Paraneoplastic neurological disorder (PND) is a rare complication of cancers. We report a 25-year-old woman presenting with generalized tonic–clonic seizure one week after vaginal delivery. No underlying etiology for the seizure was detected on initial evaluation. On re-evaluation three months later, an invasive breast carcinoma with the diagnosis of infiltrating ductal carcinoma (IDC) was revealed. To our knowledge, this is the first case report of PND that presented with generalized tonic–clonic seizure, decreased visual acuity, and acute loss of consciousness. This study briefly outlines PND and its association with breast tumors. In addition, it highlights the importance of high clinical suspicion to detect PND.
El trastorno neurológico paraneoplásico (PND) es una complicación poco común de los cánceres. Presentamos una mujer de 25 años con convulsión tónico-clónica generalizada una semana después del parto vaginal. No se detectó una etiología subyacente de la convulsión en la evaluación inicial. En la reevaluación tres meses después, se diagnosticó un carcinoma de mama intraductal invasivo. Hasta donde sabemos, este es el primer informe de caso de PND que se presentó con convulsiones tónico-clónicas generalizadas, disminución de la agudeza visual y pérdida aguda del conocimiento. Este estudio describe brevemente la PND y su asociación con los tumores de mama. Además, destaca la importancia de una alta sospecha clínica para detectar PND.
Breast cancer is the second most common malignancy and the second most common cause of mortality due to cancer in female individuals in the United States. Breast cancers may be invasive carcinomas (infiltrating ductal carcinoma (IDC) (about 80%) and invasive lobular carcinoma) or non-invasive/in situ carcinomas (over 80% are ductal and approximately 10% are lobular).1
Paraneoplastic neurological syndrome is a rare complication of malignancies and those cancers that originate outside the nervous system can directly or indirectly affect the central nervous system (CNS) and peripheral nervous system (PNS).2,3 Paraneoplastic neurological disorders (PNDs) are referred to all of the neurological complications of non-metastatic cancers when no specific etiology is found. PNDs may influence any part of the CNS or PNS and may present with significant clinical manifestations.4–6 A small percentage of patients with cancer are diagnosed with PND. Therefore, differentiating PNDs from the more common neuro-oncological disorders, such as metastases or neurological complications associated with cancer therapy, is critical.7,8 PNDs that affect the nervous system are unique compared to the other disorders that are associated with the immune system. This is because the target of the immune response is known. Some proteins that normally exist in the neurons (or other parts of the immune system) are inappropriately expressed in some types of cancer. As a result, the immune response results in a high titer of targeted antibodies (against oncological antigen).9,10
PNDs present in less than 1% of the patients who have breast cancer. However, about one-third of these patients do not have detectable onco-neural antibodies.11 PNDs do not have a specific diagnostic criterion, and therefore, are a diagnostic challenge for neurologists. The clinical manifestations of progressive encephalomyelitis reflect a multifocal inflammatory condition that occurs in a neoplasm base.12 On the other hand, PERM (progressive encephalomyelitis with rigidity and myoclonus) is a severe life-threatening condition and is characterized by rigidity, brain stem symptoms, autonomic symptoms, muscle spasms, sensory symptoms, respiratory features, and myoclonus which occurs in breast cancer.13–15
We report a 25-year-old woman presenting with generalized tonic–clonic seizure one week after vaginal delivery which on re-evaluation three months later, was diagnosed with an infiltrating ductal carcinoma (IDC) of the breast. This study highlights the importance of high clinical suspicion to detect PND and its association with breast tumors.
Case reportA young female (25 years of age) was admitted to our hospital with complaints of nausea, fever (low-grade), vomiting, and headache. She had a history of vaginal delivery one week before admission. She did not have any significant past medical history and did not use any medications.
She developed a generalized tonic–clonic seizure (GTCS) on the next day of admission. Two days later, she developed vision loss with no light perception (NLP) in her left eye and, within 24 h, the right eye was also involved. In addition, within a week she became quadriplegic and the level of her consciousness decreased. Physical examination revealed normal skin, cardiovascular system, lung, head, and neck. However, both eyes had NLP with spontaneous movements. Her pupils were bilaterally fixed with mydriasis but both disks were sharp and normal. Other cranial nerves were normal on examination. Moreover, the muscle strength of all four limbs was 1/5, and Golgi-tendon and bilateral plantar reflexes were absent. Her symptoms progressed within a week.
Brain MRI (magnetic resonance imaging) was unremarkable. PCR test was performed to rule out meningoencephalitis. Empiric treatment with ceftriaxone 4 g/daily, vancomycin 2 g/daily, and acyclovir 750 mg/daily was initiated. After five days, this treatment regimen was discontinued following the negative PCR report for infections and no improvement of the patient's clinical status. Consequently, the patient was transferred to the intensive care unit (ICU), and endotracheal intubation was performed.
To rule out the possibility of malignancy-induced encephalomyelitis, a workup to detect a tumor was initiated with chest, abdominal, and pelvic CT (computerized tomography) scans. However, the results were normal. Subsequently, empirical therapy with methylprednisolone 1 g/daily for five consecutive days was started, but there was no change in the patient's condition for the next two weeks. Therefore, intravenous immunoglobulin (IVIG) was administered (30 g/daily for five days). She had developed bulbar symptoms and respiration dysfunction. Therefore, tracheostomy was performed. After one month of admission, the patient showed improvement in vision (ophthalmoplegia), muscle strength, and consciousness status (obtundation). She had partially responded to IVIG therapy and was discharged with a tracheostomy. Following one month of home-care, the patient's condition improved and the tracheostomy was removed.
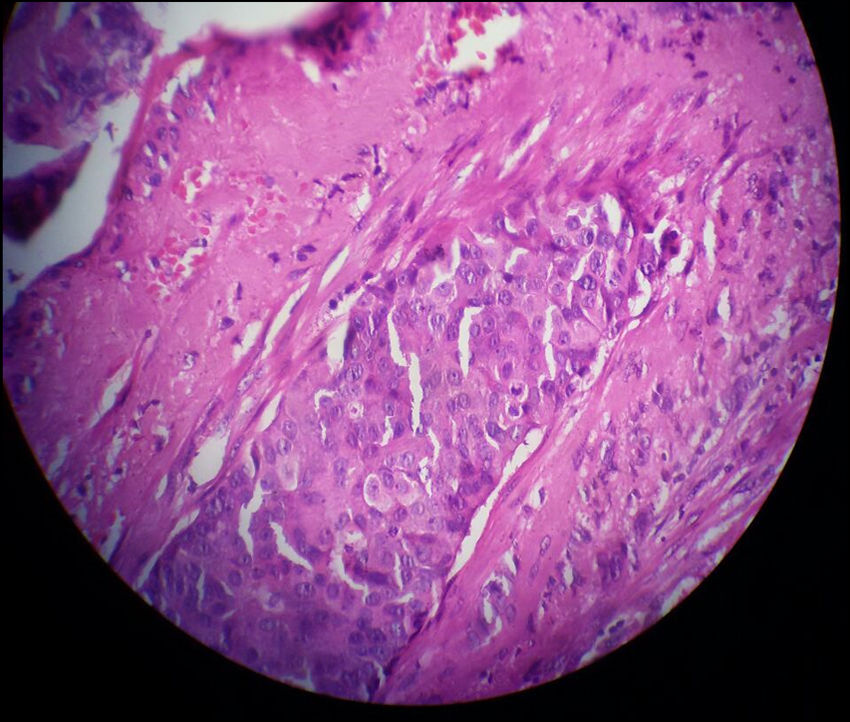
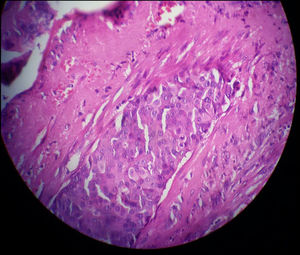
Three months later, a thorough re-evaluation to find an underlying cancer was performed with an examination, sonography, and core needle biopsy. Eventually, breast cancer was diagnosed. The pathology report described the mass (2.5 × 1.5 cm in size) as an IDC (not otherwise specified (NOS) type). The microscopic grading with the Nottingham modification of the Bloom-Richardson system was II/III (6/9) and vascular invasion was also reported (Fig. 1). As a result, modified radical mastectomy and lymph node dissection was performed on the right breast and right axilla. All surgical margins were free of tumor and five lymph nodes were involved. Subsequently, the patient underwent treatment with chemotherapy and then radiotherapy. However, metastasis to the brain was detected six months after the onset of the first symptoms of breast cancer. The patient expired two months following her brain surgery due to complications of brain and lung metastases.
DiscussionOlder age and female gender are the main risk factors of breast cancer. However, less than a third of patients with breast cancer present with a palpable breast mass. Some of the other clinical findings in breast cancer include erythema, edema, or peau d'orange appearance of the breast or discharge and retraction of the nipple. Furthermore, the most common type of breast cancer is IDC which accounts for about 80% of invasive breast cancers. Some of the imaging findings of invasive breast cancer include irregular mass, axillary lymphadenopathy, microcalcification, and acoustic shadowing (posterior).1 In addition, PND occurs in about 1% of breast cancer cases.11,16 It has no clear criteria and presents with various neurologic symptoms; therefore, the diagnosis is a challenge for neurologists. Although this is a rare disorder, diagnosis of this syndrome is very critical for clinical neurologists.17,18 Several studies have demonstrated that a lot of PNDs are mediated by the immune system. Since the manifestations mostly have an acute onset and precede the tumor diagnosis, detection of a paraneoplastic syndrome is difficult.12 Neurologists encounter paraneoplastic antibodies against the nervous system with unknown etiology which leads to various presentations. These manifestations in some studies have been reported as PERM.19,20
To our knowledge, this is the first case report of PND that presented with generalized tonic–clonic seizure, decreased visual acuity, and acute loss of consciousness. These features have not been reported in previous cases. In the previous studies, serological evidence of infection, vasculitis tests, neuroimaging, and lymphocytic pleocytosis in the patients' CSF (cerebrospinal fluid) were normal.15,21 In our case, the negative detection of a tumor after initial evaluations and the patient's response to treatment encouraged us to follow up with the patient more carefully. She was eventually diagnosed with IDC of the breast. Most patients show satisfactory results with early diagnosis and appropriate treatment with immunotherapy (corticosteroids, IVIG, plasma exchange, and cyclophosphamide). However, our patient had responded partially to IVIG therapy. She underwent brain surgery six months after the onset of the first manifestations of breast cancer due to metastasis to the brain. She expired two months following her brain surgery due to complications of brain and lung metastases.
ConclusionIn conclusion, we report a rare case of IDC of the breast in a young female who presented with rare manifestations of PND. PND is recommended to be considered as a differential diagnosis whenever patients present with generalized tonic–clonic seizure, decreased visual acuity, and acute loss of consciousness.
FundingThis work has not received any funding.
Ethical StatementThis study was conducted following the principles of the World Medical Association Declaration of Helsinki. Informed consent was obtained from the patient for publication of this case report and the accompanying image.
Authors' contributionsAll authors passed four criteria for authorship contribution based on recommendations of the International Committee of Medical Journal Editors.