The purpose of this study was to compare the shear bond strength of orthodontic brackets with two systems of hydrophilic adhesives: (I) a cyanoacrylate adhesive (Smartbond, International Gestenco) and (II) a composite system (Transbond XT and Transbond™ IPM) in two enamel conditions: dry and artificial saliva contaminated.
Materials and methods100 extracted premolars were stored in distilled water at 4 degrees Celsius. The teeth were cleaned, polished, and convenience distributed into 5 groups: (1) composite resin in enamel under dry conditions, (2) cyanoacrylate adhesive in dry enamel condition, (3) composite resin in enamel condition contaminated with artificial saliva before the primer, (4) composite resin enamel condition contaminated with artificial saliva after the primer, and (5) cyanoacrylate adhesive in artificial saliva contaminated enamel condition. The results showed that the adhesive system Transbond XT™ and Transbond MIP obtained the highest values of resistance to debonding in the dry enamel surface.
ConclusionsThe adhesive system Transbond XT™ and Transbond MIP I provide an adequate in vitro resistance to debonding in every enamel condition. The system based on cyanoacrylate adhesive Smartbond obtained proper values of resistance to debonding in dry enamel, however it obtained the lowest values in contaminated with enamel artificial saliva conditions, unsuitable for orthodontics, and even some samples were not cemented successfully in vitro under these conditions.
El propósito de este estudio fue comparar la resistencia al cizallamiento de brackets ortodóncicos de dos sistemas adhesivos hidrofílicos, éstos son: (I) adhesivo a base de cianoacrilato (Smartbond, Gestenco Internacional) y (II) una resina compuesta (Transbond XT y Transbond™ MIP) en dos condiciones del esmalte, seco y contaminado con saliva artificial.
Materiales y métodos100 premolares extraídos fueron almacenados en agua destilada a cuatro grados centígrados. Los dientes fueron limpiados, pulidos y distribuidos a conveniencia en 5 grupos, los cuales son: (1) resina compuesta en condición del esmalte seco; (2) adhesivo de cianoacrilato en condición del esmalte seco; (3) resina compuesta en condición del esmalte contaminado con saliva artificial antes del adhesivo líquido; (4) resina compuesta en condición del esmalte contaminado con saliva artificial después del adhesivo líquido; y (5) adhesivo de cianoacrilato en condición del esmalte contaminado con saliva artificial. Los resultados arrojaron que el sistema adhesivo Transbond XT y Transbond™ MIP obtuvo los valores de resistencia al desprendimiento más alto con brackets cementados en la superficie del esmalte seco.
ConclusionesEl sistema adhesivo Transbond XT y Transbond™ MIP proporciono adecuada resistencia al desprendimiento in vitro en todas las condiciones del esmalte. El sistema adhesivo a base de cianoacrilato Smartbond obtuvo valores adecuados a la resistencia al desprendimiento en condiciones del esmalte seco, sin embargo, obtuvo los valores más bajos en condiciones del esmalte contaminado con saliva artificial, no adecuados para la ortodoncia, e inclusive algunas muestras no fueron cementados con éxito in vitro bajo dichas condiciones.
BIS-GMA (bisphenol-glicidil-methacrylate) resins were successfully introduced in the 1960’s and then applied in clinical practice as orthodontic adhesives,1developing an organic molecule polymer with less dimensional changes and that the addition of inorganic particles further reduces the dimensional deformation thus increasing its resistance. This blend of organic material and inorganic material treated with a functional organic silane in order to be able to bond with the organic material is called composite resin,2 becoming the most used bonding technique in contemporary orthodontics.
The mechanical union effectiveness of conventional composite adhesives to the enamel requires that the enamel is completely dry after etching to allow the penetration of the hydrophobic primer and achieve an adequate retention. Humidity contamination (by gingival crevicular fluid or water) reduces the adhesion strength significantly and is considered the most common cause of adhesion failure of composite resins.3 While some manufacturers claim an acceptable performance of their intensive hydration products in a humid environment, others introduce active humidity adhesives. Recently a new cyanoacrylate adhesive (Smartbond, Gestenco International, Gothenburg, Sweden), was approved by the Food and Drug Administration for use in orthodontics in 1999. This adhesive system removed the application of liquid adhesive and the photocuring steps in addition to reducing the acid etching time to 10 seconds. According to the manufacturer the presence of humidity and pressure acts as an activator of the polimerization reaction.4
In 1966, in the Department of Orthodontics Eastman Dental Center,5 a direct bonding technique was developed and used for the first time in several patients. The adhesive resin was the same used in the earlier experiments of Cueto and Bounocore for sealing pits and fissures. This experiment was carried out to see whether it was feasible to bond a bracket directly to the enamel of the teeth without the use of orthodontic bands. The adhesive consisted in a methyl-2-cyanoacrylate liquid monomer (Eastman 910, Eastman Kodak, Rochester, N.Y.) and a silicate filling.5
A disadvantage of bracket direct bonding has been the humidity control in the oral cavity, that is to say that a dry field is of the utmost importance for a successful adhesion. In response to the needs that an orthodontist faces under humid environments that are difficult to control, manufacturers have developed hydrophilic adhesives. This suggests the possibility of obtaining success in the direct bonding in enamel surfaces contaminated with humidity.
This protocol aims to determine the variations in the resistance to shearing forces of two adhesion systems: a cyanoacrylate-based resin, Smartbond (Gestenco International, Guthenburg, Sweden) in two conditions of enamel surface: dry and moistened with artificial saliva and a resin with an organic component Bis-GMA, Transbond TX (3M Unitek) with an hydrophilic adhesive (MIP, 3M Unitek, Monrovia, Calif) in two enamel conditions: dry and moistened with artificial saliva, the latter in two moments of contamination. This is useful when considering the adhesive material in cases of poor humidity control that do not allow an ideal isolation at the bonding site thereby optimizing results while maintaining low costs and time of attention in the dental chair; that alone justifies this need.
Materials and methods100 caries-free premolars, extracted for reasons beyond our study and with the informed consent of the patient, were used. The teeth were washed with tap water after their extraction to eliminate traces of blood. Subsequently they were stored in distilled water that was changed regularly to prevent deterioration and were kept at a 4 degrees Celsius temperature until the time of bonding to the brackets. In no case the teeth remained stored more than six months after the extraction.6 The inclusion criteria for tooth selection were: intact enamel without cracks caused by the extraction prcedure, without caries and not have undergone any previous treatment with chemical agents (for example, the hydrogen peroxide).6
The orthodontic adhesive systems used were:
- 1.
Smartbond (Gestenco Internacional, Gothenburg, Sweden). Smartbond is a cyanocrilate esther. Its composition is 85-90% ethyl-cianocrylate, 5-10% polimethyl metacrylate, amorfous silica 5-10% and 0.1-0.5% hydroquinone. The etching gel is 37% phosphoric acid in a gel of amorfous silica.4
- 2.
Transbond XT and Transbond™ MIP Moisture Insensitive Primer (3M Unitek, Monrovia, California). Transbond TX is a hybrid resin of photopolymerization. The basis of the resin is Bis-GMA and TEGDMA in a proportion of 1:1, with 82% of silica particles of 3 m. MIP adhesive consists of polialquenoic acid with functionalised methacrylate copolymer that form a copolymer and hydroxymethyl methacrylate. The etching gel is 35% phosphoric acid in an amorphous silica gel.
100 metal premolar brackets (Bracket Std EdGw, bicuspid, Ormco) with an average bracket base area of 10.24mm2 were used. For the brackets bonding tests with the adhesive systems Transbond XT and Transbond™ MIP Moisture Insensitive First, it was necessary to use a wired photopolimerization unit. The photopolimerization unit was tested at the beginning of bracket placement and every 10 samples. The potency was assessed with the radiometer.
Before adhesion, the buccal surface of each premolar was cleaned for 10 seconds with a mixture of water and a fluoride, flavor and color-free prophylactic paste and a polishing rubber cup for every 5 samples, with a low speed hand-piece. The enamel surface was flushed with water from the triple syringe to remove the excess of prophylactic paste and dried with water and oil-free air.6 Bonding was carried out at a controlled temperature of 21 degrees centigrade under a relative humidity of 64%.
The 100 teeth were divided by convenience in 5 groups of 20 samples:
Group 1. Teeth bonded with Transbond XT were treated with the 35% phosphoric acid gel (3M Dental Products, St Paul, MN) for 15 seconds, followed by extensive washing for 10 seconds and dried with oil and water -free air for 10 seconds. After the enamel conditioning, the MIP Transbond™ adhesive was applied over the etched enamel and the resin Transbond XT was placed in the bracket. The bracket was placed near the center of the buccal surface of the tooth with enough pressure to push the excess adhesive out that was removed from the bracket base margins with an explorer before polymerization.
The tip of the photopolymerization lamp was placed at the 4 angles of the bracket 5 seconds for each of the samples.
Group 2. The Smartbond adhesive (Gestenco International, Gothenburg, Sweden) contains ethyl cyanoacrylate. An etching with 37% phosphoric acid was applied over the enamel for 15 seconds, and the teeth were thoroughly washed for 10 seconds and dried with oil and water-free air for 10 seconds. The adhesive was applied at the bracket base placing it in the center of the buccal surface of the tooth, making pressure to cause the excess adhesive to exit. The manufacturer recommends two methods for applying the adhesive to the bracket base either directly from the syringe containing the adhesive or with a brush. In this study, the brush method was used since it allowed the controlled application of the adhesive with a uniform thickness over the bracket base.4
Group 3. Teeth bonded with Transbond TX were treated with the 35% phosphoric acid gel (3M Dental Products, St Paul, MN) for 15 seconds, followed by extensive washing for 10 seconds and dried with water and oil-free air for 10 seconds. After the conditioning, artificial saliva was applied by means of a brush applicator to moisten the enamel surface. The adhesive Transbond™ MIP was applied over the contaminated etched enamel, the Transbond XT resin was placed in the bracket which was positioned near the center of the buccal surface of the tooth with enough pressure to push the excess adhesive out, removing it from the margins of the bracket base with an explorer before polymerization.
The tip of the photopolymerization lamp was placed at the 4 angles of the bracket 5 seconds for each of the samples.
Group 4. Teeth bonded with Transbond XT were treated with 35% phosphoric acid gel (3M Dental Products, St Paul, MN) for 15 seconds, followed by extensive washing for 10 seconds and dried with oil-free air for 10 seconds. After conditioning, the MIP Transbond™ adhesive was applied over the etched enamel and after that, artificial saliva with an applicator brush to moisten the enamel surface with adhesive. Subsequently the Transbond XT resin was placed in the bracket which was positioned close to the center of the buccal surface of the tooth with enough pressure to cause the excess adhesive to exit and it was removed from the margins of the bracket base with an explorer before polymerization.
The tip of the photopolymerization lamp was placed at the 4 angles of the bracket 5 seconds for each of the samples.
Group 5. Smartbond adhesive (Gestenco International, Gothenburg, Sweden). The adhesion procedure followed the manufacturer’s instructions.4 An etching with phosphoric acid 37% was performed over the enamel for 15 seconds, the teeth were thoroughly washed for 10 seconds and dried with oil and water-free air for 10 seconds. There was an application of artificial saliva with the applicator brush to moisten the enamel surface before applying the adhesive. A brush was used for positioning the adhesive since it allows the controlled application of the adhesive with a uniform thickness on the bracket base.
The samples with the bonded brackets were fixed with two stainless steel wires gauge 0.021 “x0.025” orthogonally solded and warped towards their center. These were tied to the bonded bracket to prevent shifting and centered on PVC cylindrical containers of 28mm in outer diameter and 10mm height.7 The PVC molds were filled with self-curing rapid acrylic polymer making a fluid mixture in a beaker. The mixture was poured into PVC containers that were over a glass surface covered with a petroleum based lubricant layer. The exothermic reaction was controlled by immersing the container in water at ambient temperature.
Different colors of acrylic were used for each of the 5 sample groups in order to codify and identify them with greater ease.
The pre-assembled samples were labeled and stored in closed containers in water at a temperature of 37 degrees Celsius during a week after the bonding and assembling of the samples in each of the groups, to subsequently carry out the shear tests.
The PVC cylinders were installed in a support base on the Instron universal testing machine (Model 5567). The tip of the force applicator was placed parallel to the union interface of the bracket with the tooth in an oclusso-gingival direction at a speed of 1mm/min. The force values of each sample were recorded.7
The maximum load required to debond the bracket was recorded in Newtons and was converted into megapascals (MPa) as a Newton ratio with the surface of the bracket base.
The debonded samples were stored in closed containers with distilled water at 37 degrees Celsius for analyzing of the adhesive remnant index (ARI).8 The enamel surface of each sample was examined to determine the residual adhesive on the teeth using the adhesive remnant index (ARI) as described by Artun and Bergland.8 A scale of 0 to 3 was used where:
- 0,
was no remnant adhesive left on the enamel surface.
- 1,
less than half of the adhesive remained on the enamel surface.
- 2,
more than half of the adhesive remained on the enamel surface.
- 3,
100% of the adhesive remained on the enamel surface.
The observation of the enamel surfaces of each group was performed with stereoscopic microscope at 20x magnification.
ResultsThe Kolmogorov-Smirnov test was conducted in the shear resistance test to assess the normality of each group and perform the corresponding statistical test. A parametric test, ANOVA, was applied. The Kruskal-Wallis test was conducted for the ARI values since it is a non-parametric test and the post hoc Mann-Whitney tests were used respectively.
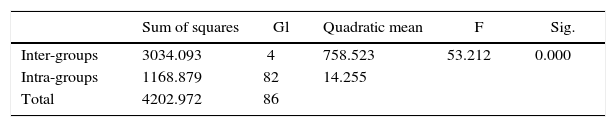
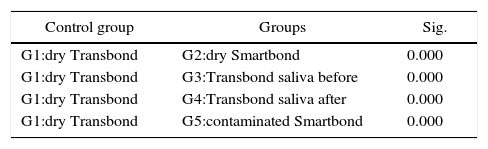
The ANOVA test showed statistically significant differences (p < 0.000). It was then proceeded to perform the Dunnett’s test as post hoc. The statistical description of the assessed groups shear test is presented in tables IandII.
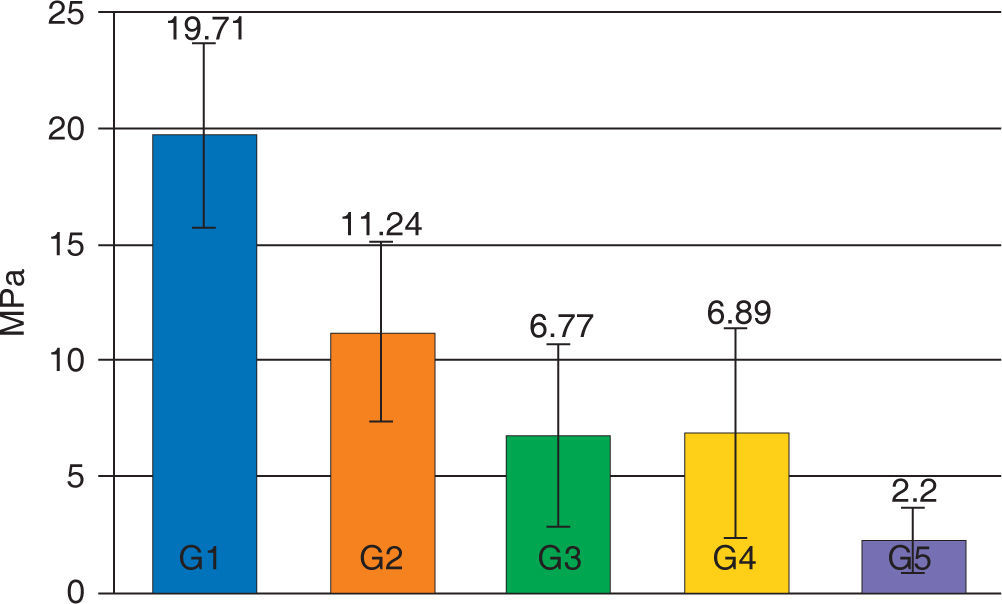
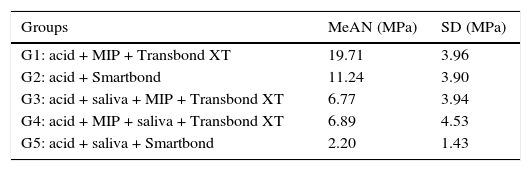
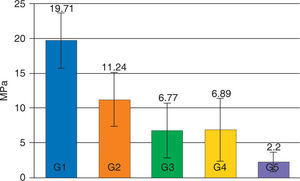
The average of the shear test for Transbond XT with the hydrophilic MIP adhesive in the dry enamel surface (group 1) presented statistically significant (p < 0.05) higher values (19.71 MPa) compared with all the assessed groups. The experimental groups of the resin Transbond XT plus the MIP adhesive (G3 and G4) did not show statistically significant differences between them (p > 0.05), however they both presented a decrease in their adhesion strength (6.77 and 6.89 MPa respectively) that was statistically significant (p < .05) compared with the control group (Figure 1).
The values of the shear test decreased in all groups contaminated with artificial saliva, being the contaminated group of Smartbond (G5) the one with the statistically significant (p < 0.05) lowest mean (2.20±1.43 MPa) with regard to the other groups (Table III).
Descriptive table that includes the mean, the standard deviation and the minimum and maximum values calculated for the 5 assessed groups.
| Groups | MeAN (MPa) | SD (MPa) |
|---|---|---|
| G1: acid + MIP + Transbond XT | 19.71 | 3.96 |
| G2: acid + Smartbond | 11.24 | 3.90 |
| G3: acid + saliva + MIP + Transbond XT | 6.77 | 3.94 |
| G4: acid + MIP + saliva + Transbond XT | 6.89 | 4.53 |
| G5: acid + saliva + Smartbond | 2.20 | 1.43 |
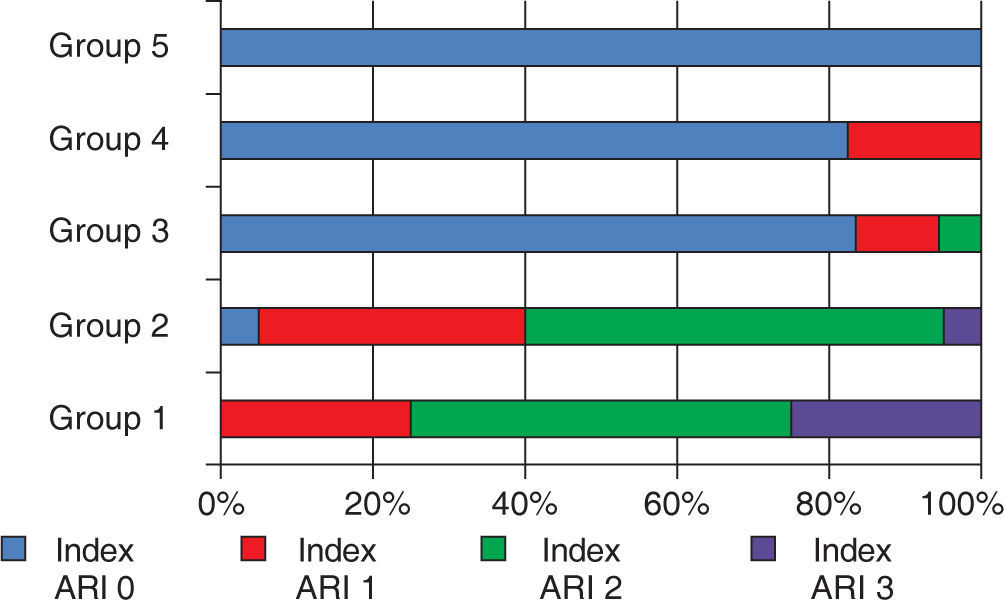
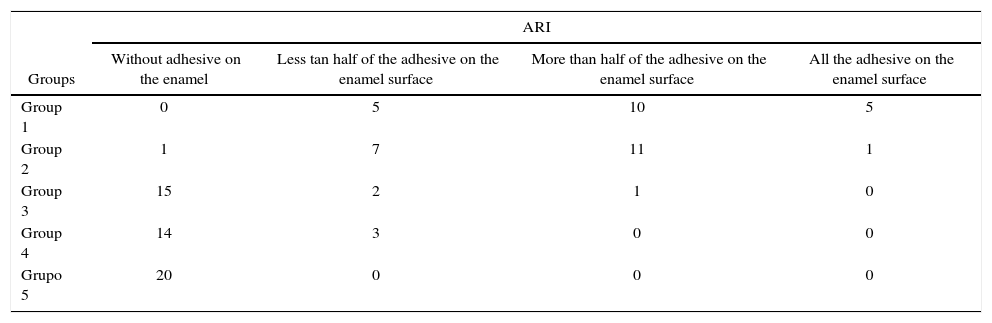
For the ARI values, the Kruskal-Wallis test showed significant differences (p < 0.000), and in the Mann-Whitney’s test, compared with the control group (G1), statistically significant differences were found when compared with groups 3, 4 and 5 (p=0.00) (Table IV).
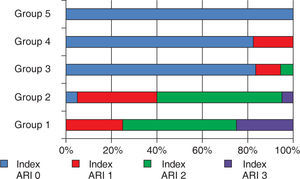
Frequency and distribution of the adhesive remnant index (ARI).
| Groups | ARI | |||
|---|---|---|---|---|
| Without adhesive on the enamel | Less tan half of the adhesive on the enamel surface | More than half of the adhesive on the enamel surface | All the adhesive on the enamel surface | |
| Group 1 | 0 | 5 | 10 | 5 |
| Group 2 | 1 | 7 | 11 | 1 |
| Group 3 | 15 | 2 | 1 | 0 |
| Group 4 | 14 | 3 | 0 | 0 |
| Grupo 5 | 20 | 0 | 0 | 0 |
Both adhesive systems in dry enamel surface had a greater frequency of ARI 2 values with more enamel debonding in 2 samples for the Transbond XT group, and the contaminated groups showed a higher frequency in the 0 ARI value. The distribution is shown in figure 2.
Percentile distribution of the adhesive remnant index (ARI).
Scale of ARI: 0; there was no remnant adhesive on the surface of the enamel, 1; less than half of the adhesive remained on the surface of the enamel, 2; more than half of the adhesive remained on the surface of the enamel, 3; 100% of the adhesive remained on the surface of the enamel.
The adhesion of orthodontic brackets has been limited to clinical conditions of humidity isolation posing a challenge for the operator, especially because the failure can be attributed to saliva contamination. On the other hand, hydrophilic adhesives are difficult to evaluate mainly because they do not have standardized assessment protocols.
A priority objective should be to reduce brackets adhesion failure rate since replacing them is timeconsuming and expensive. As a result, the search continues for greater adhesive forces, better adhesives, and simpler techniques and materials that bond when saliva is present. However, the majority of failures are due to inconsistencies in the chosen bonding technique and not to the resins or to an inadequate adhesive force or to the quality of brackets used.
The present study assessed the performance of two orthodontic adhesive systems with affinity to humidity in two enamel conditions; dry and contaminated with artificial saliva.
The mean values of bracket debonding in the shear test for both adhesive systems in the two enamel conditions were found in a range of 0.10 -26.72 MPa.
The values for the Transbond XT adhesive system with the hydrophilic adhesive MIP on its two experimental groups (group 3 and 4): contaminated with artificial saliva after enamel acid etching (6.77±3.94) and contaminated with artificial saliva after the application of the hydrophilic adhesive (6.89±4.53) did not have significant differences between them, however the obtained values were much lower than the ones shown by Webster MJ et al.9 (20.72 and 15.28 respectively), although the results in this study are in the range that Reynols10 suggests (6-8 MPa) for orthodontic clinical needs.
The values obtained in this study for the Smartbond system (2.20±1.43 MPa) were much lower than those reported by Orthendahl and Ortengren11 (18-26 MPa). However, in our study we used artificial saliva whose consistency could have caused that the adhesive was not in contact with the etched enamel surface at the time of polymerization of the cyanoacrylate based system thus producing a poor mechanical retention.
Nemeth et al.12 and Mehmet et al.13 conducted resistance to debonding tests on dry enamel surfaces with the Transbond XT adhesive system (1057 MPa and 1528 MPa respectively) and on saliva-contaminated surfaces (0.14 MPa and 3.79 MPa) but both studies were carried out with the system liquid adhesive and in this study was conducted with the hydrophilic adhesive Transbond™ MIP.
The same protocols used the cyanoacrylate-based adhesive system (Smartbond), Nemeth et al.12 in both enamel conditions, contaminated and dry (3.91 MPa and 3.22 MPa) obtained results much below those found by this study in dry enamel surface. However, similar values to the ones from our study were obtained in contaminated enamel (2.20 MPa) and Mehmet et al.13 performed it in contamination with saliva (5.85 MPa) but with a wet substrate prior to the contamination. In both investigations, human saliva was used with no standard epidemiological infection control or established protocols.
M. Kusai Al-Munajed14 conducted resistance to debonding tests with the same system based on cyanoacrylate, 24 hours and 3 months after bonding (3.58 and 1.72MPa) but this was conducted with orthodontic buttons and the test was not for shear bond strength
Both the obtained values in in vitro tests for the adhesive based on Bis-GMA and for the adhesive based on cyanoacrylate in contaminated conditions may not accurately reproduce contaminated clinical situations and should be interpreted with caution since the tests were made with artificial saliva which has a different viscosity than that of the saliva secreted by the patient.
ConclusionsThe adhesive systems Transbond XT and Transbond™ MIP Moisture Insensitive First (3M Unitek, Monrovia, California) were compared with the cyanoacrylate-based adhesive system Smartbond (Gestenco International, Gothenburg, Sweden) under two enamel conditions: dry and moistened with artificial saliva, the first one in two contamination moments. The following was concluded:
- •
The Transbond XT adhesive system and Transbond™ MIP obtained the highest values for resistance to debonding with brackets bonded to a dry enamel surface.
- •
The adhesive system Transbond XT and Transbond™ MIP provided adequate in vitro resistance to debonding in all enamel conditions.
- •
The groups of Transbond XT adhesive in its two moments of contamination, before and after the application of Transbond™ MIP, did not show statistically significant differences.
- •
The Transbond XT adhesive system and Transbond™ MIP exhibited a decrease in their resistance to debonding values under conditions of contaminated enamel with artificial saliva in its two moments of contamination. However, they are considered adequate for orthodontic treatment.
- •
The Smartbond cyanoacrylate-based adhesive system obtained adequate values of resistance to debonding under dry conditions of the enamel, however, it obtained the lowest values in artificial saliva contaminated enamel conditions, not suitable for orthodontics, and even some samples were not bonded successfully in vitro under these conditions.