Breast cancer (BC) is the most common type of cancer in women and the second most common cause of death from cancer in women around the world. Local therapies, Systematic therapies and advanced systematic therapies are included in current treatment strategies. Gene therapy has become a hopeful way to treat or stop disease by changing bad genes genetically. This innovative strategy aims to replace defective genes, enhance immune defenses, or silence harmful genes. Recent advances in cancer-specific promoter systems make targeted gene treatments possible. These include using suicide genes, cancer suppressor genes, gene silencing technologies, and gene-editing techniques. Among these, CRISPR and CAR-T therapy have revolutionized genetic editing in cancer research, offering high specificity, precision, cost-effectiveness, and efficiency. This review highlights recent advancements in gene therapy targeting cancer-specific promoters, emphasizing its potential to transform BC treatment.
El cáncer de mama (CM) es la segunda causa de muerte relacionada con el cáncer en mujeres en todo el mundo, así como la neoplasia maligna más comúnmente diagnosticada. Para abordarlo, se han desarrollado una variedad de opciones de tratamiento, que incluyen cirugía, radioterapia, terapia hormonal, quimioterapia y terapias biológicas. La terapia génica ha despertado interés como una forma novedosa de tratar muchos tipos de cáncer mediante la modificación de genes defectuosos. La terapia génica es el proceso de alterar genéticamente los genes de una célula para tratar o prevenir enfermedades. Busca tratar enfermedades, aumentar la capacidad del sistema inmunológico para defenderse de las enfermedades o cambiar genes dañados por otros funcionales. Esta tecnología se utiliza para alterar genéticamente las células del paciente para mejorar o tratar su condición. Se han creado varios sistemas promotores específicos del cáncer para atacar las células cancerosas. Busca proporcionar información actualizada sobre la terapia génica dirigida a promotores específicos del cáncer. Esto fomenta ciertas terapias, como genes supresores del cáncer, genes suicidas, silenciamiento de genes y tratamientos de edición de genes. La tecnología CRISPR, un descubrimiento clave en la edición genética, ahora se utiliza ampliamente en terapia génica y en la investigación del cáncer humano. Es ampliamente considerado por su bajo riesgo, sus cualidades de ahorro de tiempo, su rentabilidad, su especificidad y su precisión.
Breast cancer (BC) is the most common cancer in women and the second most common cause of death from cancer in the world.1 There are glandular and stromal cells in the breast. A glandular tissue has lobules that make milk and tubes that carry milk to the nipple.2 The stromal tissue comprises fatty and fibrous connective tissues, and the lymphatic tissue aids in removing cellular fluids and waste products through the immune system.3,4 BC can develop in various parts of the breast, with ductal carcinomas being the most common type, originating in the cells lining the milk ducts. Lobular carcinomas, arising from the lobules, are less common but still significant.5,6 While most changes in the breast are benign, malignant transformations, though rare, can occur in breast cells, with ductal and lobular carcinomas being the primary forms of BC.7
The causes of BC are multifactorial, involving both intrinsic and extrinsic factors. Lifestyle, environmental, psychological, and social factors contribute significantly to its prevalence. About 20–30% of BC cases are caused by risk factors that can be changed. The other 5–10% are caused by genetic changes or a family history of the disease.8,9 With 2.08 million new cases and 626,000 deaths in 2018, BC was responsible for 6.6% of all cancer-related deaths around the world.10–12 Several lifestyle factors increase BC risk, including obesity, physical inactivity, and alcohol consumption. Hormonal and reproductive factors such as early menarche, delayed childbirth, fewer pregnancies, lack of breastfeeding, and the use of hormonal therapies or oral contraceptives also contribute to the risk.13–19 Genetic factors are very important. Changes in high-risk genes like BRCA1, BRCA2, PALB2, ATM, CHEK2, and TP53 make people much more likely to get BC.20–22
Current treatment modules for BCVarious treatment modules for breast cancer are summarized in Fig. 1.
Local therapiesLocal therapy for BC is an essential component of the therapeutic strategy, designed to specifically target cancer cells in the breast and adjacent tissues while reducing effects on the remainder of the body.23,24 The primary modalities of local therapy include surgical intervention and radiation therapy, frequently employed in conjunction with systemic treatments such as chemotherapy, hormone therapy, or targeted therapy, contingent upon the particularities of the malignancy. Surgery is generally the primary local intervention for BC. The selection of a surgical technique is contingent upon aspects including tumor size and location, alongside the patient's preferences and overall health status. The two principal categories of surgical procedures are lumpectomy and mastectomy.25,26 A lumpectomy entails excising the tumor together with a narrow margin of adjacent healthy tissue. This operation is commonly known as breast-conserving surgery, as it preserves the majority of the breast tissue. After a lumpectomy, the majority of patients will have radiation therapy to eradicate any residual cancer cells that may be undetectable yet provide a risk of recurrence. Alongside these surgical alternatives, radiation therapy is essential in localized treatment.27–29 Radiation employs high-energy photons, including X-rays, to precisely target and eradicate cancer cells. Post-surgery, radiation therapy is frequently employed to diminish the probability of cancer recurrence, particularly in instances involving lumpectomy or when there is a danger of residual cancer cells in the breast or adjacent lymph nodes.30,31 Radiation may be advised for patients with bigger tumors or those whose cancer has metastasized to the axillary lymph nodes. The process entails directing controlled radiation treatments at the affected area over several weeks, generally five days per week, while the specific treatment plan is contingent upon individual conditions.32,33
Systematic therapiesSystemic therapy for BC is a treatment modality that addresses cancer cells throughout the body, rather than focusing solely on the breast tumor. This therapy is particularly crucial for addressing malignancies that may have metastasized beyond the breast, affecting adjacent lymph nodes or distant organs. The primary forms of systemic therapy encompass chemotherapy, hormone therapy, targeted therapy, and immunotherapy. Chemotherapy employs potent pharmaceuticals to eradicate rapidly proliferating neoplastic cells. It may be provided before surgery (neoadjuvant therapy) to diminish tumor size, after surgery (adjuvant therapy) to mitigate recurrence risk, or in instances of metastatic BC to manage the disease.34–38 Hormone therapy is employed for hormone receptor-positive breast tumors, in which tumor cells depend on hormones such as estrogen or progesterone for proliferation. Medications such as tamoxifen or aromatase inhibitors inhibit or diminish these hormones, aiding in the prevention of cancer recurrence.39,40 Targeted therapy concentrates on particular attributes of cancer cells, exemplified by HER2-positive tumors, where agents such as trastuzumab (Herceptin) specifically inhibit the HER2 protein on cancer cells to impede their proliferation. Immunotherapy enhances the body's immune system to more effectively identify and combat cancer cells and is frequently employed for specific aggressive types of BC, such as triple-negative BC.31,41
Advanced systemic therapiesAdvanced systemic therapies, such as Antibody-Drug Conjugates (ADCs), immune checkpoint inhibitors and Selective estrogen receptor degraders (SERDs) or emerging technologies like PROTACs exemplify innovative strategies in the management of advanced BC, providing renewed optimism for patients with metastatic or treatment-resistant conditions.
Antibody-drug conjugates (ADCs) therapyAntibody-drug conjugates (ADCs), exemplified as trastuzumab deruxtecan (Enhertu), integrate the specificity of monoclonal antibodies with the efficacy of chemotherapeutic agents. Trastuzumab deruxtecan is an antibody that specifically targets the HER2 protein, which is overexpressed in certain breast tumors.31,42 Upon binding to the HER2 receptors on cancer cells, the antibody administers a chemotherapeutic agent directly to the tumor. This facilitates a more focused assault on cancer cells while reducing harm to adjacent healthy tissues, rendering it particularly efficacious in HER2-positive BC that has developed resistance to alternative therapies. ADCs provide a more accurate and potent method for cancer treatment, enhancing response rates in individuals with metastatic or previously resistant BC.43–46
Immune checkpoint inhibitorsImmune checkpoint inhibitors, including pembrolizumab (Keytruda) and atezolizumab (Tecentriq), represent a groundbreaking class of pharmaceuticals that function by augmenting the body's immune system to more effectively identify and target cancer cells. Cancer cells frequently avoid immune system identification via immunological checkpoints, such as PD-L1, which function as inhibitors of immune response.47,48 Immune checkpoint inhibitors obstruct these checkpoints, enhancing the immune system's capacity to identify and eliminate cancer cells. This strategy has demonstrated considerable potential, especially in triple-negative BC (TNBC), which is frequently more aggressive and devoid of targeted medicines such as hormone receptor or HER2-based therapy. These inhibitors enhance the immune system's capacity to combat cancer, resulting in better survival and response rates, particularly when used in conjunction with other therapies.49
Selective Estrogen Receptor Degraders (SERDs) and PROTACs therapiesSelective Estrogen Receptor Degraders (SERDs) and innovative technologies such as PROTACs (Proteolysis-Targeting Chimeras) exemplify advanced methodologies in the management of BC, especially for estrogen receptor-positive (ER+) variants. SERDs, including fulvestrant, function by attaching to the estrogen receptor and facilitating its degradation, so obstructing estrogen signaling that stimulates tumor proliferation.50 SERDs are particularly advantageous in instances where cancer cells have acquired resistance to conventional hormone treatments. Conversely, PROTACs are a novel category of small compounds that utilize the body's intrinsic protein degradation mechanisms to selectively target and eradicate certain proteins, including those implicated in the advancement of BC. PROTACs function by enlisting the cell's ubiquitin-proteasome system to eliminate cancer-related proteins, including mutant variants of the estrogen receptor and other critical molecules implicated in tumor viability.51,52
Advanced gene therapy for BCGene therapy involves altering a cell's DNA to treat or prevent diseases.53 Genes, composed of DNA, control many body functions, from growth to vital processes.54 Diseases often arise from faulty genes, and gene therapy aims to replace defective genes with functional ones, address illnesses, or boost the immune system.55,56 This innovative technology modifies a patient's cells to improve or treat their condition.57,58 Introducing therapeutic gene material with regulatory components into the nucleus, either restores the normal expression of a defective gene or recovers lost function due to mutations.59,60 However, implementing this approach requires careful biosafety governance. Assessing and mitigating risks to human and environmental health is crucial, underscoring the importance of stringent oversight in gene therapy applications.61,62
As a result, both viral and nonviral vectors are being used in experimental and clinical settings to change genes and treat diseases.63 By 2019, more than 2600 gene therapy clinical trials had been accepted or concluded. In the US, four gene therapy products have been approved by the FDA.64,65 Of the global 232 approved gene and cell therapy trials, approximately 70% used viral vectors.66 More information about gene therapy for BC will be provided in the next section.
Method of gene therapyThere are two methods for introducing genetic material into cells (Fig. 2). Ex-vivo gene therapy involves taking cells from a patient (autologous) or a volunteer (allogeneic), changing their genes in a lab using a vector, and then putting the changed cells back into the patient after activation or expansion.67,68 In-vivo gene therapy, on the other hand, puts a vector straight into the body of the patient, so cells do not have to be taken out. The advantages and downsides of each method are different.
Ex-vivo gene transfer allows for precise targeting of specific collected cells, ensuring they possess the desired characteristics and offering multiple vector options for enhancement before transplantation. While this method requires advanced laboratory facilities, extensive research, and modern equipment, it provides greater control over the process. In contrast, in vivo, gene modification requires less lab work and is the preferred method when cells cannot be extracted or maintained outside the body. It is ideal for accessible tissues, though nonspecific interactions between vectors and cells, as well as the potential for immune reactions, limit its use.69–71
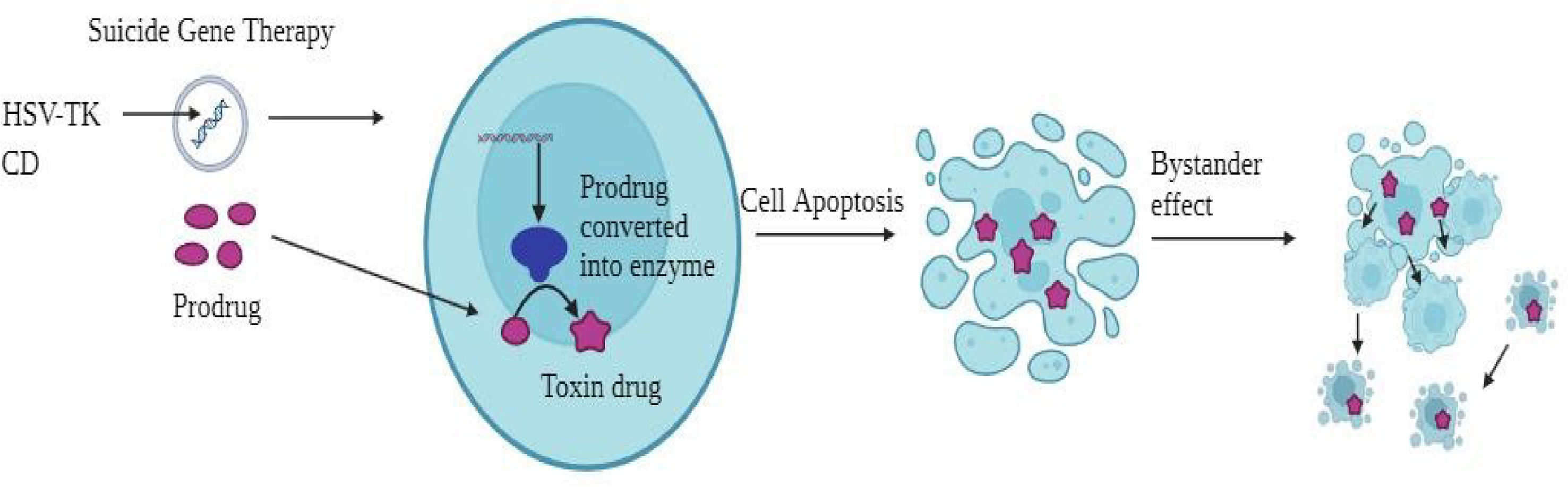
Mechanism of action of gene therapy for BCSuicide geneSuicide gene therapy is designed to induce tumor cell death by introducing specific genes that trigger cytotoxic effects, either directly or indirectly (Fig. 3). In direct suicide gene therapy, genes encoding proteins that cause tumor cell death are introduced. Among these is the diphtheria toxin, which is well known. A gene damages the cell membrane, making tumor cells less likely to survive.72–74 Indirect suicide gene therapy, on the other hand, involves adding genes that make enzymes like Herpes Simplex Virus Thymidine Kinase (HSV-tk), which turn on prodrugs like 5-fluorocytosine (5-FC). This change creates 5-fluorouracil (5-FU), a chemotherapy drug that can help fight cancers of the breast, prostate, stomach, and hepatocellular carcinoma (HCC).75,76 Another method involves synthetic molecules like B/B Homodimer (AP20187) and a modified human Caspase 9 (iCas9) gene, which, when delivered to cancer cells or mesenchymal stromal cells (MSCs), trigger apoptosis by inducing dimerization.77–79 Additionally, therapies targeting specific promoters like the H19 RNA gene can regulate cancer cell proliferation. It has been shown that blocking H19 can cause tumors to shrink and cells to die.80,81 Specifically targeting the hTERT gene, which helps tumor cells live forever, has also shown promise in encouraging tumor regression.82 Therapies such as OBP-301 (Telomelysin) have shown promise in preclinical tests.83
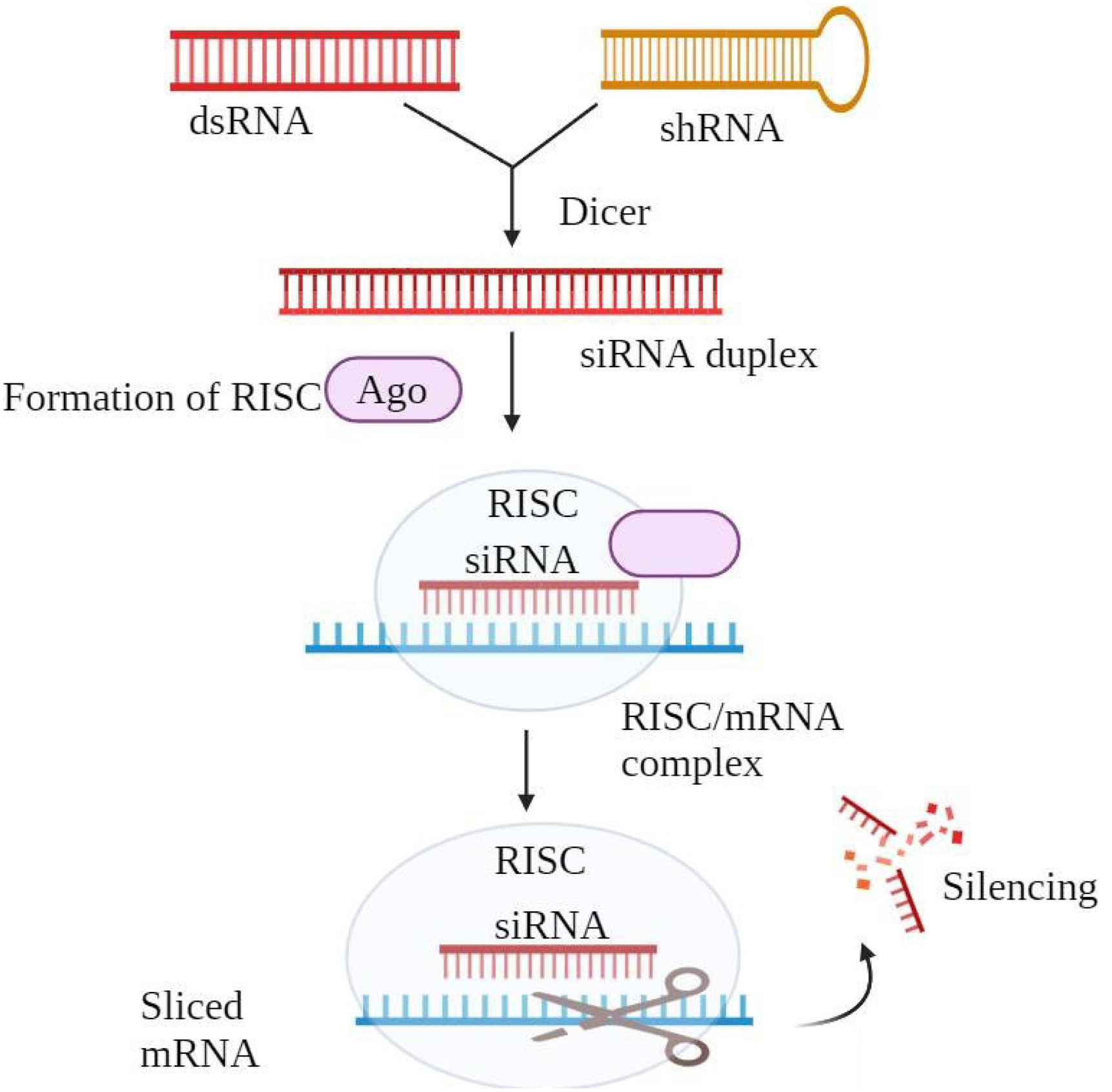
Gene silencingTransferring particular small interfering RNA (siRNA) into cells allows targeted gene silencing (Fig. 4). The siRNA forms a duplex with the RNA-induced silencing complex (RISC), which causes messenger RNA (mRNA) breakdown and disturbs protein synthesis, therefore silencing the intended gene. Targeting any gene, siRNA is a potential treatment method for many diseases including cancer, liver cirrhosis, hepatitis B, hypercholesterolemia, and human papillomavirus. Unlike other methods, siRNA does not bind to chromosomal DNA, reducing the risk of gene alterations or mutations. Its high specificity helps prevent drug resistance with minimal side effects. siRNA has demonstrated the ability to suppress cancer-related genes, shrinking tumors without eliminating faulty genes. However, systemic delivery remains challenging due to rapid clearance. To deal with this, delivery systems like CALAA-01 for melanoma and ALN-VSPOI for liver cancer are currently going through clinical studies. However, the approach faces challenges because it is toxic and does not perform effectively at transfection.84–87
Gene editing techniques for cancerThe advancement of gene editing tools has revolutionized the ability to make precise changes to specific gene sequences in eukaryotic cells, with broad applications in research, clinical settings, and biotechnology (Fig. 5).88 ZFNs, TALENs, and CRISPR/Cas are examples of customizable nucleases that have sped up the move from lab studies to clinical use. ZFNs target DNA sequences by putting together a Cys2-His2 protein and the Fok1 restriction endonuclease domain.89–91 Similarly, TALENs use Fok1's catalytic domain and a DNA-binding domain for targeted cleavage.92 These methods have been successful in inhibiting the proliferation of acute lymphoblastic leukemia and cervical cancer cells,93–95 and addressing treatment resistance in BC cells.95 The CRISPR/Cas system, which is a defense mechanism for bacteria, has made gene editing even better by using guide RNA (gRNA) to target specific DNA sequences and Cas proteins for cleavage. This system has a lot of promise for improving immunity, fighting cancer, and fixing mutations.96,97 CRISPR has shown effectiveness in reducing resistance, tumor size, and metastasis in cancers like pancreatic, prostate, colon, and BC.98–103
Directing gene therapy in cancerGene therapy has made significant strides in recent years; however, one of the biggest problems still is getting therapeutic nucleic acids (TNAs) to the right cells to make them function.104 Developing a carrier that can specifically target and deliver TNAs to cancer cells, while controlling their expression within those cells, is crucial for ensuring precise and effective treatment. Such a carrier must be specifically designed to transport genetic material efficiently.105,106
Regulatory sequences are another important part of gene therapy. They control gene expression in target cells, even if the recombinant DNA cannot get into healthy cells.107 The success of cancer gene therapy often hinges on these regulatory elements. Promoters, which are cis-acting sequences that control the initiation of mRNA transcription, are critical in ensuring that therapeutic genes are expressed only in the desired tissues.108 Located before the coding region of a gene, promoters bind transcription factors and regulate gene activity.109
Promoters are an active area of biotechnology research due to their ability to enhance transcription and provide additional control over gene expression. They can target specific organs or tissues, helping to control gene production and minimize unwanted side effects. Ideally, a promoter must be both strong enough to ensure robust gene expression and specific enough to limit expression to the desired tissue, ensuring safety and efficacy. Three main categories of promoters tissue-specific, cancer-specific, and tumor-specific can be utilized to precisely target cancer cells at the transcriptional level.110,111
Role of CRISPR/Cas9CRISPR/Cas9 technology has changed the way researchers study BC by allowing exact gene modifications. These changes have helped us learn a lot more about how tumors grow and cells multiply (Fig. 6). CRISPR, which stands for “Clustered Regularly Interspaced Short Palindromic Repeats” was first found in the IAP gene of Escherichia coli by Yoshizumi Ishino and his coworkers in 1987. Bacteria use it to protect themselves against viruses.112 This finding laid the groundwork for gene-editing applications in molecular biology. In the early 2000s, Dr. Jennifer Doudna and her team adapted the CRISPR system for DNA editing, leading to the development of the CRISPR/Cas9 technology.113 By 2013, the first successful human gene editing was achieved using CRISPR/Cas9,114 marking a pivotal moment in biotechnology. These days, CRISPR/Cas9 is one of the most useful tools for both science and medicine.
The CRISPR/Cas9 system is made up of three main parts: a noncoding RNA (crRNA) that targets the DNA sequence; a tracrRNA that, when combined with crRNA, creates a single-guide RNA (sgRNA); and a Cas protein that cuts the target DNA.115 The CRISPR mechanism allows prokaryotes to acquire foreign DNA fragments from viruses, which are integrated into the host genome as memory sequences known as spacers. These spacers enable bacteria to recognize and disable foreign DNA during subsequent infections.116 The insertion of these foreign DNA sequences into the genome is done by Cas1 and Cas2 proteins, with Cas1 having the appropriate enzyme activity.117–119 Once spacers are added, the CRISPR locus is turned into a long RNA molecule that has both spacer and repeat sequences. At the 3′ end of this pre-crRNA, the tracrRNA binds. This makes a double-stranded RNA that RNase III cuts. This process makes a fully developed gRNA complex that can direct the Cas protein to its target.120–123 The gRNA complex sets off the Cas9 protein, which then looks for foreign DNA sequences that match and cut the DNA at the target spot. This process is what makes CRISPR-mediated defense in bacteria work.124 CRISPR/Cas9 is a very important tool in genetic research because it is easy to use and can do many different things. It is especially useful for learning how diseases like BC are caused genetically. The need for a PAM sequence for targeting is a drawback of CRISPR/Cas9, despite its great efficiency.125 Researchers have come up with Cas9 variants like spCas9 to tackle this issue. These variants can recognize a wider range of PAM sequences, making the mechanism more versatile.126,127 Additionally, modified versions of Cas9, known as deactivated Cas9 (dCas9), are employed in applications such as transcriptional suppression or activation via CRISPR interference (CRISPRi) and CRISPR activation (CRISPRa).128,129 These innovations allow for more precise gene regulation, providing powerful tools for cancer research and therapies, especially in correcting mutations and offering potential treatment options for BC and other diseases.
The conflicting clinical findings suggest that CRISPR technology may serve as an efficient way to validate medication specificity in preclinical research prior to clinical testing. In comparison to other gene modification techniques, such as antagonists or RNA interference (RNAi), CRISPR-mediated gene manipulation is more precise and likely exhibits less off-target effects.130 Recently, a study employing CRISPR technology to disrupt Maternal Embryonic Leucine Zipper Kinase (MELK) in vitro refuted the belief that MELK is essential for the viability of basal BC cells, despite continuing clinical trials investigating MELK inhibitors as chemotherapeutics. This study supports the necessity of employing CRISPR technology in preclinical target validation by challenging the justification for existing clinical trials.131 Motivated by the previously mentioned findings, they enhance the CRISPR/Cas9 system to specifically target the PARP1 gene to validate the selective synergism between PARP1 disruption and chemotherapy in TNBC cells. It evaluated several BRCA1 and PARP1 genetic profiles in both an in vitro 2D environment and a 3D tumor-on-a-chip system to more accurately replicate a physiological context.132
CAR-T cellsThe most prevalent technique for generating tumor-specific T cells involves genetically altering T cells using CARs. Chimeric Antigen Receptors (CARs) typically comprise an extracellular ligand-binding domain derived from a single-chain antibody (scFv), a hinge region, a transmembrane domain, a cytoplasmic signaling chain, and/or costimulatory components. CAR-engineered T cells integrate the specificity of monoclonal antibodies with the homing and cytotoxic capabilities of T cells. CAR-T cell treatment is seen as having multiple advantages relative to other cellular immunotherapies. Initially, CAR-T cells are produced by nonspecifically activated polyclonal T cells. Consequently, they surmount the challenges associated with the isolation and amplification of natural tumor-specific CD4+ and CD8+ T cells.133,134 Secondly, CAR-T cells identify target antigens in a manner that is independent of MHC. This characteristic allows CAR-T cells to identify target cells with diminished HLA expression or antigen processing, which are regarded as significant contributors to tumor immunological evasion.135–137 Thirdly, CAR-T cells may actively and selectively migrate to tumor locations and can proliferate and endure long-term following tumor detection in vivo. Consequently, CAR-T cells directed against tumor-associated antigens (TAAs) may surpass monoclonal antibodies (mAbs) in eliciting enduring tumor responses.138
CAR-based therapy for solid tumors utilizes CARs aimed at colorectal cancer, ovarian cancer, prostate cancer, and metastatic renal cell carcinoma, among others.139 Research is also focused on HER-2, Lewis Y, mesothelin, folate receptor alpha (FR-α), and Muc1 for BC in vitro and in animal models.140,141 HER-2 expression is recognized to influence BC recurrence and overall survival. The application of anti-HER2 monoclonal antibodies has markedly enhanced BC prognosis. HER-2-targeted medicines are currently a fundamental aspect of the treatment for HER-2 overexpressing BC.142 Numerous clinical trials involving CAR-T cells aimed at HER-2 are currently underway, including a phase I/II study assessing HER-2-targeted CAR-T cells in conjunction with chemotherapy or HER-2 antibody inhibitor therapy for refractory HER-2 advanced BC, and a phase II study evaluating anti-CD3 x anti-HER2/Neu armed activated T cells following second-line chemotherapy in women with HER2/Neu (0, 1+ or 2+) metastatic BC.143
ConclusionOnly 5% of females experiencing breast discomfort are ultimately diagnosed with BC. The current BC treatment options encompass local therapies, systemic therapies and advanced systemic therapies. Due to advancements in screening, diagnosis, and treatment, the mortality rate from BC has decreased, with approximately 90% of newly diagnosed women surviving for at least five years. Ongoing research continues to improve the efficacy of screening and treatment methods. Future gene therapy candidates will be chosen based on tumor genetic data and the host's immune responses. With breakthroughs in safe gene delivery vectors and nuclease technology, genome editing holds promise as a novel approach for treating otherwise untreatable cancers. The trend in cancer treatment is shifting toward personalized care tailored to each patient's genome, immune system, and tumor profile. While CRISPR/Cas9 and CAR-T cell therapy show promise in stopping tumor growth, ethical questions, off-target consequences, and delivery challenges demand more study.
FundingThe authors have not received any funding for the study.
Ethical disclosuresIt is a review article, and ethical disclosure is not applicable to this study.
Authors have no competing interests.