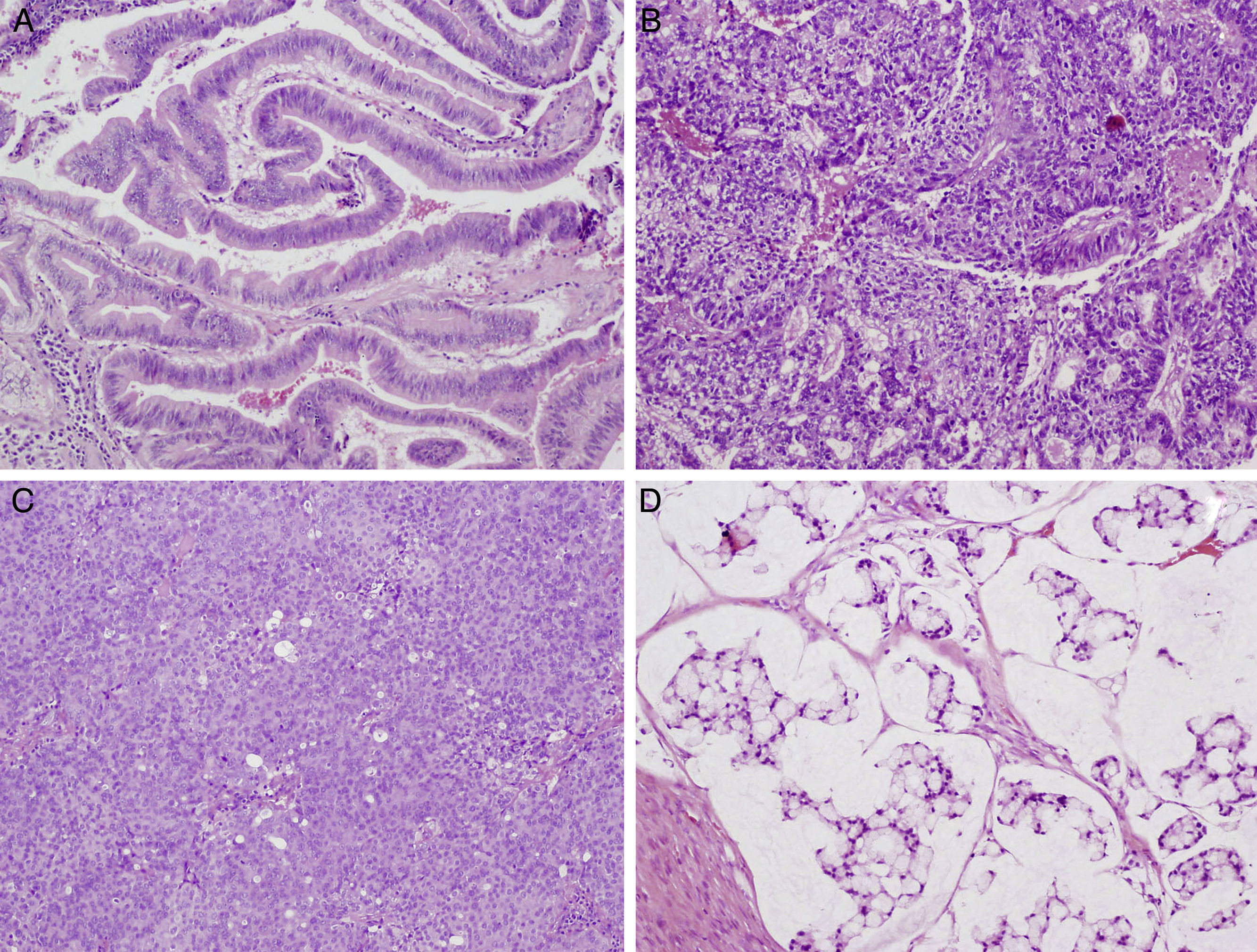
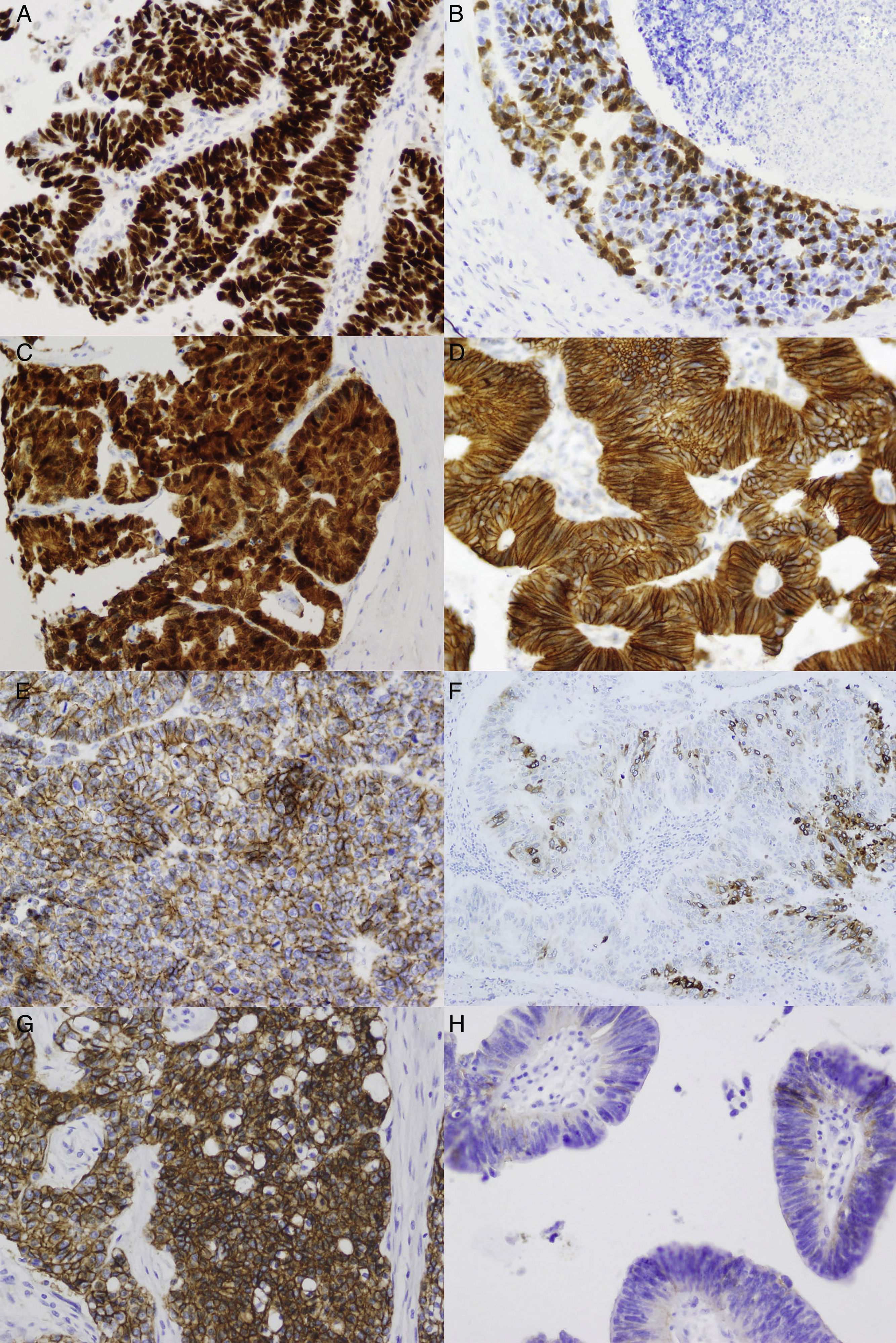
Intestinal-type sinonasal adenocarcinomas are malignant epithelial tumours. Around 8%–25% of all sinonasal malignant tumours are intestinal-type adenocarcinomas, which are related to wood dust exposure. Four histological subtypes have been described: papillary, colonic, solid and mucinous. We performed a pathological and immunohistochemical study in order to describe characteristics with prognostic, diagnostic and therapeutic value, and also to compare our results with previous studies.
MethodsSixty-six tumour samples were analysed and protein expression of p53, p16, E-cadherin, β-catenin, epidermal growth factor receptor (EGFR), human epidermal growth factor receptor 2 (HER2/neu) and cyclooxygenase-2 (COX-2) was performed by tissue microarray blocks.
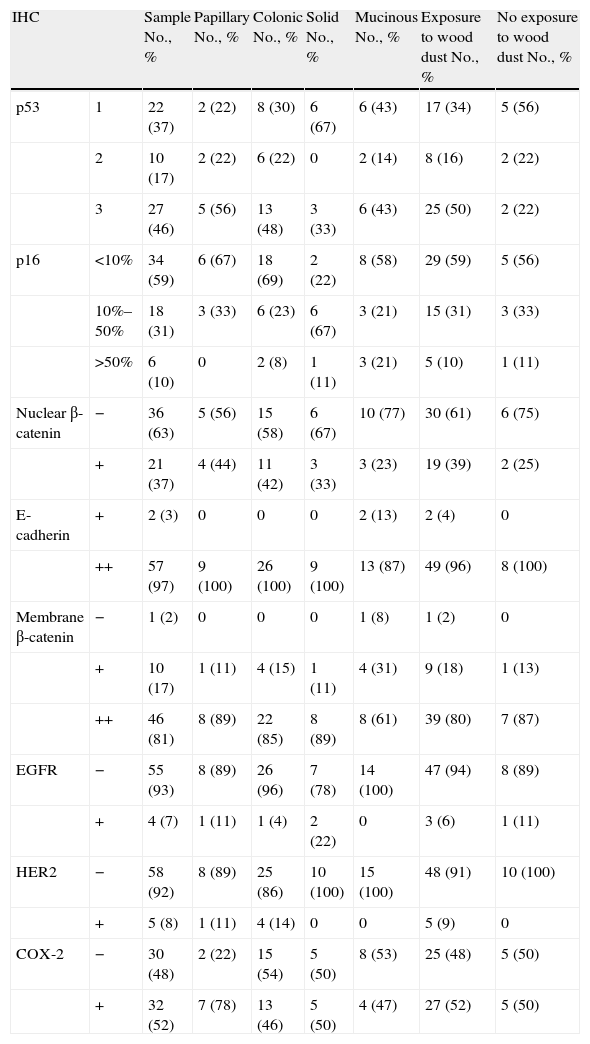
ResultsThe 63% of cases were p53 positive; 37% showed nuclear staining with β-catenin and 100% with E-cadherin, while 98% showed membrane staining with β-catenin, 7% with EGFR, 8% with HER2/neu and 52% with COX-2; and 59% of the cases lost p16 expression.
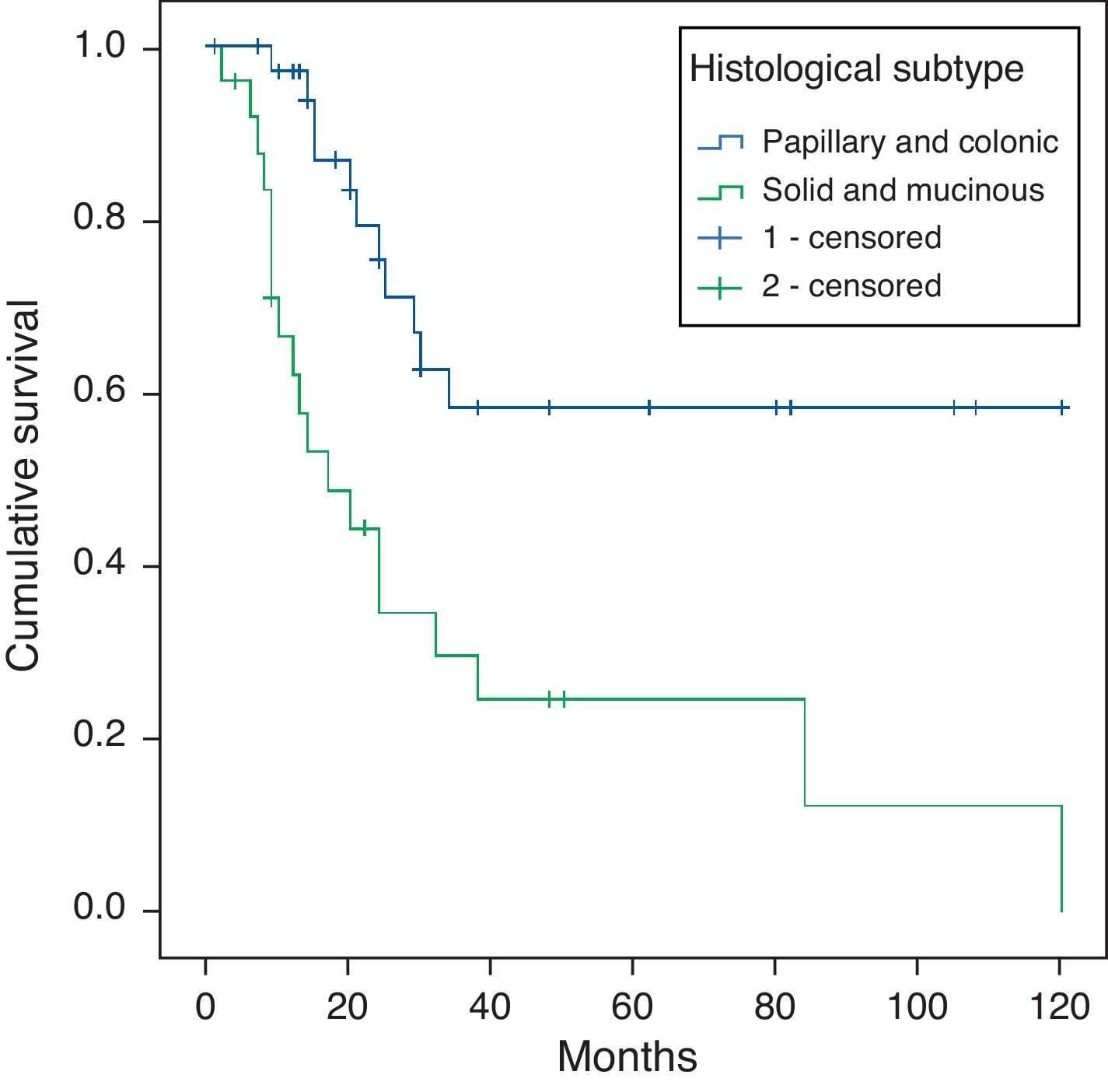
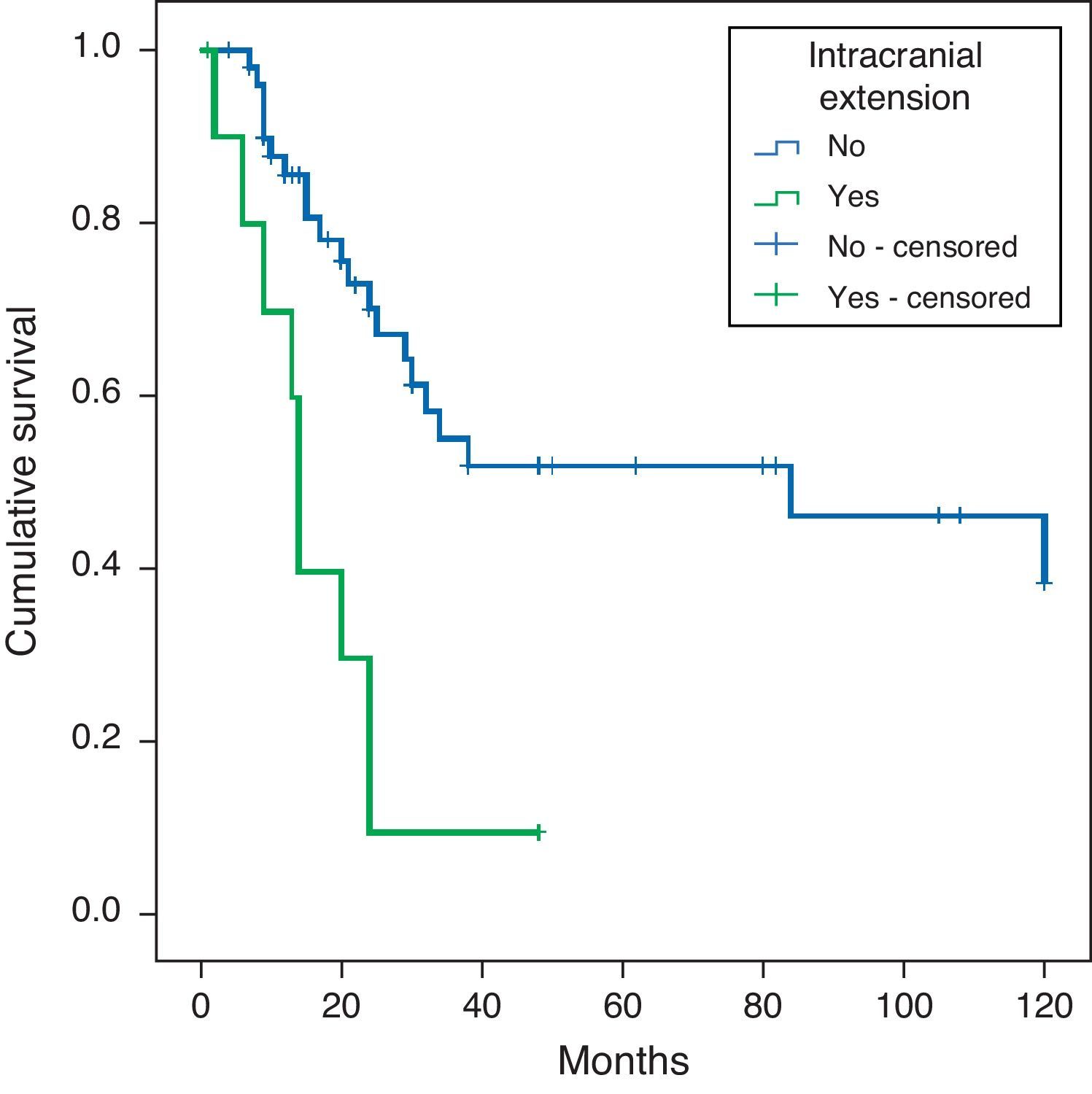
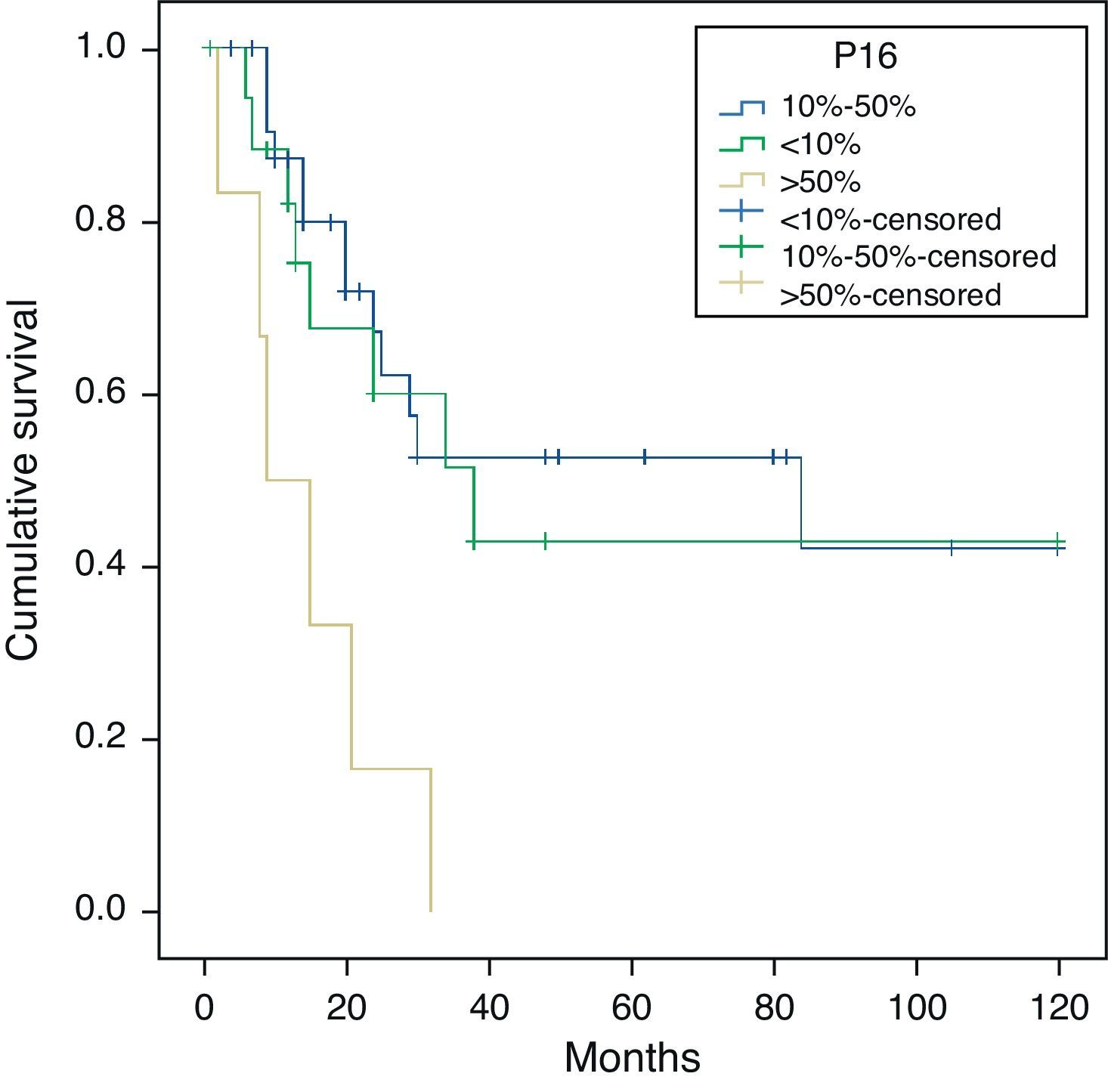
ConclusionsIntracranial invasion was the worst prognostic associated event. Solid and mucinous tumours were the most aggressive histological subtypes. Intracranial invasion was more frequent in mucinous subtype tumours. Immunohistochemical results were similar in all tumour subtypes, except for mucinous tumours, which showed weak expression of E-cadherin and β-catenin. Comparing with previous studies, we found a lower expression of EGFR, HER2/neu and COX-2. The p16 expression was associated with worse survival and metastatic disease.
Los adenocarcinomas nasosinusales tipo intestinal son tumores epiteliales malignos, que suponen el 8–25% de los tumores malignos nasosinusales. Se relacionan con la exposición al polvo de la madera. Se subdividen histológicamente en papilares, colónicos, sólidos y mucinosos. Realizamos un estudio patológico e inmunohistoquímico con el fin de establecer características con significado pronóstico, diagnóstico e incluso terapéutico, así como comparar con estudios previos.
MétodosEstudiamos 66 muestras tumorales mediante matrices tisulares. Realizamos tinciones inmunohistoquímicas para p53, p16, β-catenina, E-cadherina, receptor del factor de crecimiento epidérmico (EGFR), receptor 2 de factor de crecimiento epidérmico humano (HER2/neu) y ciclooxigenasa 2 (COX-2).
ResultadosUn 63% de los casos son positivos para p53, el 37% para β-catenina nuclear, el 100% para E-cadherina, el 98% para β-catenina membranosa, el 7% para EGFR, el 8% para HER2/neu, el 52% para COX-2 y el 59% pierden la expresión de p16.
ConclusionesLa invasión intracraneal es el factor clínico pronóstico más importante. Los tumores de tipo sólido y mucinoso son los que muestran un comportamiento más agresivo, siendo los mucinosos los que mayor invasión intracraneal muestran. No existen diferencias inmunohistoquímicas entre los distintos subtipos histológicos, únicamente la tinción débil para E-cadherina y β-catenina, más frecuente en los de tipo mucinoso. El EGFR, HER2/neu y COX-2 muestran una positividad menos frecuente que en series previas. La positividad para p16 se asocia a una menor supervivencia y mayor frecuencia de enfermedad metastásica.