There are no agreed protocols on hospital management of hyperglycemic decompensation induced by pharmacological doses of glucocorticoids (GCs). The study objective was to assess the efficacy and safety of an insulin therapy protocol specific for patients treated with glucocorticoids (CP) as compared to a general protocol (GP) in diabetes decompensation secondary to glucocorticoids.
Materials and methodsAn experimental study in patients with glucocorticoids-induced decompensated diabetes admitted to a respiratory ward including a non-randomized control group. Two protocols (CP and GP), both based on basal-bolo insulin regimens, but with different insulin doses and distribution, were compared.
The difference in mean blood glucose (MBG) levels between both protocols was measured during hospital stay, as was the risk of having MBG levels>200mg/dL, adjusted for potential confounding factors (related to patients and to the glucocorticoid therapy used).
ResultsA total of 131 patients were included, 60 assigned to the GP and 71 to the CP groups. Seventy-four percent of patients had been admitted due to COPD exacerbation. There was a significant difference in the total daily insulin dose used between the CP and GP groups (29.4 vs. 57.4 IU; p<0.0001).
The adjusted difference in MBG levels (CP-GP) was −14.8 (95% CI, −26.2 to −3.3) mg/dL. Patients in the CP group had a lower adjusted risk of having MBG levels >200mg/dL during hospital admission (OR=0.31; 95% CI, 0.11–0.91; p=0.033). There were no differences in the risk of severe hypoglycemia between the CP and GP groups (0% vs. 1.4%; p=0.36).
ConclusionsThe study protocol has been shown to decrease MBG levels in patients with glucocorticoids-induced decompensation of diabetes during hospital admission without compromising their safety.
No existen protocolos consensuados de manejo hospitalario de las descompensaciones hiperglucémicas inducidas por dosis farmacológicas de glucocorticoides (GC). Nuestro objetivo fue evaluar la eficacia y la seguridad de un protocolo de insulinización específico para corticoides (PC) frente a un protocolo general (PG) en diabetes descompensada por GC (DDG).
Materiales y métodosEstudio experimental con grupo control, no aleatorizado, en pacientes con DDG ingresados en neumología. Se compararon 2 protocolos (PC y PG), ambos basados en terapia basal-bolo pero con diferentes dosis y distribución de insulina.
Se evaluó la diferencia de glucemia media (GM) durante la hospitalización entre el PC y el PG, así como el riesgo de presentar una GM > 200mg/dl, ajustado para potenciales factores de confusión (relacionados con el paciente y con la terapia de GC empleada).
ResultadosSe incluyó a 131 pacientes, 60 asignados al PG y 71 al PC. Un 74% de los pacientes estaban ingresados por exacerbación de enfermedad pulmonar obstructiva crónica. Hubo diferencia significativa en la dosis total de insulina entre el PG y el PC (29,4 vs. 57,4 unidades; p < 0,0001).
La diferencia ajustada de GM (PC-PG) fue de –14,8 (IC del 95%, –26,2 a –3,3) mg/dl. Los pacientes del PC tuvieron menor riesgo ajustado de presentar GM > 200mg/dl durante la hospitalización (OR = 0,31; IC del 95%, 0,11-0,91; p = 0,033). No hubo diferencias en el riesgo de hipoglucemia grave entre el PG y el PC (0% vs. 1,4%; p = 0,36).
ConclusionesEl protocolo estudiado ha demostrado reducir la GM de pacientes con DDG durante la hospitalización sin comprometer su seguridad.
Glucocorticoids (GCs), administered at supraphysiological doses, are potent therapeutic agents used in many diseases. One of the most common indications in acute situations is the management of exacerbated chronic obstructive pulmonary disease (COPD).1–3
Although these drugs are very useful in daily clinical practice, they are not without risks and side effects. The development of hyperglycemia in patients with no prior diagnosis of diabetes, or of hyperglycemic decompensation in patients with known diabetes, is the most common adverse effect. In a meta-analysis conducted by Liu et al., the incidence of hyperglycemia among all patients receiving corticosteroid treatment was found to be 32.3%, while 18.6% developed glucocorticoid-induced diabetes (GID).4 In a study specifically analyzing patients administered GCs for the treatment of respiratory disease, the incidence of GID was found to be 14.7%.5 Despite these data, the true frequency of GID may be underestimated, since it is conditioned to the criteria used to diagnose the disorder. Bearing in mind the mechanism of action of GCs (increased insulin resistance with a predominantly postprandial effect) and their use in many cases for short periods of time, it is better to base the diagnosis on the detection of glycemia≥200mg/dl at any time of day rather than on fasting glycemia or glycosylated hemoglobin (HbA1c).1,6,7
An association has been described between the development of hyperglycemia and the occurrence of complications upon hospital admission: increased nosocomial infections, a risk of developing diabetic ketoacidosis or hyperosmolar nonketotic syndrome, the prolongation of the hospital stay, and increased mortality.1,2,8 It has been established that for every 18mg/dl increase in blood glucose, the mortality rate increases by 10%.9 At present, acceptable control during hospital admission is defined as mean glycemia 140–180mg/dl.10
The effects upon carbohydrate metabolism depend on the type of GC, its dose and the administration regimen. In general, on administering a single morning dose of methylprednisolone or prednisone, peak hyperglycemia will be observed after the midday meal and before the evening meal, and fasting blood glucose monitoring may prove normal. However, when dexamethasone or multiple doses of methylprednisolone are administered, hyperglycemia will manifest similarly throughout the day. Therefore, the insulin dosing regimen should be adaptable to the type of GC and the administration regimen used, with due anticipation of the need for dose modification with the purpose of ensuring a good balance between the prevention of both hyperglycemia and hypoglycemia. Few data are available in the literature on the best therapeutic approach to secure good in-hospital glycemia control in patients treated with pharmacological doses of GCs. Although mean glycemia (MG)<180mg/dl during admission defines good glycemic control, values<200mg/dl may be considered acceptable in complex patients.11,12
The objective of the present study was to explore the efficacy and safety of a new protocol for the management of diabetes decompensation secondary to glucocorticoids (DDG) in the course of hospital admission.
Material and methodsStudy designA prospective, non-randomised, control group intervention study was carried out to assess the efficacy of an insulin treatment protocol in patients with DDG during their hospital stay. The study was conducted between 4 October 2015 and 6 April 2017 in patients admitted to the Pneumology ward of a third-level hospital. Up until 16 October 2016 we collected data from the patients in the control group, and from that date until study closure, we collected data from the patients in the intervention group. The duration of the intervention was the same as that of admission to hospital, though only the data corresponding to the first 15days of admission were collected.
Ethical considerationsInformed consent was obtained from all patients after we had described the treatment they would be receiving, and emphasized that the study involved a modification of the standard in-hospital insulin administration protocol. The study was approved by the Research Ethics Committee of Aragón (Ref. 18/2014).
Study populationInclusion criteria: a previous diagnosis of type 2 diabetes, age over 18 years, GC treatment at an initial dose equivalent to ≥0.5mg/kg/day of methylprednisolone, and an expected hospital stay of more than three days.
Exclusion criteria: pregnant women, patients with a glomerular filtration rate (GFR)<15ml/min/1.73m2 and ketoacidosis or hyperosmolar nonketotic syndrome upon admission.
Clinical endpointsPrimary: Mean glycemia during admission.
Secondary:
- –
The standard deviation (SD) of all glycemia values during admission.
- –
The risk of presenting MG during hospitalisation>180mg/dl.
- –
The risk of presenting MG during hospitalisation>200mg/dl.
Demographic: age, gender, patient origin.
Anthropometric: weight and height, with calculation of the body mass index (BMI) as weight in kg/height in m2.
Clinical: personal history (arterial hypertension, dyslipidemia, chronic kidney disease, neoplasms, stroke, ischemic heart disease), previous treatment for diabetes (classified into two categories according to whether insulin was included or not), blood pressure upon admission, the Charlson comorbidity index, the total daily insulin dose (TDD) during hospitalization (basal and prandial), the maximum insulin dose per kilogram body weight and day reached, the type and daily doses of GCs (the different types of GCs were transformed into their equivalent in mg of methylprednisolone, as this is the most frequently used drug), and complications during admission.
Laboratory tests: plasma glucose, creatinine (with calculated GFR in ml/min/1.73m2 based on the CKD-EPI formula) and HbA1c (obtained during admission or in the previous 3 months).
Related to glycemia control during admission: capillary blood glucose values were recorded to determine the following parameters as measures used to assess in-hospital glycemic control: maximum glycemia (the highest capillary blood glucose value recorded during admission), MG during admission (defined as the mean of all capillary blood glucose values of the patient), the standard deviation (SD) of all capillary blood glucose values of the patient and the coefficient of variation, defined as the ratio between SD and MG (SD/MG). Good control was defined as MG≤180mg/dl, and acceptable control as MG≤200mg/dl. We recorded the number of hypoglycemic episodes (<70mg/dl) and their severity (severe hypoglycemia being defined as <40mg/dl or accompanied by a loss of consciousness).
Glycemic control interventionControl group. Upon admission, all oral antidiabetic drugs (OADs) and premixed insulins were discontinued, and treatment involving a corrective regimen was decided upon, with basal insulin plus a corrective regimen, or with basal-bolus treatment, following the general hyperglycemia management protocol (GP) used at our hospital.13 In both groups the insulins used were insulin glargine or detemir as basal replacement insulins, and insulin aspart as prandial and corrective insulin. When basal insulin was given in two doses it consisted of insulin detemir, while insulin glargine was used in the case of a single daily dose. The pneumologist in charge of the patient could apply the GP personally or request collaboration from the Endocrinology Department. Collaboration from Endocrinology was requested in 45% of the control patients.
Intervention group. All OADs were suspended in the patients in the experimental (intervention) group, and a new insulin utilization protocol adapted to corticosteroid treatment (CP) was introduced, supervised by a member of the Endocrinology Department. Like the GP, the CP was based on the use of basal-bolus therapy, but with higher insulin doses and with 5 daily capillary blood glucose controls (fasting, before lunch, afternoon snack and evening meal, and at midnight) instead of only three. Under fasting conditions, we only administered basal insulin plus a corrective regimen every four hours. In the case of normal food intake, we administered 50% of the TDD calculated as basal insulin in two doses, and the other 50% as preprandial boluses distributed into breakfast (15%), lunch (15%), afternoon snack (10%), and dinner (10%). In addition, the CP allowed for a flexible adaptation to changing GC doses during the hospital stay. If GC was administered as a single morning dose, basal insulin was administered as a single dose at the time of GC administration, and the preprandial boluses were distributed differently: breakfast (10%), lunch (20%), afternoon snack (15%), and dinner (5%). The aim was to achieve glycemia levels during hospitalization of between 100 and 200mg/dl, given the complexity of reaching stricter control targets in this group of patients.
As rescue therapy in both groups, in the event of glycemia values>500mg/dl, or two consecutive recordings>400mg/dl, we started continuous intravenous insulin infusion.
The GP and the CP are attached as annexes (A and B, respectively).
Laboratory methodsPlasma blood glucose was measured using an enzymatic method with hexokinase; HbA1c was recorded by high-performance chromatography; and capillary blood glucose was measured using an Optium Xceed® system (Abbott) with a precision of 3–3.6%, an accuracy r=0.98 with respect to plasma glycemia, and 99% compliance with the ISO standard.
Statistical analysisThe sample size needed to detect a clinically important difference in MG between the two groups (18mg/dl), with a statistical power of 80% and a 95% confidence level, assuming a MG standard deviation of 35mg/dl, was 60 patients per group.
Quantitative variables were reported as the mean and standard deviation, while qualitative variables were described with their frequency distribution. Quantitative variables were compared between the two groups (GP and CP) using the nonparametric Mann–Whitney U-test. The comparison of qualitative variables was carried out with the chi-squared test or Fisher's exact test.
Since there was no randomization of the intervention, we calculated an adjusted estimate of the influence of the patient inclusion group upon in-hospital glycemic control based on multivariate linear regression analysis. The dependent variables considered included MG and the SD of all blood glucose levels during admission, with the results being expressed as adjusted differences (glucose values [mg/dl] in the CP, glucose values [mg/dl] in the GP). Multivariate adjustment was made for the variables according to their clinical and statistical significance (the presence of significant differences between the 2 groups). The correcting variables were age, the BMI, the Charlson index, the GFR, baseline blood glucose, HbA1c, home treatment for diabetes, the mean dose of GCs, and the maximum dose of GCs per kilogram body weight and day used during admission.
The impact of CP use upon the risk of experiencing MG>180mg/dl or MG>200mg/dl during admission was assessed by multivariate logistic regression analysis adjusted for the same variables as above. A sequential exclusion procedure was used to determine the main independent predictors of the adequacy of hospital glycemic control.
Associations with p<0.05 were regarded as statistically significant. The SPSS version 22.0 statistical package was used throughout.
ResultsGeneral description of the sampleThe study comprised 131 patients (74% males) with a mean age of 72.2 years (SD 11.2). The mean BMI was 30.6 (SD 5.4) kg/m2, the mean Charlson index score was 3.44 points (SD 1.9), and the mean GFR 69.4 (SD 26.6) ml/min/1.73m2. Approximately half of the patients (50.4%) had been treated at home with insulin before admission, and the mean HbA1c was 7.7% (SD 1.1).
Sixty patients were assigned to the control group applying the GP and 71 to the intervention group applying the CP. The most frequent reason for admission and treatment with GC was COPD exacerbation (74%), followed by asthma exacerbation (13.7%). Intravenous methylprednisolone was the most commonly used GC, followed by oral prednisone. Regarding the GC doses received by the patients, no statistically significant differences were found between the two groups in terms of either the mean dose during admission (56.9mg/day in the control group versus 53.3mg/day in the intervention group) or the maximum dose per kilogram body weight and day (1.29 versus 1.15, respectively).
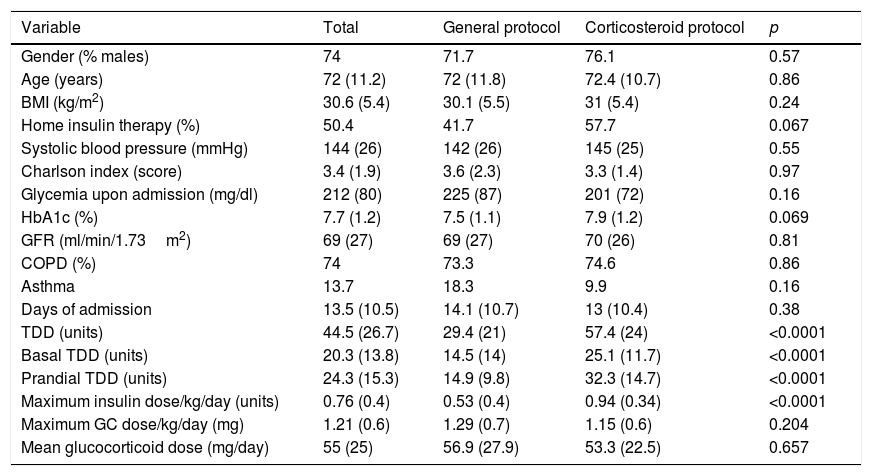
Patient characteristics according to the allocation group (Table 1)As a result of the application of the different protocols (GP and CP), we observed significant differences between the control group and the intervention group in the number of daily glycemia measurements (2.8 versus 3.4; p<0.0001), the proportion of patients subjected to glycemia measurement on the occasion of the afternoon snack (51.7% versus 100%; p<0.0001), the mean basal insulin dose (14.5 versus 25.1 units; p<0.0001), the prandial insulin dose (14.9 versus 32.3 units; p<0.0001) and the total insulin dose (29.4 versus 57.4 units; p<0.0001), the maximum insulin dose reached per kilogram body weight and day (0.53 versus 0.94 units; p<0.0001), and the percentage of patients who were receiving basal-bolus treatment at the end of their stay (35% versus 100%; p<0.0001). The patients in the control group received treatment with programmed insulin after an average of 6 days of admission, while in the experimental group this time was shortened to 0.65 days (p=0.001).
Patient characteristics expressed as the mean (standard deviation) or frequency distribution (%).
| Variable | Total | General protocol | Corticosteroid protocol | p |
|---|---|---|---|---|
| Gender (% males) | 74 | 71.7 | 76.1 | 0.57 |
| Age (years) | 72 (11.2) | 72 (11.8) | 72.4 (10.7) | 0.86 |
| BMI (kg/m2) | 30.6 (5.4) | 30.1 (5.5) | 31 (5.4) | 0.24 |
| Home insulin therapy (%) | 50.4 | 41.7 | 57.7 | 0.067 |
| Systolic blood pressure (mmHg) | 144 (26) | 142 (26) | 145 (25) | 0.55 |
| Charlson index (score) | 3.4 (1.9) | 3.6 (2.3) | 3.3 (1.4) | 0.97 |
| Glycemia upon admission (mg/dl) | 212 (80) | 225 (87) | 201 (72) | 0.16 |
| HbA1c (%) | 7.7 (1.2) | 7.5 (1.1) | 7.9 (1.2) | 0.069 |
| GFR (ml/min/1.73m2) | 69 (27) | 69 (27) | 70 (26) | 0.81 |
| COPD (%) | 74 | 73.3 | 74.6 | 0.86 |
| Asthma | 13.7 | 18.3 | 9.9 | 0.16 |
| Days of admission | 13.5 (10.5) | 14.1 (10.7) | 13 (10.4) | 0.38 |
| TDD (units) | 44.5 (26.7) | 29.4 (21) | 57.4 (24) | <0.0001 |
| Basal TDD (units) | 20.3 (13.8) | 14.5 (14) | 25.1 (11.7) | <0.0001 |
| Prandial TDD (units) | 24.3 (15.3) | 14.9 (9.8) | 32.3 (14.7) | <0.0001 |
| Maximum insulin dose/kg/day (units) | 0.76 (0.4) | 0.53 (0.4) | 0.94 (0.34) | <0.0001 |
| Maximum GC dose/kg/day (mg) | 1.21 (0.6) | 1.29 (0.7) | 1.15 (0.6) | 0.204 |
| Mean glucocorticoid dose (mg/day) | 55 (25) | 56.9 (27.9) | 53.3 (22.5) | 0.657 |
TDD: total daily insulin dose; GFR: glomerular filtration rate; BMI: body mass index.
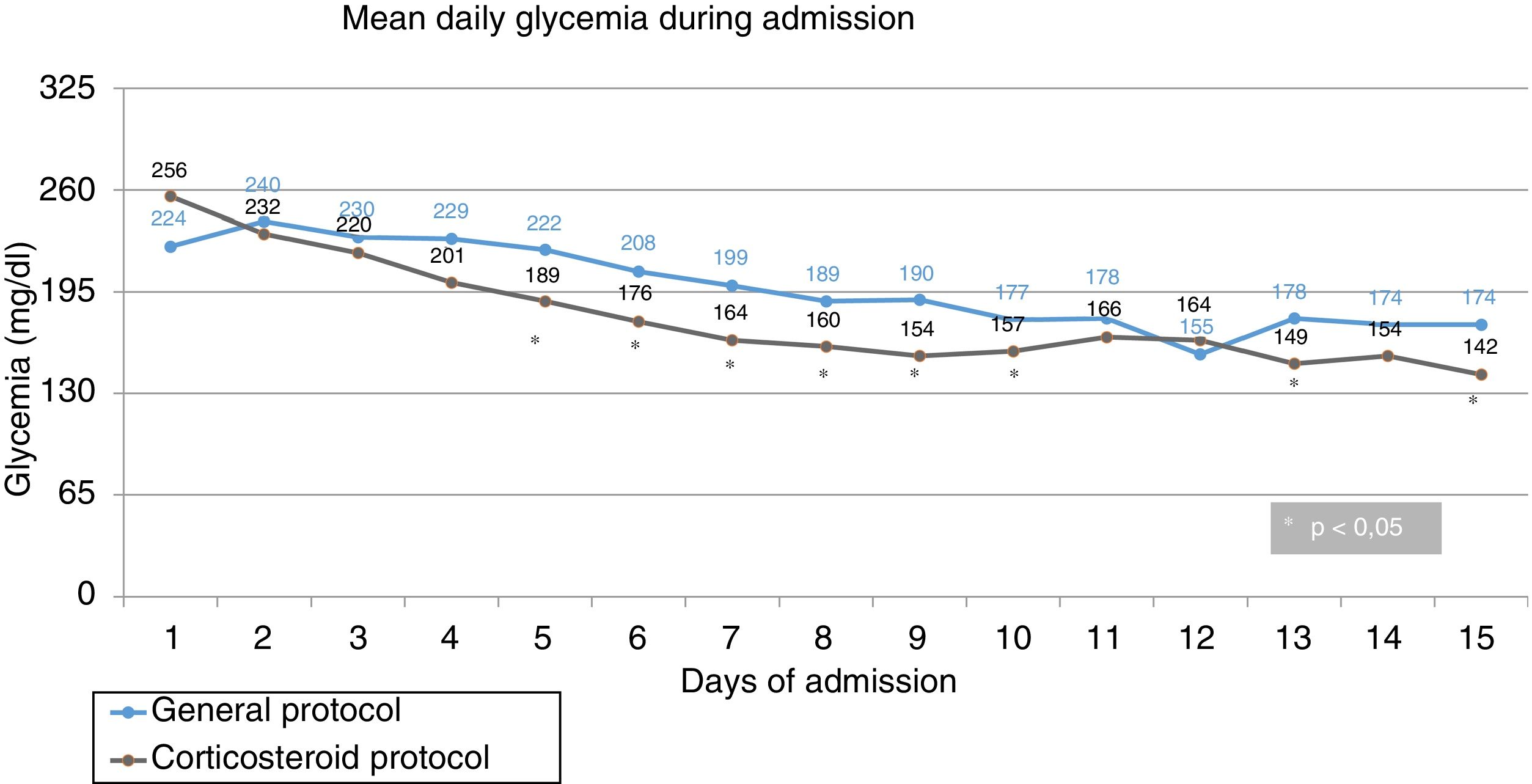
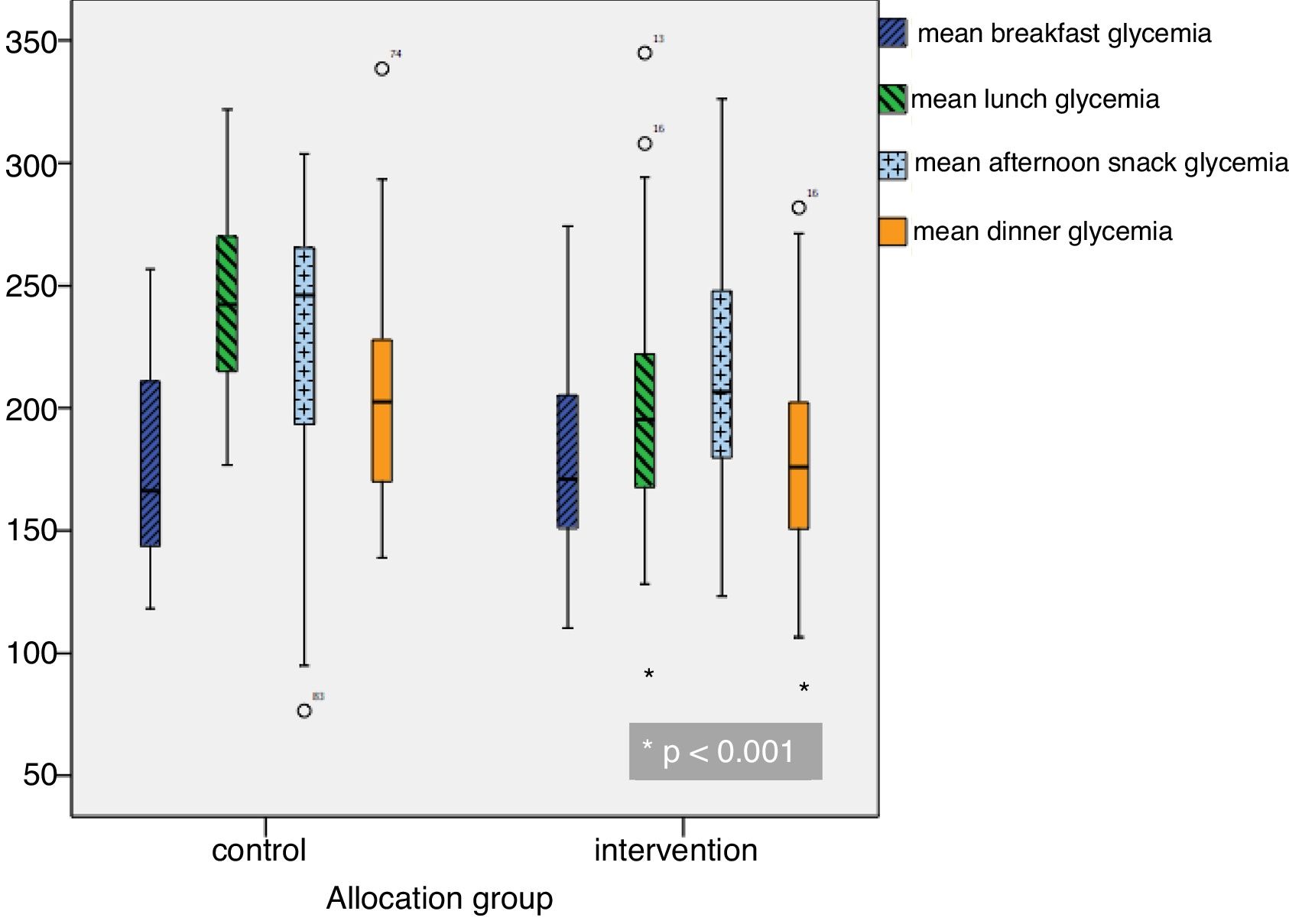
Mean daily glycemia levels. From the second day of admission, the MG values were lower in the intervention group, with statistically significant differences being reached from the fourth day (Fig. 1). Mean glycemia<180mg/dl was reached from the tenth day in the control group and from the fifth day in the intervention group. In turn, MG<200mg/dl was reached from the seventh day in the control group and from the fourth day in the intervention group. The MG values were lower in the intervention group at lunch and dinner, but no significant differences were observed at breakfast or the afternoon snack (Fig. 2).
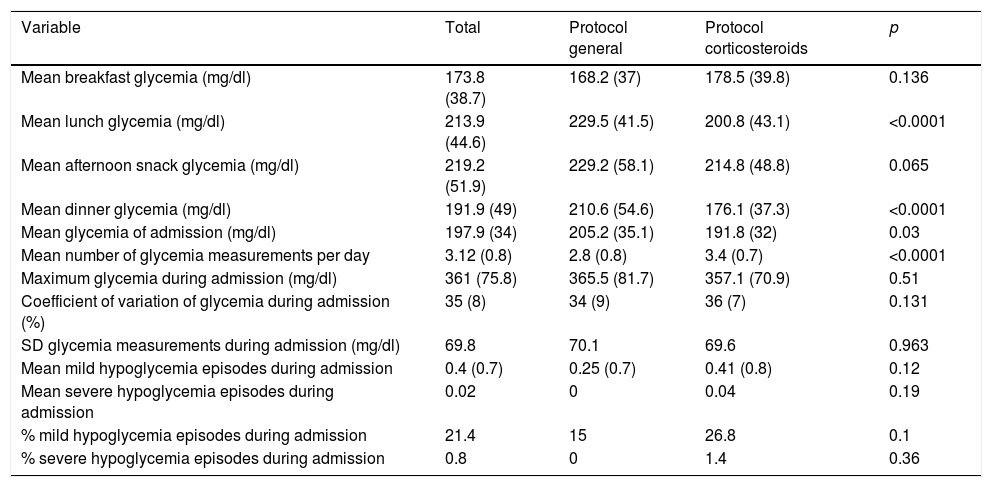
Comparison of glycemic parameters between the two groups.
| Variable | Total | Protocol general | Protocol corticosteroids | p |
|---|---|---|---|---|
| Mean breakfast glycemia (mg/dl) | 173.8 (38.7) | 168.2 (37) | 178.5 (39.8) | 0.136 |
| Mean lunch glycemia (mg/dl) | 213.9 (44.6) | 229.5 (41.5) | 200.8 (43.1) | <0.0001 |
| Mean afternoon snack glycemia (mg/dl) | 219.2 (51.9) | 229.2 (58.1) | 214.8 (48.8) | 0.065 |
| Mean dinner glycemia (mg/dl) | 191.9 (49) | 210.6 (54.6) | 176.1 (37.3) | <0.0001 |
| Mean glycemia of admission (mg/dl) | 197.9 (34) | 205.2 (35.1) | 191.8 (32) | 0.03 |
| Mean number of glycemia measurements per day | 3.12 (0.8) | 2.8 (0.8) | 3.4 (0.7) | <0.0001 |
| Maximum glycemia during admission (mg/dl) | 361 (75.8) | 365.5 (81.7) | 357.1 (70.9) | 0.51 |
| Coefficient of variation of glycemia during admission (%) | 35 (8) | 34 (9) | 36 (7) | 0.131 |
| SD glycemia measurements during admission (mg/dl) | 69.8 | 70.1 | 69.6 | 0.963 |
| Mean mild hypoglycemia episodes during admission | 0.4 (0.7) | 0.25 (0.7) | 0.41 (0.8) | 0.12 |
| Mean severe hypoglycemia episodes during admission | 0.02 | 0 | 0.04 | 0.19 |
| % mild hypoglycemia episodes during admission | 21.4 | 15 | 26.8 | 0.1 |
| % severe hypoglycemia episodes during admission | 0.8 | 0 | 1.4 | 0.36 |
Values are reported as the mean (standard deviation).
Adjusted MG differences and the SD of all glycemia measurements during hospital admission. There was a significant difference in MG between the control and intervention groups (205.2 versus 191.8mg/dl; p=0.030). The difference in MG, adjusted for potential confounding factors, in the intervention group versus the control group was −14.8mg/dl (95% confidence interval [95%CI] −26.2 to −3.3) (p=0.012). There was no significant difference between the control and intervention groups regarding the SD (70.1 versus 69.6mg/dl; p=0.96). The difference in the SD, adjusted for potential confounding factors, in the intervention group versus the control group was −1.5mg/dl (95%CI −7.9 to 4.8) (p=0.63).
Risk of developing elevated MG during hospital admission. The patients in the intervention group had a lower adjusted risk (bordering on statistical significance) of presenting MG>180mg/dl during their hospital stay (odds ratio [OR]=0.34; 95%CI 0.10–1.15; p=0.083). Likewise, the patients in the intervention group had a significantly lower adjusted risk of presenting MG>200mg/dl during their hospital stay (OR=0.31; 95%CI 0.11–0.91; p=0.033).
Predictors of MG>200mg/dl during hospital admission. Through sequential exclusion we found the predictors of presenting MG>200mg/dl to be: allotment to the intervention group (protective factor with OR=0.36; 95%CI 0.15–0.86; p=0.018), higher glycemia upon admission (OR1mg/dl=1.007; 95%CI 1.002–1.013; p=0.010) and a likewise higher HbA1c concentration (OR1%=1.86; 95%CI 1.24–2.77; p=0.001).
ComplicationsThe most common complications were infections (5% in the control group versus 0% in the intervention group) and vascular disorders (5% versus 7%), with no significant differences between the groups. A total of 6 patients (4.6%) died during the study: four in the control group (6.7%) and two in the intervention group (2.8%), the difference likewise being non-significant. In all cases death was considered to be derived from the disease that had caused admission, with no relation to the protocol used.
No acute complications in the form of diabetic ketoacidosis or hyperosmolar nonketotic syndrome were observed. As regards the risk of hypoglycemia, there were no significant differences between the two groups: mild hypoglycemia was noted in 15% of the controls versus in 26.8% of the patients in the intervention group (p=0.1), while severe hypoglycemia was recorded in 0% versus 1.4% (p=0.36).
DiscussionThe present study shows that by using a specific insulin protocol (CP) for patients with DDG, the MG value during admission can be reduced by approximately 15mg/dl without compromising patient safety. The use of the CP reduced the risk of presenting MG>200mg/dl during hospitalization by over 60%.
The application of specific protocols has resulted in highly variable MG values; this may be due to a number of factors mainly related to the patient inclusion criteria applied and to the GC therapy used. With regard to the profile of the included patients, the studies that recruited subjects with and without previous diabetes (a mixture of patients with GID and DDG) recorded lower MG values. In this regard, Lakhani et al., in their study with a DDG prevalence of 42%, observed an MG value in the experimental group of 170mg/dl.14 With respect to the type and dose of the GCs used, the MG values were higher in studies such as that published by Gosmanov et al., in patients with hematological malignancies requiring treatment with potent and long-acting GCs such as dexamethasone (MG in the group assigned to basal-bolus insulin treatment 219mg/dl).15
In our study we obtained an MG value of 192mg/dl with the CP versus 205mg/dl in the GP group. Ruiz de Adana et al.16 recorded an MG value of 205mg/dl in the group assigned to basal-bolus insulin therapy, with an insulin dose per kg body weight of 0.64 units. This finding reinforces the message that the insulin dose reached is a fundamental factor, since in our CP it reached 0.94 units per kilogram of weight per day.
Few studies have analyzed different DDG treatment regimens in hospitalized patients, and our literature review found no studies prospectively assessing complete results over a two-week time horizon. Lakhani et al. compiled data over the entire admission period, but discarded all capillary blood glucose values corresponding to days with glycemia<70mg/dl or >400mg/dl, which may not accurately describe the reality of patient admission.14 Grommesh et al. analyzed the results without taking into account the capillary blood glucose values of day 1, which is a day when the values in both groups are high, probably because the protocol has not had enough time to start working.17 In fact, in our study we found no significant differences in MG values between the groups until the fourth day. Dhital et al. conducted a retrospective study, analyzing capillary blood glucose values on a single admission day.18
Several published protocols advocate the management of GID or DDG using NPH insulin. Although it has been argued that the use of NPH insulin may be associated with a lesser risk of hypoglycemia versus insulin glargine or detemir,17 in our study based on basal-bolus therapy we recorded only one severe hypoglycemia episode in the CP group, despite the high insulin doses administered. Radhakutty et al., in their analysis of glycemic control on days 1 and 3 of admission using a basal-bolus regimen versus NPH insulin, found no significant differences in glycemic control or in the incidence of hypoglycemia.19 Other studies have also found a similar efficacy between NPH and basal-bolus regimens.16,18 Our study used a basal-bolus regimen different from that recommended by Perez et al.1 in order to minimize the risk of hypoglycemia, shifting part of the evening insulin to the afternoon snack.
The results of our study further underline the efficacy and safety of a basal-bolus regimen adapted to the modification in the GC dose. The adjusted difference of almost 15mg/dl lies at the limit of clinical relevance, and there were no significant differences in the number of hypoglycemia episodes. We believe that the use of NPH insulin has a number of disadvantages over basal-bolus therapy with insulin analogs. Firstly, the pharmacodynamic profile of NPH insulin is less predictable, with peaks of maximum action that are difficult to overlap with the postprandial glycemia peak. Secondly, in our hospital setting, the most commonly used insulins (in the established GP) are glargine, detemir and aspart, applied in basal therapy plus corrective doses or as basal-bolus therapy. Adopting a protocol involving NPH insulin – with which the hospital staff are unfamiliar and that has not shown greater efficacy20,21 – could make implementation and management difficult.
Contrary to some published studies, in our case we discontinued all OADs upon admission. We do not advocate maintaining them, due to the acute and potentially serious condition of the hospitalized patient, the possible complications derived from some of these drugs, and their possible masking effect upon the results of the protocol being evaluated. In this regard, in the study published by Brady et al., where all the OADs were maintained or metformin was introduced upon admission if the patients were not already receiving the drug, it is difficult to know whether any particular part of the control achieved was attributable to the studied protocol or to the administered OADs.22
The advantages of this study were the possibility of assessing both the efficacy and the safety of a new dynamic insulin model in patients with DDG during their hospital stay, given the lack of published protocols referring to this clinical situation. Thanks to its prospective design, we were able to conduct an exhaustive compilation of patient glycemia values. The application of the CP was performed in all cases by the same person, thus ensuring the homogeneity of the intervention. In addition, the number of patients included was greater than in other similar studies, with adequate statistical power to detect clinically important differences in MG.
The limitations of the study fundamentally center on the lack of patient randomization. However, there were no differences between the groups except for variables related to the different protocols used (the TDD, the number of glycemia measurements), and an analysis adjusted for potential confounding factors was also performed. We therefore consider that the differences in glycemic control seen were a consequence of the application of the CP versus the GP. Secondly, although the number of patients was limited, we had adequate statistical power to detect clinically relevant differences in glycemic control. However, the number of mild hypoglycemia episodes was higher in the intervention group; it therefore cannot be ruled out that a larger sample size would have caused the difference to be significant. Thirdly, adherence to the indications of the CP regarding the number of daily glycemia measurements was not perfect, a fact that could have limited its efficacy. Finally, the CP was always implemented by the same expert in DDG care, which could have positively influenced its usefulness. In this respect, it would be necessary to assess the efficacy of the protocol when used by professionals with different degrees of experience. In fact, in the GP group the delay of 6 days in starting programmed insulin could be attributed to a certain degree of therapeutic inertia.
In conclusion, the application of a new CP is effective and safe in dealing with the frequent problem of nosocomial hyperglycemia in patients receiving GC. We regard our work as the pilot test of a CP that, if validated and improved upon by other professionals and other hospitals, could lead to the development of a consensus-based approach to DDG.
AuthorshipThe three named authors participated in the conception and design of the study and in data collection. In addition, they contributed to the statistical analysis and interpretation of the data, and reviewed and approved the final submitted manuscript version.
Financial supportThe present study did not receive specific financial support from public agencies, the commercial sector or from non-profit entities.
Conflicts of interestNone.
Please cite this article as: Agudo-Tabuenca A, Gimeno-Orna JA, Sáenz-Abad D. Evaluación de la eficacia y la seguridad de un protocolo de manejo de pacientes con diabetes descompensada por glucocorticoides durante la hospitalización. Endocrinol Diabetes Nutr. 2019;66:353–360.