Los objetivos de la resección transuretral (RTU) del tumor vesical son la resección completa de las lesiones y la realización de un diagnóstico correcto con el objetivo de estadificar adecuadamente al paciente. Es bien sabido que la presencia de músculo detrusor en el espécimen es un requisito previo para minimizar el riesgo de infraestadificación.
La persistencia de enfermedad tras la resección de los tumores vesicales no es infrecuente, y es la razón por la que las guías europeas recomiendan una re-resección transuretral (re-RTU) para todos los tumores T1. Recientemente se ha publicado que, en los casos con inclusión de músculo en el espécimen, la re-RTU no afecta la progresión ni la supervivencia específica del cáncer.
Presentamos aquí los factores relacionados con el paciente y el tumor que pueden influir en la presencia de enfermedad residual en la re-RTU.
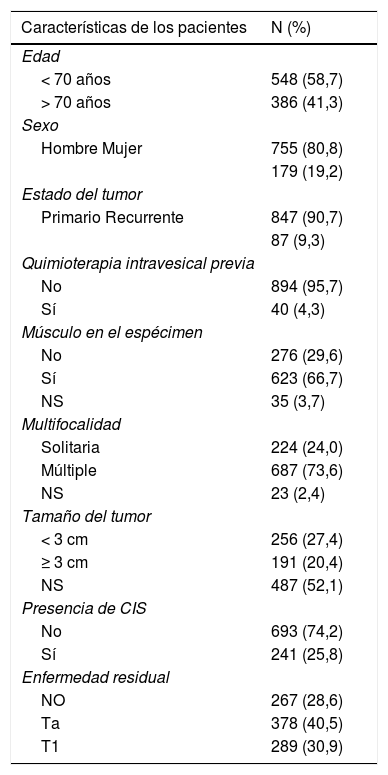
Material y métodosDe nuestra cohorte retrospectiva de 2.451 pacientes con tumores T1G3 primarios tratados inicialmente con bacilo de Calnette-Guérin (BCG), están disponibles los resultados patológicos de 934 pacientes (38,1%) que se sometieron a una re-RTU. El 74% tenía tumores multifocales, el 20% de los tumores tenía más de 3 cm de diámetro y el 26% tenía carcinoma in situ (CIS) concomitante. En este subgrupo de pacientes que se sometieron a una segunda RTU, no hubo enfermedad residual en 267 pacientes (29%) y se presentó enfermedad residual en 667 pacientes (71%): Ta en 378 (40%) y T1 en 289 (31%) pacientes. Se analizaron la edad, el sexo, el estado del tumor (primario/recurrente), la terapia intravesical previa, el tamaño del tumor, la multifocalidad del tumor, la presencia de CIS concomitante y la inclusión de músculo en el espécimen para evaluar los factores de riesgo de enfermedad residual en la re-RTU, tanto en los análisis univariantes, como en las regresiones logísticas multivariantes.
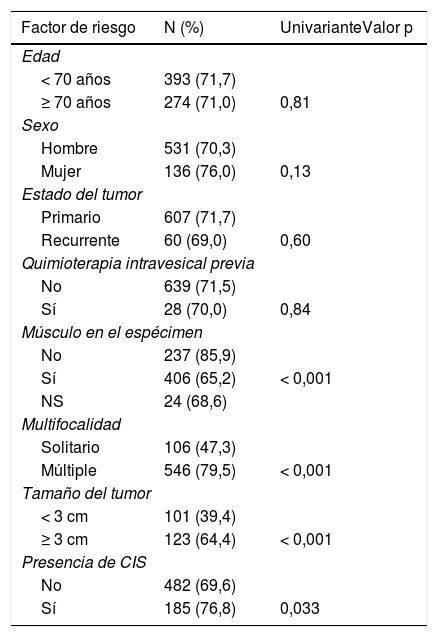
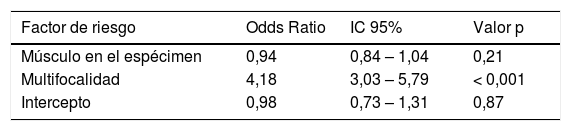
ResultadosLa edad, el sexo, el estado del tumor y la quimioterapia intravesical previa no fueron factores de riesgo de enfermedad residual, mientras que la ausencia de músculo en la RTU, los tumores múltiples, los tumores > 3 cm y la presencia de CIS concomitante sí fueron factores de riesgo univariantes para la presencia de enfermedad residual. Debido a la correlación entre la multifocalidad y el tamaño del tumor, el modelo multivariante retuvo el número de tumores o el diámetro del tumor (pero no ambos), p < 0,001. La presencia de músculo en el espécimen dejó de ser significativa, mientras que la presencia de CIS sólo fue significativa en el modelo con el tamaño del tumor, p < 0,001.
ConclusionesLos factores más significativos para un mayor riesgo de enfermedad residual en la re-RTU en pacientes con tumores T1G3 son los tumores multifocales y los tumores de más de 3 cm. Los pacientes con CIS concomitante y los que no tienen músculo en la muestra también tienen un mayor riesgo de enfermedad residual.
The goals of transurethral resection of a bladder tumor (TUR) are to completely resect the lesions and to make a correct diagnosis in order to adequately stage the patient. It is well known that the presence of detrusor muscle in the specimen is a prerequisite to minimize the risk of under staging.
Persistent disease after resection of bladder tumors is not uncommon and is the reason why the European Guidelines recommended a re-TUR for all T1 tumors. It was recently published that when there is muscle in the specimen, re-TUR does not influence progression or cancer specific survival.
We present here the patient and tumor factors that may influence the presence of residual disease at re-TUR.
Material and methodsIn our retrospective cohort of 2451 primary T1G3 patients initially treated with BCG, pathology results for 934 patients (38.1%) who underwent re-TUR are available. 74% had multifocal tumors, 20% of tumors were more than 3 cm in diameter and 26% had concomitant CIS.
In this subgroup of patients who underwent re-TUR, there was no residual disease in 267 patients (29%) and residual disease in 667 patients (71%): Ta in 378 (40%) and T1 in 289 (31%) patients. Age, gender, tumor status (primary/recurrent), previous intravesical therapy, tumor size, tumor multi-focality, presence of concomitant CIS, and muscle in the specimen were analyzed in order to evaluate risk factors of residual disease at re-TUR, both in univariate analyses and multivariate logistic regressions.
ResultsThe following were not risk factors for residual disease: age, gender, tumor status and previous intravesical chemotherapy. The following were univariate risk factors for presence of residual disease: no muscle in TUR, multiple tumors, tumors > 3 cm, and presence of concomitant CIS. Due to the correlation between tumor multi-focality and tumor size, the multivariate model retained either the number of tumors or the tumor diameter (but not both), p < 0.001. The presence of muscle in the specimen was no longer significant, while the presence of CIS only remained significant in the model with tumor size, p < 0.001.
ConclusionsThe most significant factors for a higher risk of residual disease at re-TUR in T1G3 patients are multifocal tumors and tumors more than 3 cm. Patients with concomitant CIS and those without muscle in the specimen also have a higher risk of residual disease.