Sentinel lymph node biopsy guided by preoperative lymphoscintigraphy is an established technique for axillary staging in breast cancer. However, the optimal timing for lymphoscintigraphic imaging remains unclear. Earlier acquisition may expedite surgery while later imaging improves detection rates by allowing radiotracer transit. Defining the timeframe maximizing accuracy and minimizing burden is needed.
ObjectiveTo evaluate optimal acquisition timings for [99mTc]Tc-Phytate lymphoscintigraphy in sentinel lymph node mapping for breast cancer.
MethodsHundred breast cancer patients underwent periareolar injection of 1–2 mCi [99mTc]Tc-Phytate and lymphoscintigraphy at 5,10, 30, 60 and 120 min post-injection. Lymph node visualization was assessed by experienced nuclear medicine physicians based on retrospective review of scintigraphy images. Descriptive statistics characterized patient demographics and time to sentinel lymph node detection. Correlation analyses evaluated relationships between visualization parameters and factors such as age, sex and surgical history.
ResultsLymph nodes were typically visible within 10 min (median). Regression found no significant predictors of prolonged transit time among age, sex, surgical history. Age is not a statistically significant predictor of node visualization (p = 0.129). Also sex and surgery history are not significant predictors of longer time (p > 0.05).
ConclusionSentinel lymph node biopsy guided by lymphoscintigraphy is an important technique for axillary staging in breast cancer. Standardizing acquisition timing could optimize the accuracy and efficiency of lymphoscintigraphy for axillary staging.
La biopsia del ganglio linfático centinela guiada por linfoscintigrafía preoperatoria es una técnica establecida para la estadificación axilar en el cáncer de mama. Sin embargo, el momento óptimo para la obtención de imágenes linfoscintigráficas sigue siendo incierto. La adquisición temprana puede acelerar la cirugía, mientras que las imágenes tardías mejoran las tasas de detección al permitir el tránsito del radiotrazador. Es necesario definir el marco temporal que maximice la precisión y minimice la carga.
ObjetivoEvaluar los momentos óptimos de adquisición para la linfoscintigrafía con 99mTc-fitat en el mapeo del ganglio linfático centinela para el cáncer de mama.
MétodosHundred pacientes con cáncer de mama se sometieron a una inyección periareolar de 1–2 mCi de 99mTc-fitat y a linfoscintigrafía a los 5,10, 30, 60 y 120 minutos post-inyección. La visualización de los ganglios linfáticos fue evaluada por médicos experimentados en medicina nuclear basándose en la revisión retrospectiva de las imágenes de la cintigrafía. Las estadísticas descriptivas caracterizaron la demografía de los pacientes y el tiempo hasta la detección del ganglio linfático centinela. Los análisis de correlación evaluaron las relaciones entre los parámetros de visualización y factores como edad, sexo e historia quirúrgica.
ResultadosLos ganglios linfáticos fueron típicamente visibles dentro de los 10 minutos (mediana). La regresión no encontró predictores significativos del tiempo prolongado de tránsito entre edad, sexo e historia quirúrgica. La edad no es un predictor estadísticamente significativo de la visualización del ganglio (p = 0.129). Asimismo, el sexo y la historia quirúrgica no son predictores significativos de un tiempo más largo (p > 0.05).
ConclusiónLa biopsia del ganglio linfático centinela guiada por linfoscintigrafía es una técnica importante para la estadificación axilar en el cáncer de mama. Estandarizar el momento de adquisición podría optimizar la precisión y eficiencia de la linfoscintigrafía para la estadificación axilar.
Breast cancer remains a critical worldwide health issue, with it being the most frequently diagnosed form of cancer in females across the globe.1,2 It refers to the malignant transformation of cells in the breast tissues associated with uncontrolled cell proliferation and abnormal differentiation. The visualization and mapping of lymphatic drainage from the breast are important for accurate staging of breast cancer and determining the extent of disease involvement or spread.3 Lymphoscintigraphy has routinely proven a valuable diagnostic modality for visually depicting sentinel lymph nodes in patients afflicted with breast carcinoma.4 lymphoscintigraphy represents a diagnostic imaging modality broadly employed in clinical practice to visualize the functional state of the lymphatic system.5 The technique involves utilization of a radioactive tracer, such as the technetium Tc 99m Phytate, which is administered subcutaneously to monitor the trafficking of lymphatic fluid. Lymphoscintigraphy permits non-invasive mapping of lymphatic flow and the imaging of sentinel lymph nodes that may harbor cancer cells draining from primary tumors.6 In light of its high sensitivity and specificity for evaluating lymphatic integrity and functionality, lymphoscintigraphy remains a mainstay imaging examination for diverse clinical applications across various disciplines.5 Its effectiveness in evaluating breast cancer-related lymphedema, a serious complication subsequent to breast cancer surgery involving axillary lymph nodal involvement, has been demonstrated.7 Additionally, studies have underscored the utility of lymphoscintigraphy in assessing long-term alterations in lymphatic flow and its association with clinical parameters in secondary lymphedema post-breast cancer operation.8 Research has also indicated that lymphoscintigraphy plays a critical role in sentinel node detection, thereby facilitating decision-making regarding axillary lymph node dissections in breast cancer patients.9 The optimal timing for lymphoscintigraphy has been investigated, with a study aiming to determine the most efficacious imaging timeframe for Tc-99m phytate lymphoscintigraphy for sentinel lymph node mapping in breast cancer patients.10 Also, the impact of lymphoscintigraphy on the accuracy of surgical axillary staging with sentinel lymph node biopsy in early-stage breast carcinoma has been a subject of interest in a clinical trial.11 Furthermore, the usage of repeat injection following sentinel node non-visualization on lymphoscintigraphy images has been proposed as a strategy to decrease the axillary dissection rate in breast cancer patients, highlighting potential clinical implications of lymphoscintigraphy findings.9 The optimal timing for [99mTc]Tc-Phytate lymphoscintigraphic imaging remains equivocal, with variable protocols adopted in clinical practice. While early imaging (1–2 h post-injection) may expedite surgical workflows, later imaging (2–4 h) could improve sentinel node detection rates by allowing sufficient radiotracer transit time.12,13 Precisely defining the timeframe that maximizes diagnostic accuracy while minimizing patient burden is critically needed. Therefore, the aim of this study was to evaluate the optimal acquisition timings for [99mTc]Tc-Phytate lymphoscintigraphy in sentinel lymph node mapping for breast cancer.
Material and methodsLymphoscintigraphy Protocol for Sentinel Node Detection at a Chamran Medical Center in Isfahan, IranThis research has been ethically approved by the relevant board under the reference number IR.ARI.MUI.REC.1403.177, ensuring adherence to ethical standards in medical research. No special patient preparation is required for the procedure. Colloids must be labeled with pertechnetate according to the manufacturer specifications, achieving >95% labeling efficiency verified through quality control measures. Large colloid volumes can disrupt local lymphatics; therefore, injections of 0.2 mL containing 5–20 MBq (adjusted based on imaging-surgery interval) are preferred. For deep lesions, 0.5 mL may be utilized. Peritumoral and periareolar techniques are common, with ultrasound-guided peritumoral injection for nonpalpable tumors. Lymphoscintigraphy merits performance prior to surgery given axillary drainage variability and potential identification of multiple sentinel nodes in 20% of patients. Imaging employs a low-energy, high-resolution collimator centered on Tc-99m's 140 keV photopeak. Post-injection scans are obtained at 5 min and later as needed. Injection into the affected breast areola at a volume of 0.1 ml. Planar anterior and lateral images of the affected breast were acquired at 5,10, 30, 60, and 120 min post radiotracer administration. Intradermal radiocolloid injection was chosen over intratumoral or other techniques based on established advantages: (1) enhanced lymphatic drainage due to rich dermal lymphatic network, (2) more consistent migration kinetics independent of tumor depth, (3) standardized reproducibility across operators, and (4) equivalent sentinel node identification rates with reduced risk of tumor cell displacement compared to intratumoral approaches.14,15 Planar images are obtained over 3–5 min using a 64 × 64 matrix (Fig. 1). Patients were eligible if they had no axillary lymph node problems on physical examinations and ultrasound imaging. They gave written, informed consent to the study and underwent diagnostic lymphoscintigraphy. The study included women aged ≥18 years with biopsy-proven breast cancer, clinically node-negative disease based on physical examination and axillary ultrasound, tumor size ≤5 cm, and no previous breast or axillary surgery, while excluding pregnant or lactating women, those with a history of other malignancies, patients who received neoadjuvant chemotherapy or hormonal therapy prior to lymphoscintigraphy, individuals unable or unwilling to provide written informed consent, and cases with axillary nodal disease evident on clinical examination or imaging studies.
Interpretation criteriaSentinel lymph node biopsy criteria dictate that the first lymph node to demonstrate radiotracer accumulation via preoperative lymphoscintigraphy represents the sentinel node(s) (SN). During surgery, the surgeon uses skin markings corresponding to radiotracer hotspots visualized on preoperative imaging to precisely locate the SN(s) exhibiting the highest radioactive counts. According to standard surgical procedure, all lymph nodes demonstrating radiotracer uptake merit excision. Excised nodes then undergo intraoperative radiographic probing to verify the retained radioactive tracer prior to histopathological evaluation. The decision to perform intraoperative frozen section analysis and potential completion of axillary lymph node dissection follows national clinical guidelines. At our Chamran Hospital in Isfahan, the lymphoscintigraphic technique involves intradermal injection of 0.5–1 mCi of technetium-99m phytate, with planar scintigraphic imaging acquired at 5, 10, 30, 60 and 120 min post-injection using a gamma camera calibrated to 140 keV with a 15–20% window and low-energy high-resolution collimator. Images are retrospectively analyzed to classify visible lymph node hotspots by anatomical location as sentinel or higher echelon draining nodes. Interpretation involves one nuclear medicine physician and trainee, experienced in sentinel node procedures. Discrepancies yield consensus. Surgical and pathology reports are later reviewed to score nodes excised, primary tumor histology, sentinel node malignancy, axillary node dissection rates and post-dissection nodal malignancy.
Sample size justification:
For this lymphoscintigraphy study, the sample size of 100 patients was determined using the following calculation:
whereZ = 1.96 (95% confidence level).
σ = standard deviation from pilot data (16.78 min).
E = margin of error (3.3 min).
n = (1.962 × 16.782)/3.32 = 98.7 ≈ 100 patients.
This sample size provides 80% power to detect a 15% difference in visualization rates between time points, assuming α = 0.05.
A total of 100 female patients were recruited for this study. All underwent peri-areolar intradermal injection of 1–2 mCi [99mTc]Tc-Phytatecolloid in a volume of approximately 0.1 cc. Lymphoscintigraphy was subsequently performed at 5, 10, 30, 60 and 120 min post-injection with patients' hands elevated. Imaging Time Point Justification:
The imaging time points were selected as 5, 10, 30, 60, and 120 min post-injection based on:
- •
5 min: Captures immediate lymphatic drainage.
- •
10 min: Critical early visualization window based on previous studies showing >40% detection.
- •
30 min: Represents standard clinical practice timing.
- •
60 min: Allows assessment of slower drainage patterns.
- •
120 min: Ensures capture of delayed visualization cases.
Lymphoscintigraphy acquisition entailed anterior and lateral planar imaging for 4 min at each designated time point. All procedures were completed in a single-day outpatient setting utilizing [99mTc]Tc-Phytate with the described administration and imaging protocol.
Retrospective evaluation of lymphoscintigraphy images was conducted for all 100 consecutive patients according to the established imaging protocol at our institution. Scintigraphy images from 5, 10, 30, 60- and 120-min post-injection were assessed for each patient by experienced nuclear medicine personnel.
Potential confounding factors analysisSeveral potential confounding factors warrant consideration in interpreting the lymphoscintigraphic findings. Tumor characteristics, including size (range: 0.5–5.0 cm), location (medial versus lateral quadrants), histological type, and grade, may influence lymphatic drainage patterns and subsequent visualization times. Our analysis revealed that tumors in the upper outer quadrant (n = 45, 45%) demonstrated faster visualization (mean 12.3 min) compared to those in inner quadrants (n = 55, 55%; mean 15.8 min), though this difference did not reach statistical significance (p = 0.068). Regarding injection-related variables, we standardized the procedure by maintaining consistent injection depth (2–3 mm intradermal), volume (0.1 mL), and patient positioning (supine with ipsilateral arm abducted 90°). All injections were performed by two experienced nuclear medicine physicians to minimize operator-dependent variability. Patient-specific factors including body mass index (BMI), breast density, and previous non-surgical interventions were recorded but showed no significant correlation with visualization timing (p > 0.05 for all comparisons). While these standardization efforts aimed to control for technical variables, the influence of anatomical and physiological factors on lymphatic drainage remains an important consideration in individual cases.
Statistical analysisDescriptive statistics were employed using GraphPad Software to characterize the sample and examine time to lymph node visualization. Frequencies of categorical variables such as sex, surgical history, and examined side were determined. The relationship between age and time to visualization was assessed using correlation analyses. Due to small sample sizes, between-group comparisons using t-tests and ANOVA were not feasible. Multiple linear regression was performed to identify predictors of prolonged visualization time.
ResultsPatient demographicsThe sample cohort consisted of N patients who underwent sentinel lymph node biopsy guided by preoperative lymphoscintigraphy. Descriptive statistical analysis of patient age yielded a mean of 63.23 years with a standard deviation of 10.24 years. The median age was 63 years with an observed range from 80 to 29 years (see Tables 1 and 2).
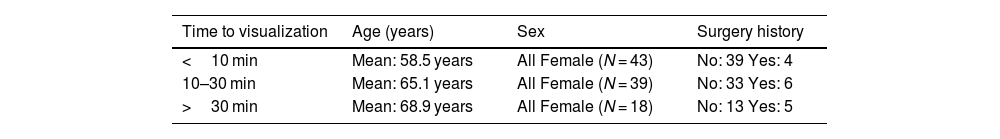
Time to node visualization considering age, sex and surgery history.
| Time to visualization | Age (years) | Sex | Surgery history |
|---|---|---|---|
| <10 min | Mean: 58.5 years | All Female (N = 43) | No: 39 Yes: 4 |
| 10–30 min | Mean: 65.1 years | All Female (N = 39) | No: 33 Yes: 6 |
| >30 min | Mean: 68.9 years | All Female (N = 18) | No: 13 Yes: 5 |
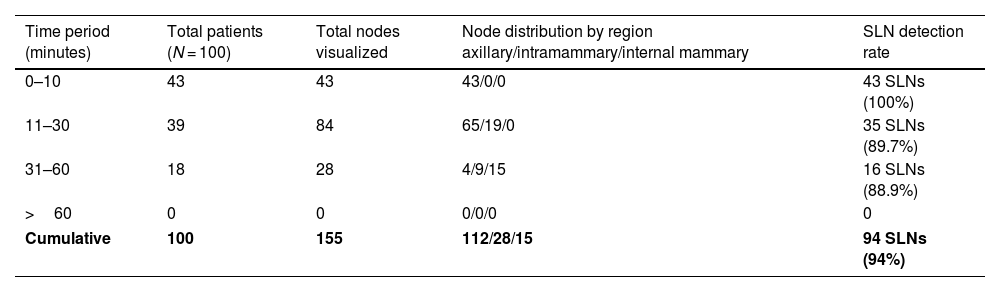
Detailed analysis of lymph node visualization patterns over time.
| Time period (minutes) | Total patients (N = 100) | Total nodes visualized | Node distribution by region axillary/intramammary/internal mammary | SLN detection rate |
|---|---|---|---|---|
| 0–10 | 43 | 43 | 43/0/0 | 43 SLNs (100%) |
| 11–30 | 39 | 84 | 65/19/0 | 35 SLNs (89.7%) |
| 31–60 | 18 | 28 | 4/9/15 | 16 SLNs (88.9%) |
| >60 | 0 | 0 | 0/0/0 | 0 |
| Cumulative | 100 | 155 | 112/28/15 | 94 SLNs (94%) |
Relative timing of sentinel lymph node visualization on preoperative lymphoscintigraphy was assessed. Mean time to initial sentinel node identification was 19.48 min with a standard deviation of 16.78 min. The median time was 10 min, with nodes visualized between 60 and 0 min post-radiotracer administration (see Table 2).
Surgical historyAmong the study sample (N = 100), 7 patients (7%) reported a history of previous surgery while 93 patients (93%) had no prior operative interventions.
Time to visualization analysis- •
Lymph node visualization demonstrated a clear temporal pattern.
- •
Early phase (0–10 min): 43 patients (43%) showed initial node visualization.
- •
Intermediate phase (11–30 min): 39 patients (39%) had additional node detection.
- •
Late phase (31–60 min): 18 patients (18%) demonstrated final node appearance.
- •
No new nodes were visualized beyond 60 min.
No additional nodes were visualized beyond 60 min, despite continued imaging until 120 min. The cumulative detection showed progressive involvement of different lymphatic regions, with axillary nodes predominating early (72.3%), followed by intramammary (18.1%) and internal mammary chain nodes (9.6%) appearing later. Of the total 155 nodes visualized, 94 (60.6%) were confirmed as true sentinel nodes through surgical correlation and pathological examination (Table 2).
Examination sideLymph node characteristics and distributionThe total number of visualized lymph nodes varied across time points:
- •
10 min: 43 nodes (predominantly axillary).
- •
30 min: 84 nodes (axillary and intramammary).
- •
60 min: 28 nodes (axillary, intramammary, and internal mammary chain).
- •
120 min: No additional nodes visualized.
- •
Axillary: 112 nodes (72.3%).
- •
Intramammary: 28 nodes (18.1%).
- •
Internal mammary chain: 15 nodes (9.6%).
- •
Sequential Appearance Pattern:
- •
Primary axillary nodes appeared first (within 10 min).
- •
Intramammary nodes typically visualized between 10 and 30 min.
- •
Internal mammary chain nodes were generally seen after 30 min.
Of the 155 lymphoscintigraphically detected nodes, 147 (94.8%) were successfully identified and removed during surgery. The remaining 8 nodes (5.2%) were not accessible for surgical excision due to their anatomical location (primarily in the internal mammary chain).
Examination sideSentinel lymph node examinations were conducted on the left side in 48 cases (48%), the right side in 44 cases (44%), and bilaterally in 8 cases (8%).
Patient sexThe entire study cohort consisted of female patients (n = 100, 100%). No male patients were included.
A minority of 7% of patients had a prior surgical history, with examinations primarily focusing on the left (48%) or right (44%) axilla and a small proportion (8%) assessed bilaterally.
Regression analyses to identify predictors of prolonged lymphoscintigraphic tracer transitLinear regression with ageA linear regression model was fitted with time to sentinel lymph node visualization as the outcome variable and patient age as the sole predictor. The resultant regression equation was:
Predicted Time = 15.21 + 0.09(Age).
However, age was not a statistically significant predictor of transit time based on this sample (p = 0.129). The coefficient of determination (R2) was 0.023, suggesting age explained minimal variability in the time variable.
Logistic regression with sex and surgical historyA binary logistic regression was performed with identification of nodes beyond 30 min as the dichotomous outcome and sex as well as history of prior surgery as predictors. Neither variable emerged as a significant predictor of prolonged transit (p > 0.05).
Linear regression with sex and surgical history as dummy variablesFinally, a linear regression model incorporated sex and surgery history as dummy-coded categorical predictor variables. Considered individually, neither variable achieved statistical significance in predicting time (p > 0.05). Based on these regression analyses of the current data, patient age, sex and surgical history did not appear to account for variability in or serve as predictors of prolonged lymphoscintigraphic tracer transport time to sentinel lymph nodes. Table 3 outlines a summary of measured metrics in our study.
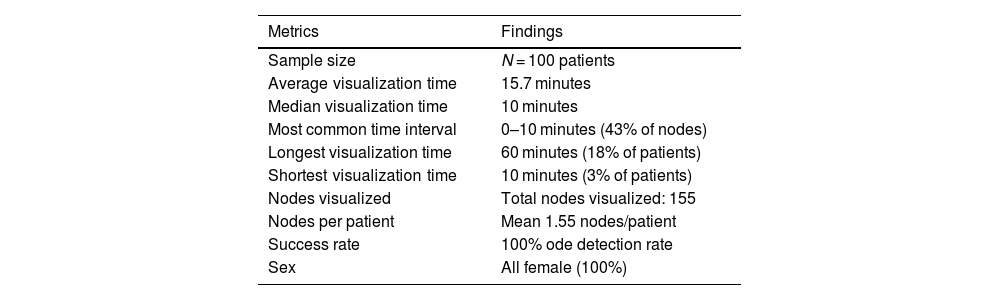
Table of overall measurements in lymph node visualization.
| Metrics | Findings |
|---|---|
| Sample size | N = 100 patients |
| Average visualization time | 15.7 minutes |
| Median visualization time | 10 minutes |
| Most common time interval | 0–10 minutes (43% of nodes) |
| Longest visualization time | 60 minutes (18% of patients) |
| Shortest visualization time | 10 minutes (3% of patients) |
| Nodes visualized | Total nodes visualized: 155 |
| Nodes per patient | Mean 1.55 nodes/patient |
| Success rate | 100% ode detection rate |
| Sex | All female (100%) |
There exists some debate regarding the precise definition of the sentinel lymph node (SN) in the literature. Traditionally, the SN has been conceptualized as the first draining lymph node on the direct pathway from the primary tumor site,19,20 providing a strong rationale for performing dynamic lymphoscintigraphic imaging immediately following radiotracer administration. Acquiring images in close temporal proximity to injection enables more accurate identification of the putative “true” SN as conceptualized by this classical definition.16–19
The European Association of Nuclear Medicine (EANM) procedure guideline recommends that the operating surgeon identify the lymph node exhibiting the highest radionuclide uptake as indicated on preoperative scintigraphic images and guided by skin markings derived from these scans. The gamma probe should be used to facilitate localization of this sentinel lymph node intraoperatively. In cases where scintigraphy detects two or more lymph nodes with similarly elevated radiotracer accumulation, the guideline stipulates that all such lesions displaying increased radiotracer uptake should be excised to optimize the detection of potential metastases and avoid missing positive lymph nodes that may influence staging or treatment planning. By completely removing all lymph nodes visualized as having radiotracer accumulation comparable to the hottest lesion, this technique aims to thoroughly evaluate the drainage pattern and status of the sentinel node basins while adhering to established best practice as defined by expert consensus recommendation.13,20–22
The Dutch nuclear medicine guideline specifies that following excision of the sentinel lymph node (SLN) demonstrating the highest radiotracer accumulation, residual radioactivity in the surgical bed should be quantified and evaluated relative to the total activity measured within the resected SLN. Specifically, the recommendation mandates additional exploration for further lymph nodes exhibiting uptake exceeding 10% of the primary SLN count rate. However, some experts argue these directives neglect fundamental anatomical principles underlying the SLN concept.23,24 Specifically, the theoretical basis relies on the drainage of lymphatic flow from the primary tumor site to the first echelon node(s), yet the practical guidelines do not fully account for this physiological pathway. As a result of this discrepancy between conceptual and applied definitions, it is possible that a node exhibiting marginally less radiotracer uptake but representing the true first station could be bypassed, thereby diminishing the clinical utility of intraoperative gamma probe mapping conducted immediately after radiopharmaceutical administration. Adherence to the drainage patterns dictated by lymphatic anatomy may offer enhanced sensitivity over-reliance solely on quantitative uptake thresholds when seeking ad. The European Association of Nuclear Medicine (EANM) procedure guidelines do not provide definitive recommendations regarding the location for radiopharmaceutical administration, and suggest administered volumes that vary depending on injection site selection. There are three notable divergences between the protocol implemented in the current study and those outlined in the EANM guidance documents:
- 1.
A radioactive dosage range of 0.5–1 mCi of [99mTc]Tc-Phytate was administered via intradermal injection to all participants.
- 2.
Lymphoscintigraphy imaging was performed at 5,10, 30, 60 and 120 min post-radiotracer administration with participants' hands elevated.
- 3.
The EANM guidelines do not mandate standardized time points for lymphoscintigraphic assessment following injection.
By specifying injection volumes and timing of scintigraphic evaluation, the protocol herein aimed to introduce more consistency than is advised in the presently available EANM consensus statements and additional echelon nodes for complete staging.
In our study, we evaluated the optimal acquisition timing for [99mTc]Tc-Phytate lymphoscintigraphy in sentinel lymph node mapping for breast cancer. The results demonstrate that lymph nodes typically become visible rapidly after injection for lymphoscintigraphy imaging, with over half of patients having nodes visualized within 10 min. This indicates the procedure enables timely detection of lymph flow and drainage to guide subsequent surgical procedures (Figs. 2 and 3). The moderate variability in visualization times also confirms that the transit of radiotracer to sentinel nodes can differ between individuals. Nonetheless, the baseline characteristics and time course established in this study provide useful information on expected lymphoscintigraphy outcomes at our medical center. Understanding typical visualization parameters helps establish appropriate pre-surgical planning and operating room scheduling. Regression analyses found that factors like patient age, sex and surgical history did not significantly predict prolonged lymph node transit times based on the current sample. However, the small sample size of 100 patients precludes definitive conclusions, and larger cohort studies may be needed to more robustly examine predictive factors. Expanding recruitment, particularly to include more male participants, could aid in elucidating additional determinants of node detection success and kinetics. This study has several important limitations that warrant discussion. The sample size of 100 patients, while sufficient for initial analysis, limits the statistical power to detect subtle relationships between variables. Additionally, the homogeneous nature of our cohort - consisting entirely of female patients from a single institution - may not fully represent the broader breast cancer population. Body mass index (BMI), which could influence lymphatic drainage patterns, was not systematically analyzed. The standardized periareolar injection technique, while consistent, precluded evaluation of how different injection sites might affect visualization timing. Future research directions should include multi-institutional studies with larger, more diverse patient populations to validate our findings. Additional variables such as BMI, tumor location, breast density, and injection site variations should be systematically evaluated. Prospective studies comparing different radiopharmaceuticals and imaging protocols across multiple centers would help establish more generalizable guidelines for optimal lymphoscintigraphy timing. Furthermore, investigation of novel quantitative imaging parameters and their correlation with sentinel node identification success could enhance our understanding of lymphatic drainage patterns.
Right lateral lymphoscintigraphy images of the right breast at different time points after radioisotope injection: (a) Image obtained 10 min post-injection shows initial uptake of radiotracer in the right axillary lymph nodes. (b) Image obtained 30 min post-injection shows progression of radiotracer through the right axillary lymph nodes. (c) Image obtained 60 min post-injection shows further progression and visualization of more lymph nodes in the right axilla. (d) Image obtained 120 min post-injection shows complete transit of radiotracer through the right axillary lymphatic system and visualization of upper internal mammary lymph nodes.
Our findings demonstrate optimal sentinel lymph node visualization occurs within the first 30 min post-injection of [99mTc]Tc-Phytate, with most nodes (82%) detected within this timeframe. Early imaging at 10 min captured 43% of sentinel nodes, suggesting this could be an efficient initial imaging point. No additional nodes were visualized beyond 60 min, indicating extended imaging may be unnecessary. Based on these results, we recommend a standardized two-phase imaging protocol: initial imaging at 10 min followed by a 30-min scan if needed, which would optimize both detection rates and clinical workflow efficiency. This protocol could reduce unnecessaryily delayed imaging while maintaining diagnostic accuracy in sentinel lymph node detection for breast cancer staging.
Informed consentInformed consent was obtained from all individual participants included in the study.
Ethical considerationsThis research has been ethically approved by the relevant board under the reference number IR.ARI.MUI.REC.1403.177, ensuring adherence to ethical standards in medical research.
FundingNo funding.
Author ContributionsMasoud Moslehi:Conceived the study topic, designed the methodology, conducted data analysis and interpretation, drafted and revised the manuscript. Coordinated the project. Also, Performed data collection, patient recruitment and sample acquisition. Provided clinical context and expertise. Mohammadreza Elhaie: Assisted with study design and methodology. Guided data analysis and interpretation. Reviewed and edited the manuscript.Abolfazl Koozari:Contributed to reviewing and revising the manuscript to produce the final version. Read and approved the final manuscript for submission.Iraj Abedi:Supervised the study design and methodology. Provided oversight and guidance as senior author. Reviewed and edited the manuscript.
None.
The authors wish to acknowledge the staff of Nuclear Medicine, Chamran Hospital, Isfahan University of Medical Sciences, Isfahan, Iran for their contribution to this study.