lists the minimum essential information that must be included in the report on microorganisms and antimicrobials of special epidemiological and clinical interest.
- 3
To reflect temporal trends in susceptibility, it is more useful to present the data in the form of a figure (histogram).
- 4
Use referenced abbreviations for antimicrobials in tables18.
- 5
As a rule, it is recommended that in the susceptibility tables at the very least the isolates obtained from blood cultures be separated from those obtained from urine cultures, following the same criteria recommended in the general guidelines for the elimination of duplicates.
- 6
Separate the isolates in the susceptibility tables according to the type of patient (hospitalised, Intensive Medicine, Paediatrics, Primary Care and others that may be of clinical interest)19–21.
- 7
Provide percentages of patients with microorganisms with resistance mechanisms of special clinical relevance, such as methicillin-resistant Staphylococcus aureus, glycopeptide-resistant enterococci, ESBL- or carbapenemase-producing enterobacteriaceae or multidrug-resistant or extensively drug-resistant Acinetobacter baumannii and Pseudomonas aeruginosa22.
The PNT-IASA-2 offers a tool to facilitate the calculation of the statistical values proposed in procedure no. 51 of the SEIMC in Excel spreadsheets (95% CI of the %S, comparison of percentages with the 95% CI of the difference) and trend analysis. The Excel workbook can be downloaded from the following link: www.seimc.org/contenidos/documentoscientificos/procedimientosmicrobiologia/seimc-procedimientomicrobiologia51.pdf
- 1
Susceptibility percentages allow for a better estimate of the true proportion in the actual population of isolates. The report does not need to include the values of the confidence intervals that indicate the accuracy of the percentage presented so as not to detract from the report's clarity. However, these values must be available to anyone who needs them through specific consultation with the Microbiology Department/Unit.
- 2
The statistical significance of the difference between susceptibility percentages from two time periods must be distinguished from clinical or epidemiological importance (or relevance).
- 3
The analysis of susceptibility trends over time presumes that the original database contains the real values of the minimum inhibitory concentrations (MIC) or halo diameter, in order that the results may be reinterpreted, given that the clinical cut-off points may vary throughout the period that is to be studied.
Traditionally, accumulated susceptibility reports have been presented in paper format, often in the form of triptychs so they can be consulted on a day-to-day basis by clinicians and thereby contribute to the adequacy of empiric treatments. With today’s new computer tools, these reports can be posted in different places or platforms such as on the hospital intranet or even in microbiological results viewers. Regarding the latter, when a microbiological result is issued with the identification of a microorganism for which the result of its antimicrobial susceptibility is not yet known, it is very useful to insert a link that leads directly to the hospital’s cumulative susceptibility report through which the clinician can ascertain the microorganism’s usual sensitivity and therefore choose the best empiric therapy for the microorganism in question more precisely and conveniently. This functionality is possible today with some laboratory computer systems and allows the accumulated susceptibility reports to be more practical and available to many more professionals.
ConclusionsThe need to understand the proportion of microorganisms that are susceptible to antimicrobials, together with the rise in multi-resistance in recent years, makes global surveillance that goes beyond the limits of a single centre essential.
In order to be able to work with data jointly, it is essential that susceptibility reports be prepared in a homogeneous and standardised way at all centres.
These reports must be readily accessible to professionals whose work is related to the use of antibiotics, thus contributing to a better optimisation of this type of treatment and the preservation of its activity, avoiding the generation of resistances due to inappropriate use.
FundingNo specific funding has been received for the preparation of this document. COESANT activities are partially funded by the Sociedad Española de Enfermedades Infecciosas y Microbiología Clínica (SEIMC) and the Agencia Española de Medicamentos y Productos Sanitarios (AEMPS).
The Plan Estatal de Investigación Científica y Técnica y de Innovación [State Plan for Scientific and Technical Research and Innovation] 2017-2020 and the Instituto de Salud Carlos III [Carlos III Health Institute], General Subdirectorate for Cooperative Research Networks and Centres, Ministry of Science and Innovation, funds research by MNL, RC, FFC, LMM and AO through the Red de Investigación en Patología Infecciosa [Infectious Pathology Research Network] (REIPI) and CIBER for Infectious Diseases (CIBERINFEC) and research by EC, JFD and JG through CIBER for Respiratory Diseases (CIBERES).
Conflicts of interestThe authors have no conflicts of interest to declare.