Semen analysis is a clinical method aimed at determining the fertility of a male individual. The traditional subjective method lacks the reliability that can be achieved by computer-assisted sperm analysis (CASA) technology. Unfortunately, this technology has only been used when taking into consideration individually different sperm characteristics. The aim of this work is to present an integrative mathematical approach that considers different seminal variables to establish human sperm subpopulations.
MethodsSamples were obtained from thirteen volunteers via masturbation and were analyzed by the routine subjective method and two objective systems, CASA Motility (CASA-Mot) and CASA Morphology (CASA-Morph).
ResultsSeminogram variables were reduced to three principal components (PC) showing two subpopulations. Kinematics and morphometric variables each rendered three PCs for four subpopulations.
ConclusionsThese results lay the foundations for future studies including different geographical, social, ethnic and age range conditions with the aim of achieving a definitive view of the human semen picture.
El análisis de semen es el método clínico para determinar la fertilidad masculina. El método subjetivo tradicional carece de la fiabilidad, que se puede obtener con el uso de la tecnología del análisis de semen asistido por ordenador (CASA). Desafortunadamente, esta tecnología se ha venido utilizando únicamente teniendo en cuenta de forma independiente las diversas características de los espermatozoides. El objetivo del presente estudio es presentar una aproximación matemática que incluye diversas variables seminales para definir las posibles subpoblaciones espermáticas.
MétodosLas muestras se obtuvieron por masturbación de 13 voluntarios, que se analizaron de forma subjetiva, así como con 2 sistemas objetivos, para el análisis de la movilidad (CASA-Mot) y la morfología (CASA-Morph).
ResultadosTanto las variables cinemáticas como las morfométricas rindieron 3 componentes principales y 4 subpoblaciones.
ConclusiónEstos resultados sientan las bases para estudios futuros que incluyan diferencias geográficas, sociales, étnicas o de rango de edad con el ánimo de obtener una definición concluyente sobre las características seminales de la especie humana.
During recent decades it has become increasingly evident that reproductive problems affect a large proportion (8–12%) of human populations worldwide, leading to important socio-economic consequences. Of all infertility cases, approximately 40–50% is due to “male factor” infertility and as many as 2% of all men will exhibit suboptimal sperm parameters.1
Semen analysis is usually the first and most commonly performed test during male infertility consultations. These tests started in the middle of the last century.2 However, it was only in 1971 that Eliasson3 established the first well-defined criteria for a full semen analysis. Subsequently, the World Health Organization (WHO) defined their criteria with the aim of spreading them universally in accordance with successive WHO manuals in 1980, 1987, 1992, 1999 and 20104–6 and making them prevail over the criteria established by the European Society for Human Reproduction and Embryology (ESHRE).7–9 All these criteria were based on individual seminal characteristics from subjective analyses (basically concentration, motility and morphology). These manuals were based on scientific approaches designed to identify standards for normal semen measurements.10 Some were based on comparisons between couples who conceived with those who did not.11 Others merely compared fertile men with infertile ones12 or followed couples after they had discontinued their use of contraception.13 One problem associated with these kind of approaches is in how to define male fertility.14 For this purpose, some papers have focused on the level of success following different assisted reproduction techniques.15,16
In any case, it is necessary to point out that the prognostic interpretation of semen analysis is not straightforward, except where there is a total abnormality such as persistent azoospermia or zero sperm motility.17,18 Semen evaluation has one important limitation in the classical approach, both subjective or using CASA systems, which treat seminal variables one-by-one. In this way, none of the classical parameters alone or in combination can be considered to be diagnostic for infertility.14 Hence, while poor semen quality is a good indicator of subfertility, good semen quality (in terms of conventional subjective analysis of sperm number, motility and morphological normality) is no guarantee of acceptable fertility, given that there are many other parameters involved, many of which involve the female partner.19
While studies using in vitro fertilization (IVF) have indicated the importance of both sperm motility and morphology for fertilization,20 the situation in vivo is more difficult to assess and results are less clear.18 Several trials to define the significance of semen analysis results for predicting fertility success after assisted reproductive techniques such as intrauterine insemination, intracytoplasmic sperm injection and IVF have produced different results.15,16,21
All this has led to more accurate measurements of the classical parameters by introducing metric data obtained by using CASA technology, instead of categories, and the study of other specific markers of sperm function.16,23 In any case, CASA technology for assessing motility (CASA-Mot) was being used without a fine system-limitation analysis and for this reason could not offer its full potential.24,25 In addition, most laboratories using CASA-Mot systems are doing so only to substitute subjective evaluations with an objective one. This has reduced technical error,26 and has allowed the development of strong quality control programs.27 However, such powerful information obtained by means of kinematic data is lost, as only mean values of the population are considered when comparing between individuals or experimental conditions, yet it is clear that all spermatozoa do not show the same motility patterns. This is probably why there are no noticeable changes between samples when only mean values are used. In this way, it is necessary to consider the total cell by cell data and distributions, which contain much more information.
The recent approach of computing a group of variables by using multivariate statistics that includes principal components and cluster analysis (subpopulations) offers a new comprehension of what a semen picture is and how it relates to male fertility. Recently, a special number of the journals Asian Journal of Andrology (2016, vol. 18) and Reproduction Fertility and Development (2018, vol. 30) were published, dealing with this approach and taking into consideration morphometric and kinematic data obtained using CASA-Morph24 and CASA-Mot,28 respectively.
In this study, we have used kinematic and morphometric data obtained from human semen samples to examine the subpopulation structure regarding motility and with the aim of presenting a conceptual mathematical approach to be applied in fertility studies.
Materials and methodsSamplesThirteen volunteers (age range 25–59 years) signed informed consent forms to participate in and provide semen for the study. Semen samples were collected by masturbation following sexual abstinence for 3–5 days. Each sample was collected in a clean 60mL wide-mouthed universal container and stored at 37°C in an incubator for 30min to allow liquefaction that could be observed at 30min after ejaculation in all cases.
Kinematic analysisSperm motility and concentration were calculated using a reusable 10-μm deep Spermtrack® counting chamber (Proiser R+D S.L., Paterna, Valencia, Spain).
Analyses were conducted using the ISAS® v1 CASA-Mot system (Proiser R+D S.L.), equipped with an ISAS® CM13-ON video-camera attached to a UB203 microscope (UOP/Proiser), with a 10x negative-phase-contrast objective (Numerical aperture 0.25) and an integrated self-heated stage maintained at a constant temperature of 36°C. The final resolution of the images was 1280×1024 pixels, with an individual x and y linear pixel value of 0.482451μm. Nine 1-s captures of each sample were taken (at 100images/s).
Measured parameters were as follows: curvilinear velocity (VCL), straight line velocity (VSL), average path velocity (VAP), linearity (LIN), wobble (WOB), straightness (STR), amplitude of lateral head displacement (ALH) and beat-cross frequency (BCF).25
Morphometric analysisAfter mixing of the samples, an aliquot of 7μL was smeared on a clean slide. After air drying, samples were stained using the Diff-Quik kit (Medion Diagnostics, Düdingen, Switzerland) by 7–8 immersions (1s) in each of the three solutions (fixative, Stain 1 and Stain 2), washed free of excess colorant with distilled water and air drying again.
Slides were mounted by immersion in Neo-Clear® for 1s (Merck KGaA., Darmstadt, Germany), followed by the application of a drop of mounting medium (Neo-Mount®; Merck KGaA.) beneath a large coverslip (24mm×60mm).
Analyses were conducted using the morphometry module of the ISAS®v1 CASA-Morph system. The camera used was an ISAS® CM13-ON attached to a microscope UB203 (UOP/Proiser) using a 40x objective, with a resolution of 0.121526μm/pixels on both axes. Images from about 250 spermatozoa per sample were captured and analyzed to obtain 12 morphometric values: nine from the sperm head: length (L, μm), width (W, μm), area (A, μm2), perimeter (P, μm), acrosome (% of head area) and the unitless shape factors Ellipticity (L/W), Rugosity (4πA/P2), Elongation ([L−W]/[L+W]), Regularity (πLW/4A); and three from the midpiece: mp-width (μm), insertion distance (μm) and insertion angle (°).
Statistical analysisClustering procedures were performed to identify sperm subpopulations from the complete set of seminogram, kinematic and morphometric data independently. The first step was to perform a principal component analysis (PCA). The feasibility of factorial analysis (FA) was verified by the Bartlett's test of sphericity, to test the null hypothesis that the correlation matrix is an identity matrix, and by means of the KMO index (Kaiser–Meyer–Olkin), which determines the computation of the correlations between two variables once the influence that the other variables on them has been eliminated, and which indicates the convenience of performing the FA. To select the number of principal components that should be used in the next step of analysis, the criterion of selecting only those with an eigenvalue (variance extracted for that particular principal component)>1 (Kaiser criterion) was followed. The second step was to perform a two-step cluster procedure with the sperm-derived indices obtained after the PCA. All the seminogram, kinematic and morphometric measurements within each ejaculate were clustered using a non-hierarchical clustering procedure (k-means model and Euclidean distance). This classifies the spermatozoa of each data set into a small number of subpopulations according to their kind, concentration or vitality (seminogram), velocity and progressivity (kinematic) and head dimensions (morphometric). This analysis allowed the identification of sperm subpopulations and the detection of outliers. The results are presented as median and first quartile and third quartile. Statistical significance was considered as P<0.05. All data were analyzed using InfoStat Software (v. 2017) for Windows.
ResultsKinematic parametersThree PCs were obtained, explaining 84% of the overall variation. PC1 was more related to sperm velocity parameters and BCF, explaining 46% of the variation; PC2 was positively related with progressive motility parameters and negatively with VCL and ALH, explaining 27% of variability; and PC3 was related with the area of the sperm heads, explaining 11% of the variation (Table 1).
Eigenvestors of the three PC obtained in the study of sperm kinematic and morphometric for human semen.
| Kinematic | |||
|---|---|---|---|
| Variables | PC 1 | PC 2 | PC 3 |
| Area (μm) | 0.96 | ||
| VCL (μm/s) | 0.38 | −0.40 | |
| VSL (μm/s) | 0.47 | ||
| VAP (μm/s) | 0.42 | ||
| LIN (%) | 0.31 | 0.48 | |
| STR (%) | 0.34 | 0.36 | |
| WOB (%) | 0.42 | ||
| ALH (μm/s) | 0.31 | −0.47 | |
| BCF (Hz) | 0.38 | ||
| Explained variation (%) | 46 | 27 | 11 |
| Morphometric | |||
|---|---|---|---|
| Length (μm) | 0.45 | ||
| Width (μm) | 0.56 | ||
| Area (μm2) | 0.47 | ||
| Peri (μm) | 0.39 | 0.33 | |
| Acr (%) | 0.31 | ||
| Ellipticity | 0.43 | ||
| Rugosity | −0.43 | ||
| Elongation | 0.42 | ||
| Regularity | |||
| Width-mp | 0.36 | ||
| Dintance (h-mp) (μm) | 0.63 | ||
| Angle (h-mp)(°) | 0.62 | ||
| Explained variation (%) | 36 | 24 | 14 |
Only eigenvalues>0.3 are presented. The table shows only the values of the covariance higher than 0.3, for each PC.
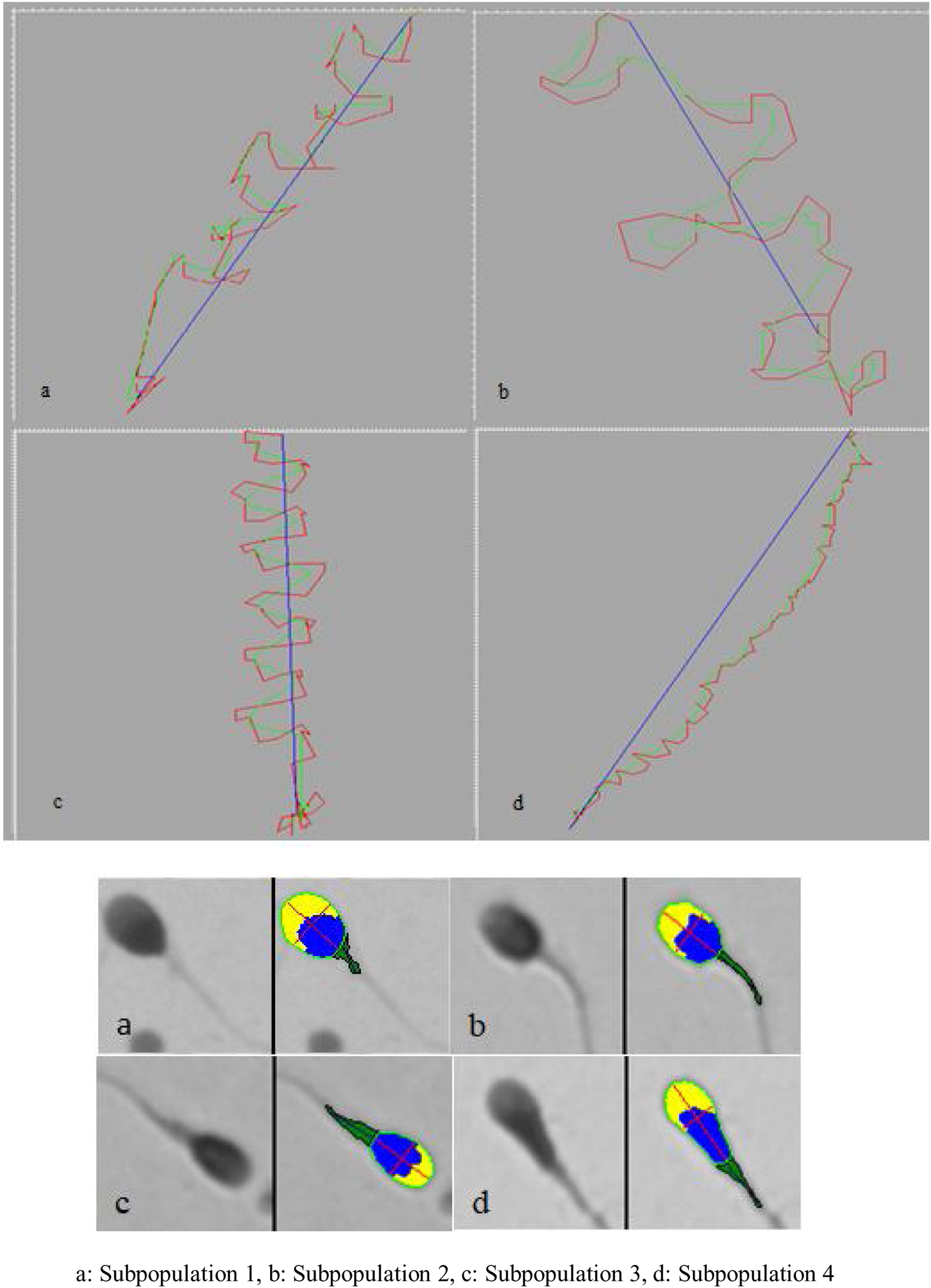
Four subpopulations were defined from these PCs. SP1 represented 31% of the total number of cells and was characterized by large, rapid and progressively motile cells; SP2 (29.3%) comprised small, fast and medium progressively motile spermatozoa with the highest ALH; SP3 (28.95%) represented the smallest and less motile and progressively motile cells; and SP4 (10.8%) included the largest spermatozoa with low motility and progressiveness (Table 2, Fig. 1).
Sperm subpopulation values from the human kinematics data.
| SP 1 | SP 2 | SP 3 | SP 4 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MD | Q1 | Q3 | MD | Q1 | Q3 | MD | Q1 | Q3 | MD | Q1 | Q3 | |
| n/% | 1243/31.0 | 1175/29.3 | 1161/28.9 | 431/10.8 | ||||||||
| Area (μm) | 16.60c | 14.60 | 19.30 | 15.90b | 14.00 | 17.90 | 15.20a | 12.70 | 17.60 | 25.60d | 23.00 | 29.60 |
| VCL (μm/s) | 110.90c | 95.00 | 128.50 | 167.90d | 146.10 | 195.70 | 68.70a | 49.10 | 92.70 | 77.50b | 53.50 | 103.30 |
| VSL (μm/s) | 64.50d | 52.50 | 78.50 | 54.00c | 38.80 | 69.30 | 16.40a | 8.60 | 26.60 | 20.50b | 10.00 | 35.40 |
| VAP (μm/s) | 77.40b | 66.00 | 88.30 | 96.50c | 84.50 | 111.50 | 44.40a | 30.90 | 60.30 | 45.50a | 31.90 | 59.30 |
| LIN (%) | 58.60d | 50.60 | 68.30 | 32.60c | 24.60 | 39.80 | 25.00a | 17.00 | 34.80 | 28.50b | 18.20 | 39.40 |
| STR (%) | 87.20d | 77.30 | 93.50 | 57.30c | 42.60 | 70.60 | 39.40a | 27.20 | 52.70 | 49.00b | 31.70 | 66.10 |
| WOB (%) | 69.90d | 64.20 | 76.20 | 57.50a | 52.50 | 62.80 | 64.70c | 56.40 | 73.80 | 58.30b | 51.80 | 66.90 |
| ALH (μm) | 1.30 | 1.10 | 1.50 | 2.10 | 1.80 | 2.40 | 1.00 | 0.80 | 1.20 | 1.10 | 0.90 | 1.40 |
| BCF (Hz) | 33.00d | 27.80 | 38.40 | 28.00c | 23.70 | 32.70 | 16.10a | 11.00 | 22.00 | 19.00b | 13.00 | 25.00 |
n (spermatozoa)=4010; MD, median; Q1, first quartile; Q3, third quartile. Different superscripts indicate significant differences between subpopulations (p<0.05), a is the lowest d is highest ordered by value.
The distribution of subpopulations among individuals was significantly different (P<0.0001, by χ2 analysis): SP1 being the most frequent in five individuals, SP2 in three and SP3 in the other five (Table 3).
Percentage of human sperm cell subpopulation for kinematic and morphometric data for different individuals.
| Kinematic | Morphometric | |||||||
|---|---|---|---|---|---|---|---|---|
| Individual | SP1 (%) | SP2 (%) | SP3 (%) | SP4 (%) | SP1 (%) | SP2 (%) | SP3 (%) | SP4 (%) |
| 1 | 9.91 | 29.13 | 18.92 | 42.94 | 3.48 | 68.16 | 22.39 | 5.97 |
| 2 | 6.83 | 37.30 | 37.46 | 18.41 | 3.55 | 35.53 | 59.39 | 1.52 |
| 3 | 10.79 | 56.27 | 17.49 | 15.45 | 2.55 | 34.18 | 8.16 | 55.10 |
| 4 | 26.45 | 31.68 | 20.11 | 21.76 | 0.50 | 30.00 | 20.00 | 49.50 |
| 5 | 5.56 | 29.17 | 41.67 | 23.61 | 0.00 | 4.00 | 66.50 | 29.50 |
| 6 | 12.13 | 20.79 | 19.31 | 47.77 | 4.61 | 28.11 | 46.08 | 21.20 |
| 7 | 16.56 | 16.34 | 41.61 | 25.49 | 0.00 | 40.91 | 54.04 | 5.05 |
| 8 | 11.40 | 7.02 | 37.72 | 43.86 | 3.23 | 45.16 | 27.19 | 24.42 |
| 9 | 2.78 | 66.67 | 19.44 | 11.11 | 2.00 | 23.50 | 72.50 | 2.00 |
| 10 | 0.56 | 23.73 | 33.90 | 41.81 | 2.50 | 48.50 | 43.00 | 6.00 |
| 11 | 14.92 | 40.33 | 24.86 | 19.89 | 2.00 | 47.00 | 44.50 | 6.50 |
| 12 | 4.57 | 17.35 | 16.89 | 61.19 | 7.93 | 56.83 | 29.96 | 5.29 |
| 13 | 2.55 | 32.77 | 32.34 | 32.34 | 7.04 | 68.34 | 24.12 | 0.50 |
Highest values are indicated in bold. Subpopulation distributions among individuals were different in all the cases (p<0.0001, χ2).
The first step of cluster analysis showed three PCs, explaining 74% of the total variation. PC1 (36%) was positive with regards to head length and ellipticity and negative regarding rugosity; PC2 (24%) with head spermatozoa size; and PC3 (14%) with midpiece characteristics (Table 1).
In this study, four subpopulations were found. The first comprised 40.9% of the total population and was characterized by the largest acrosomes and with low ellipticity of sperm heads, having a thin and well-aligned midpiece. SP2 included 39.7% of cells, which showed the smallest area with small acrosomes and the thinnest and most angulated midpiece. SP3 represent 16.3% of the cells and was characterized by narrow cells with a large acrosome and a wide and angulated midpiece. Finally, SP4 only included 3.1% of the cells, with large size, small acrosomes and the most angulated midpiece (Table 4, Fig. 1).
Median values of CASA variables for each sperm subpopulation of human.
| SP 1 | SP 2 | SP 3 | SP 4 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MD | Q1 | Q3 | MD | Q1 | Q3 | MD | Q1 | Q3 | MD | Q1 | Q3 | |
| n/% | 1085/40.9 | 1053/39.7 | 432/16.3 | 82/3.1 | ||||||||
| Length (μm) | 5.05c | 4.77 | 5.39 | 4.43a | 4.13 | 4.70 | 5.61d | 5.28 | 6.13 | 4.91b | 4.34 | 5.34 |
| Width (μm) | 3.42d | 3.24 | 3.55 | 2.94b | 2.76 | 3.10 | 2.73a | 2.51 | 2.92 | 3.30c | 2.94 | 3.49 |
| Area (μm2) | 13.75c | 12.96 | 14.88 | 10.77a | 9.75 | 11.59 | 12.77b | 11.68 | 14.15 | 13.33b | 10.93 | 15.03 |
| Peri (μm) | 14.22c | 13.69 | 14.91 | 12.54a | 11.91 | 12.99 | 14.69d | 13.90 | 15.74 | 14.12b | 12.75 | 15.14 |
| Acr (%) | 45.04c | 40.00 | 49.62 | 35.18a | 29.48 | 41.07 | 38.01b | 32.39 | 42.94 | 33.82a | 27.40 | 43.24 |
| Ellipticity | 1.48a | 1.37 | 1.63 | 1.51b | 1.39 | 1.66 | 2.07c | 1.92 | 2.29 | 1.51ab | 1.33 | 1.65 |
| Rugosity | 0.86c | 0.83 | 0.89 | 0.87d | 0.84 | 0.89 | 0.75a | 0.69 | 0.78 | 0.84b | 0.79 | 0.88 |
| Elongation | 0.19a | 0.16 | 0.24 | 0.20b | 0.16 | 0.25 | 0.35c | 0.32 | 0.39 | 0.20ab | 0.14 | 0.24 |
| Regularity | 0.97b | 0.95 | 1.00 | 0.95a | 0.92 | 0.97 | 0.94a | 0.91 | 0.97 | 0.97b | 0.94 | 1.01 |
| Width-mp | 1.44b | 1.14 | 1.76 | 1.35a | 1.08 | 1.68 | 1.52c | 1.26 | 1.82 | 2.22d | 1.50 | 3.59 |
| D (h-mp) (μm) | 0.17b | 0.09 | 0.28 | 0.17b | 0.09 | 0.26 | 0.15a | 0.09 | 0.23 | 0.84c | 0.53 | 1.29 |
| A (h-mp) (°) | 6.28a | 2.76 | 11.30 | 6.80a | 3.39 | 11.99 | 6.51a | 2.93 | 12.10 | 6.30a | 2.78 | 10.33 |
n (spermatozoa)=2652; For each CASA descriptor, significant differences between the four subpopulations are indicated by different letters (p<0.05), a is the lowest d is highest ordered by value.
As in the case of kinematics, the proportion of subpopulations varied between individuals, with SP2 being the greatest in six individuals, SP3 in five and SP4 in two. SP1 was the lowest category in all subjects, being absent in two of them (Table 3).
DiscussionFor decades, scientists focused on defining the ‘gold parameter’ of semen analysis, namely the parameter defining by itself the fertility of the sample.14,15 Following the scheme used with subjective parameters during the first attempts, quantitative variables were considered independently with the aim of finding the most significant parameter when establishing fertility.29 However, semen is a complex mixture of cells and seminal fluid and all seminal parameters are interconnected. Hence this kind of individual approach for analysing potential fertility has very limited power.30
In addition, a correlation study between different semen parameters, in bull, indicated that it is not possible to define a key parameter explaining by itself the fertility of a sample. In fact, fertility is multifactorial, not only because of individual male effects but other factors such as oocyte quality, oviductal environment, or time of insemination.31
In order to overcome this individual approach, some attempts were made considering different subjectively evaluated parameters at the same time as undertaking, for instance, logistic regression analysis. Therefore, the proportion of spermatozoa with progressive motility in the insemination medium, and linearity of sperm motion in the semen were found to be the more significant sperm variables in the logistic regression model to define fertility in vitro.32
Recently, our group has published a semen subpopulation analysis based on subjective semen analysis following the WHO criteria6 in addition to other analytical probes (basically centered on DNA fragmentation and stability). The principal component (PC) analysis rendered three components, explaining 77% of the total variation. The PC1 was basically positively related with total count and progressive motility, the PC2 was negatively related with sperm chromatin stability and the PC3 was positively related with sperm vitality and motility non-progressive. The subsequent cluster analysis rendered two subpopulations being characterized SP1 by progressive motility and high levels of sperm chromatin stability and sperm DNA maturation and SP2 with total count and vitality, poor motility and sperm DNA fragmentation.33
The use of CASA systems enables us to obtain a collection of a large number of mathematical parameters (e.g. kinematic and, morphometric measures), allowing stricter statistical approaches. For defining the quality of a sample, it is common practice simply to use the means and standard errors provided by CASA systems as the main inputs for further analysis. However, it was shown that this is completely inadequate. In fact, mean values of VSL are not correlated with maximum VSL in a variety of mammals. Another common mistake is to consider subjective limits to define different kinds of motility such as ‘subpopulations’ when they are just a subjective arbitrary distribution of kinematic limits.34
Therefore, a rational approach for the study of semen characteristics must include quantitative parameters obtained from CASA technology, such as the statistical analyses of various parameters, as has been done in a variety of species.31
The aim of this study was to define a new mathematical approach by taking a great variety of seminal quantitative characteristics into account. Owing to the relatively low number of samples available, it was not possible to perform a cluster analysis from the multiple data set obtained from kinematic and morphometric data, as this holistic approach is only possible with a higher sample size, an adequate sample size would be around an n of 200.35 It is needed to consider that there are diverse statistical procedures that can be followed in order to calculate sperm subpopulations. In the case of CASA systems that offer a large volume of data, it is necessary to perform a two-step procedure when defining the subpopulations. The first step includes PC analysis to reduce the dimensions of data, indicate redundant information and show the relative weight of each parameter in the variance explanation. The second step, using PCs as variables, consists of cluster analysis to define the structure of sperm subpopulations.29
Therefore, a big effort has dealt in the recent years with the study of subpopulations of spermatozoa in an ejaculate, changing the vision of a race between more or less equivalent cells, to a new vision of competition between different groups of spermatozoa with similar characteristics, even if the true significance of this fact is not clear at the moment.36 However, as well as following this advanced approach, most work has focused on just one kind of variable such as kinematics,37 morphometry38 or DNA fragmentation analysis.39 It is not so common to carry out a subpopulation study from different kinds of variables in the same paper as we have applied here.29
Following this approach, in a study on human adolescents subjective seminal and morphometric data were combined in a more sophisticated mathematical approach. PC analysis from an original set of nine seminal and eight sperm head morphometric parameters produced five PCs. The first two of these, which were related to sperm size and shape respectively, were most explanatory.38 In the best of our knowledge only one work using fox semen samples, combined kinematic and morphometric data in order to study sperm subpopulation structure. Upon considering eight morphometric and eight kinematic parameters, two PCs were obtained, being particularly interesting the fact that the most elucidative ones were related with morphometric parameters. Based on this PCs four SPs were obtained which proportion varied among animals, as here ir was observed in human individuals.40
In this study, a great variety of seminal quantitative data was used to define the semen characteristics from different perspectives. In order to be applied in semen diagnosis and fertility prediction future studies must consider a higher sample size to develop a mathematical model integrating all the variables.
ConclusionsHolistic approaches such as those described here could improve our knowledge of semen characteristics. This would be promising for advancing both scientific knowledge and improving the diagnostic potential of semen analysis. The utility of the conceptual approach presented in this study must be extended to a population with a wide range of variability among subjects, using different regional, socio-economic, ethnicity, age and pharmacological and toxicological conditions, making possible to define new universal criteria that improve the prediction of fertility and optimize the diagnosis for assisted reproduction techniques.
Ethical disclosuresProtection of human and animal subjectsThe authors declare that no experiments have been performed on humans or animals for this research.
Confidentiality of dataThe authors declare that they have followed the protocols of their work center on the publication of patient data.
Right to privacy and informed consentThe authors declare that no patient data appear in this article.
Conflict of interestsThe authors declare that they have no conflict of interest.