Varicocele is a common cause of male infertility associated with an elevated testicular temperature that induces apoptosis, spermatogenesis dysfunction, and affects sperm parameters. In this study, we investigate the probable therapeutic effects of resveratrol (RES), a natural phytoalexin, against varicocele.
Materials and methodsIn this study, 48 male Wistar rats randomly divided into 8 groups: normal, sham, normal+RES (20 and 50mg/kg), varicocele, varicocele+ethanol and varicocele+RES (20 and 50mg/kg). Incomplete closure of the left renal vein was used for varicocele induction and two months later, RES was administrated orally for 60 days. Then, sperm parameters, DNA fragmentations, chromatin density, and testis histopathology were analyzed. In addition, HSPA2, protamine 1, and 2 expression levels were evaluated using real-time PCR.
ResultsAccording to our results, resveratrol treatment improved sperm parameters, testis histopathology, DNA fragmentation, and chromatin maturation which damaged follow varicocele (p≤0.05). Also, it increased HSPA2, protamine 1, and 2 expression levels significantly in both doses (p≤0.05).
ConclusionResveratrol potentially attenuates varicocele-induced spermatogenic impairments by its antioxidant features and regulates spermatogenic gene expression undergoing DNA fragmentation, so leads histopathological properties of tissues to physiological parameters.
El varicocele es una causa común de infertilidad masculina asociada con una temperatura testicular elevada que induce apoptosis, disfunción de la espermatogénesis y afecta los parámetros espermáticos. En este estudio, investigamos los probables efectos terapéuticos del resveratrol (RES), una fitoalexina natural, contra el varicocele.
Materiales y métodosEn este estudio, 48 ratas Wistar macho se dividieron aleatoriamente en 8 grupos: normal, simulado, normal+ RES (20 y 50mg/kg), varicocele, varicocele+ etanol y varicocele+ RES (20 y 50mg/kg). Se utilizó el cierre incompleto de la vena renal izquierda para la inducción del varicocele y dos meses después se administró RES por vía oral durante 60 días. Luego se analizaron los parámetros espermáticos, las fragmentaciones de ADN, la densidad de cromatina y la histopatología testicular. Además, los niveles de expresión de HSPA2, protamina 1 y 2 se evaluaron mediante PCR en tiempo real.
ResultadosSegún nuestros resultados, el tratamiento con resveratrol mejoró los parámetros espermáticos, la histopatología testicular, la fragmentación del ADN y la maduración de la cromatina que dañó el varicocele posterior (p≤0,05). Además, aumentó significativamente los niveles de expresión de HSPA2, protamina 1 y 2 en ambas dosis (p≤0,05).
ConclusiónEl resveratrol atenúa potencialmente las deficiencias espermatogénicas inducidas por varicocele por sus características antioxidantes y regula la expresión de genes espermatogénicos que sufren fragmentación del ADN, por lo que conduce las propiedades histopatológicas de los tejidos a parámetros fisiológicos.
Varicocele is an abnormal dilation and torsion of pampiniform plexus in the spermatic cord. The prevalence of this pathologic condition is rare before the age of 10 years but increased significantly in ages of 10–19 years.1 Varicocele has been found in 15% of total healthy men and up to 35% of patients with primary infertility.2 One of the proposed hypothesis to explain varicocele is hyperthermia following venous stasis. The testicular temperature is 34.8°C under physiological conditions; however, it reaches 37.4°C in varicocele, leading to reactive oxygen species (ROS) generation, apoptosis induction, and ultimately causing spermatogenesis disruption.2 For eliminating the side effects of varicocele, varicocelectyomy is suggested to remove swollen veins and restore proper blood flow inside the testis. Sperm parameters, testosterone levels and testicular function improve about 3–6 months after varicocele repair which leads to increase pregnancy rate in couples with male infertility associated with varicocele.3
Spermatogenesis is a complicated process that requires precise coordination between different genes expression in each stage. 70kDa heat shock protein (Hsp70) is one of the thermal shock proteins that is detected in different parts of human getnital system such as epidydimis, prostate and seminal vesicle.4 Heat shock protein family A member 2 (HSPA2) is a dominant type of Hsp70 has crucial role in spermatogenesis and male fertility. The persence of HSPA2 on sperm cells or testicular tissue can be used as a biomarker for having a type of successful assisted reproductive technology (ART).5 HSPA2 is expressed during the first meiotic stage of spermatogenesis and elongated sperm. This protein plays an essential role to develop a complex for binding to zona plucieda. The low level of HSPA2 leads to decrease in sperm count and ability to attach to zona plucieda.4 This agent also has an essential protective function in heat response, providing a balance between synthesis, degradation, folding, transport, and accumulation of proteins in the cytoplasm, mitochondria, and endoplasmic reticulum.6
Protamine (P1 and P2), a positive charge protein, is another essential protein required for normal spermatogenesis that causes DNA–protamine complex stability by disulfide bonds formation. Histones–proteins replacement increases sperm chromatin condensation during spermiogenesis which plays an important role in DNA protection and proper sperm function.7 DNA fragmentation involved in varicocele is possibly due to protamine deficiency.8
Considering varicocele-associated spermatogenic disorders remedies, some antioxidant therapy strategies would be useful in decreasing the negative effects of varicocele on fertility.9 Resveratrol (RES), a phenolic phytoalexin, is a herbal ingredient which exist in grape skins and seeds, raspberries, peanuts, blueberries, and blackberries.10 RES has anti-inflammatory properties and exerts its function through different signaling pathways. It reduces ROS and nitric oxide production. RES also suppresses IL-1b, TNF-α and NF-кB activation.11 Therefore; it has many medical effects, particularly in cardiovascular diseases, cancers, diabetes, neurological problems.12 This antioxidant can be helpful for preserving male fertility. It could protect sperm DNA integrity from damage induced by cryopreservation when it was added to the freezing media.13 RES reduces the histopathological testicular damages following testicular torsion.14 Infertile males with hyperthyroidism can preserve sperm motility by daily RES administration.15 Despite more research is needed for understanding its functional mechanisms following varicocele. RES improves sperm parameters quality such as morphology, viability, mitochondrial activity, acrosome integrity, and DNA health in peripubertal varicocelized rats.16 In our previous work, RES reduced inflammatory responses and apoptosis in varicocele testis.17 However, the probable effects of RES on genes involved in spermatogenesis are not well understood; this study was designed to assess HSPA2, Protamine 1, and 2 genes expression as essential genes for normal spermatogenesis in RES administrated varicocele rats.
Materials and methodsAnimalsThe Ethics Committee of Arak University of Medical Sciences approved research and animal care (IR.ARAKMU.REC.1395.67). Animals were housed at 24°C and controlled conditions with free access to water and food.
48-Weeks old adult male Wistar rats (200–250g, Pasteur, Iran) randomly were divided into 8 following groups (n=6): I – normal; II – sham; III – normal+20mg/kg RES; IV – normal+50mg/kg RES, V – varicocele; VI – varicocele+ethanol; VII – varicocele+20mg/kg RES and VIII – varicocele+50mg/kg RES.
Experimental left varicocele inductionThe animals were anesthetized by intraperitoneal injection of ketamine (100mg/kg) and xylazine (50mg/kg). Following the supine position, the midline incision was applied. The left renal vein was dissected, and a 0.85mm wire was placed parallel to the renal vein and was fixed with silk string (4/0). Then the wire was carefully removed, and the abdominal wall was sutured.18 In the sham group, a similar procedure was done except for partial ligation of the left renal vein.
Resveratrol administrationTwo months after surgery, RES was administrated orally (20 and 50mg/kg) for 60 consecutive days (the chosen doses were based on our previous work).17 Animals were sacrificed under deep anesthesia and left testis and cauda epididymis were removed. Epididymis tail was transferred to a petri dish containing 5ml phosphate buffer saline (PBS; Sigma, Germany) and used for sperm parameter analysis. The left testis was transferred to the liquid nitrogen and stored at −70°C for further analysis.
Sperm parameters assessment: motility, count, morphology, and viabilityThe cauda was minced into 5ml of prewarmed Ham's F10 (Gibco, Germany) and incubated at 37°C in 5% CO2 for 30min. One drop of sperm suspension was placed in Neubauer chamber. Sperm motility and sperm count were assessed by a light microscope (40×) according to the routine protocol.19 For sperm viability index assessment, the eosin–nigrosin staining was used. Living sperm (white or faint pink head) and dead sperm (red or dark pink head) were evaluated (100×).20 Papanicolaou staining was performed to evaluate sperm morphology based on two morphological features; hook-like heads and smooth tails sperms were reported as normal.21
Assessment of DNA fragmentation and chromatin maturityAcridine orange staining was used to evaluate DNA fragmentation. This technique is based on fluorescent emission (green and red emission representing normal and denatured DNA, respectively). At first, sperm smears were fixed by fixative (methanol:acetic acid: 3:1) for 2h. Then, slides were stained and washed with PBS. Finally, the slides were covered by a cover slide and observed with a fluorescent microscope (100×).22 Acidic Aniline Blue Staining (AAB) was performed to evaluate chromatin condensation of sperm nuclei. This technique is a specific positive reaction for lysine. The immature sperm nucleus is rich in lysine, which absorbs blue color. However, the normal sperm nucleus is rich in arginine and cysteine, representing no blue color. For this assay, sperm smears were fixed in glutaraldehyde for 30min. Each slide was stained with AAB (5%) in acetic acid (4%, pH=3.5). Finally, the color variations were assessed with a light microscope (100×).23
Histopathological evaluationTestis tissue was fixed in Bouin's solution (Merck, Germany), and after the histopathology process, 5μm paraffin sections were prepared and stained with hematoxylin–eosin (Merck, Germany). Spermatogonia number, seminiferous tubules diameter, and thickness were measured by Image Tools analysis software.21
Quantitative RT-PCRHSPA2, protamine 1, and 2 genes expression was studied by quantitative reverse transcriptase-polymerase chain reaction (qRT-PCR) in all groups. Total RNA was extracted using YTzol reagent (Yekta Tajhiz, Iran) according to the manufacturer's protocols. 2μg of total RNA was used for cDNA synthesis in a total volume of 20μl by using RevertAid™ First Strand cDNA Synthesis Kit (Aryatous, Iran). qRT-PCR was performed using the Light Cycle Real-time PCR (Roche, USA). qRT-PCR was performed in a total volume of 20μl containing cDNA, 5mmol/l solutions of forward and reverse primers, and SYBR green reagent (Takara Bio, Otsu, Shiga, Japan). Each sample was loaded in duplicate. The sequences of used primers were: HSPA2 5′-AGGACCCACCATTGAGGAAGTG-3′ and 5′-TCAAGCAAATCTCCACGTACATAC-3′, protamine-1 5′-CCAGCCGCAAACTAAAGACTCATGG-3′ and 5′-AGCTCATTGCCGCATTACAAGTGGG-3′, protamine-2 5′-AGGAAAGGTGAGCAAGAGAAAGGCG-3′ and 5′-CATTCCCCTAGTGATGGCTATCTCC-3′ and β-actin 5′-TCACCCACACTGTGCCCCATCTACGA-3′ and 5′-CAGCGGAACCGCTCATTGCCAATGG-3′. All samples were normalized against β-actin (internal control) using the comparative CT method (ΔΔCT).
Statistical analysisAll obtained data were represented by the mean±standard error of the mean (SEM). Normality distribution was assessed by Kolmogorov–Smirnov test. The one-way analysis of variance (ANOVA) followed by the Tukey-test was used to analyze statistical differences between multiple groups. p-Value<0.05 was considered statistically significant.
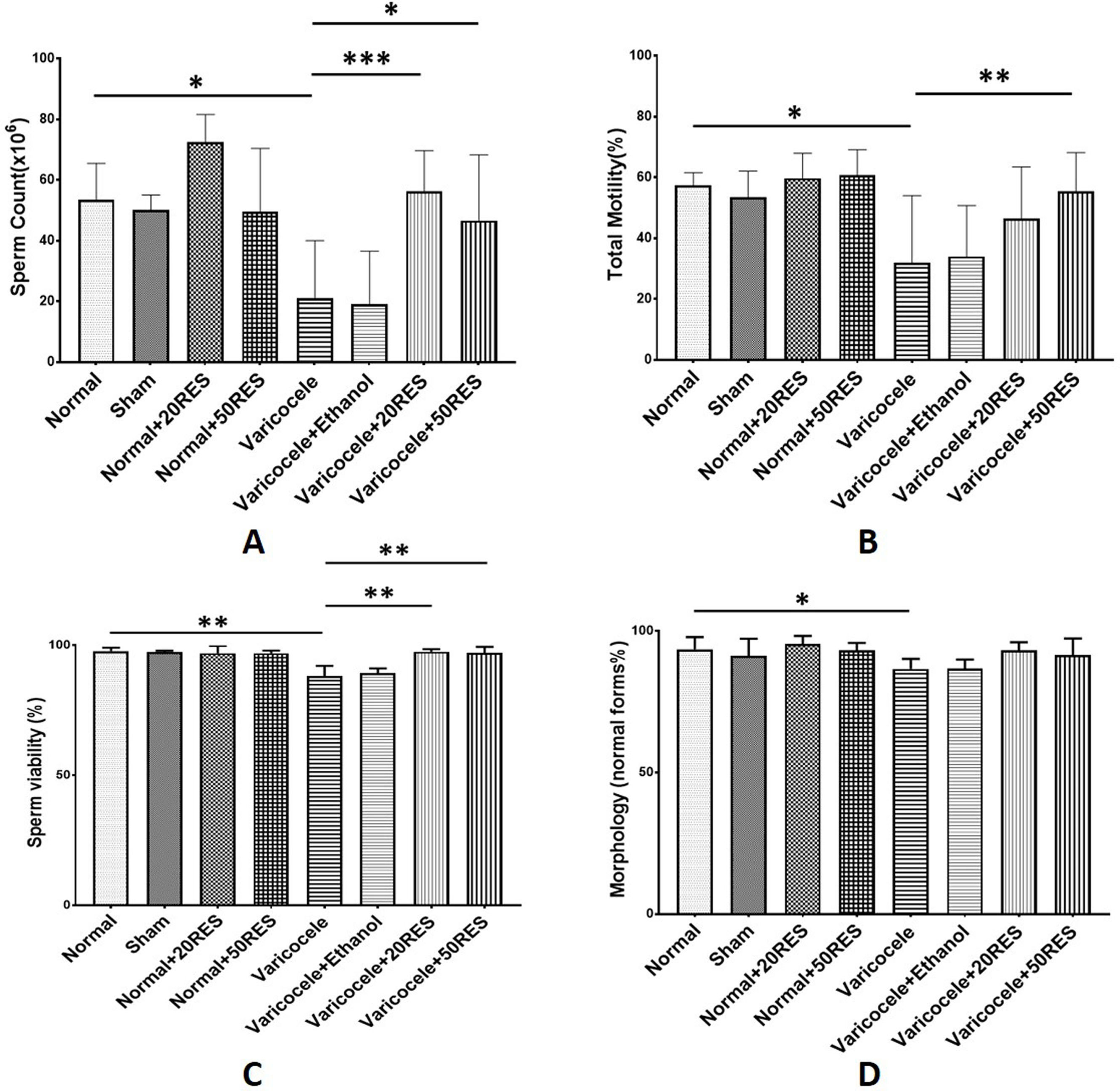
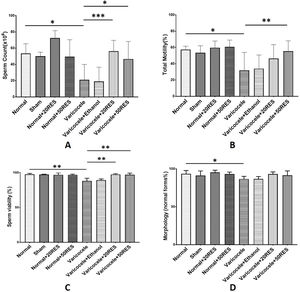
ResultsResveratrol improved sperm parametersAccording to our results, sperm parameters including sperm count, motility, morphology, and viability were significantly decreased in varicocele rats compared to the control group (p≤0.05). Resveratrol administration in both doses (20 and 50mg/kg) meaningfully increased sperm count and viability (Fig. 1A and C) (p≤0.05). A significant increase in sperm motility was observed only in the high-dose resveratrol group (Fig. 1B) (p≤0.01). Resveratrol showed no changes in sperm morphology (Fig. 1D).
Sperm parameters are represented in different experimental groups. Note the significant decrease in sperm count and viability following varicocele induction. Furthermore, note the positive effect of both doses of resveratrol on these parameters (A and B) and the significant increase in sperm motility following the high-dose administration of resveratrol (C). Sperm morphology significantly declined in the varicocele group and treatment with resveratrol administration could not improve sperm morphology. *: p≤0.05, **: p≤0.01, ***: p≤0.001.
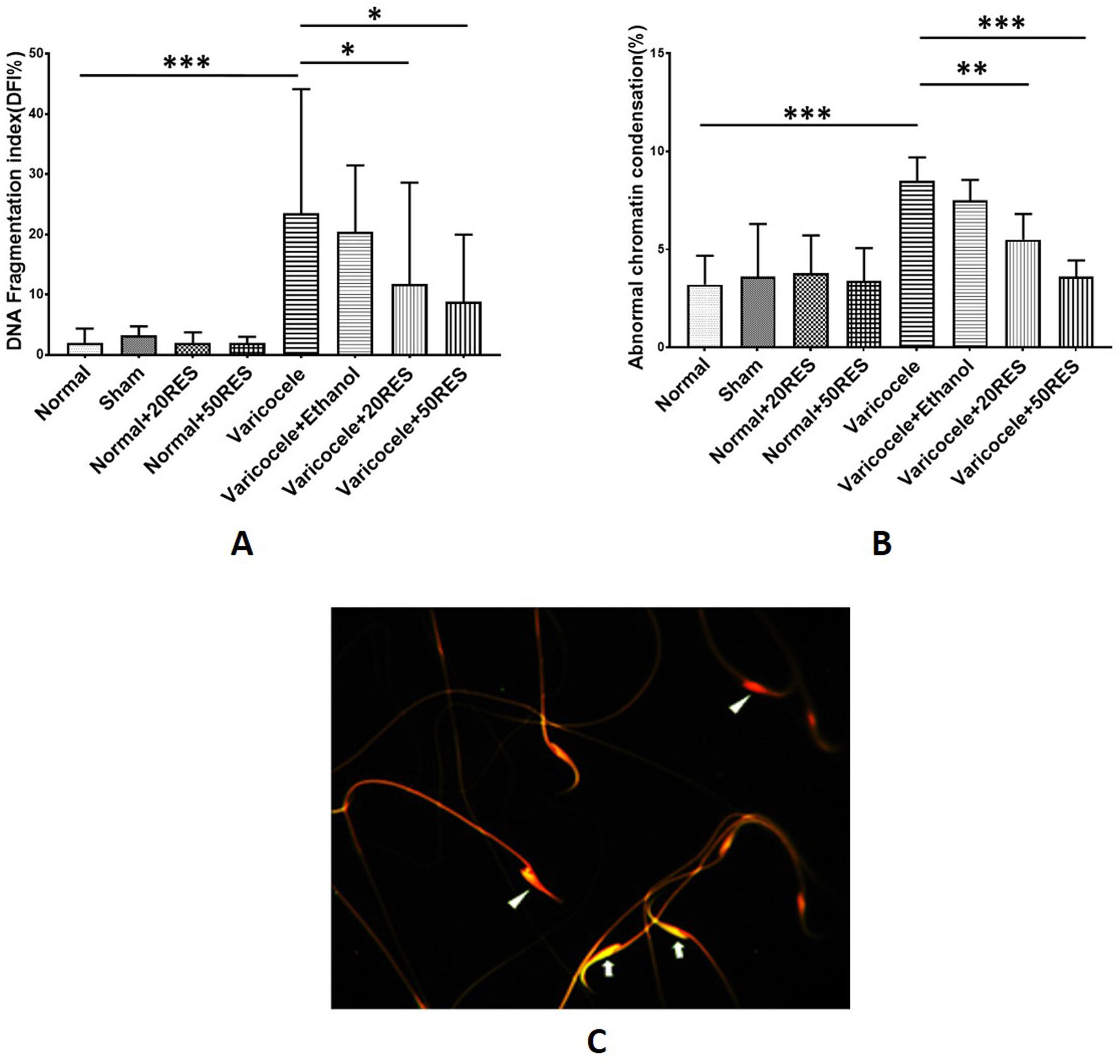
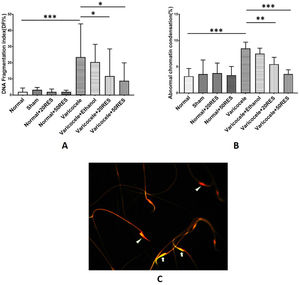
By using acridine orange and aniline blue staining, we determined sperm DNA fragmentation and chromatin condensation, respectively. According to our results, the percentage of fragmented DNA and immature sperms were increased in the varicocele group compared to the control group (p<0.001). Administration of both concentrations of resveratrol significantly improved DNA fragmentation and chromatin condensation (p≤0.05) (Fig. 2A and B). In acridine orange staining, sperm with double-strand DNA appeared green while sperm with single-strand DNA appeared red (Fig. 2C).
Comparison of DNA fragmentation index (A) and abnormal chromatin condensation (B) are represented in different experimental groups. Note the increasing DNA fragmentation and abnormal chromatin condensation in varicocele groups. Besides, note that resveratrol in both doses significantly reduced these two indices. Acridine orange staining for evaluating DNA fragmentation (C). Sperm with double-strand DNA (arrow) and sperm with single-strand DNA (arrow head). *: p≤0.05, **: p≤0.01, ***: p≤0.001.
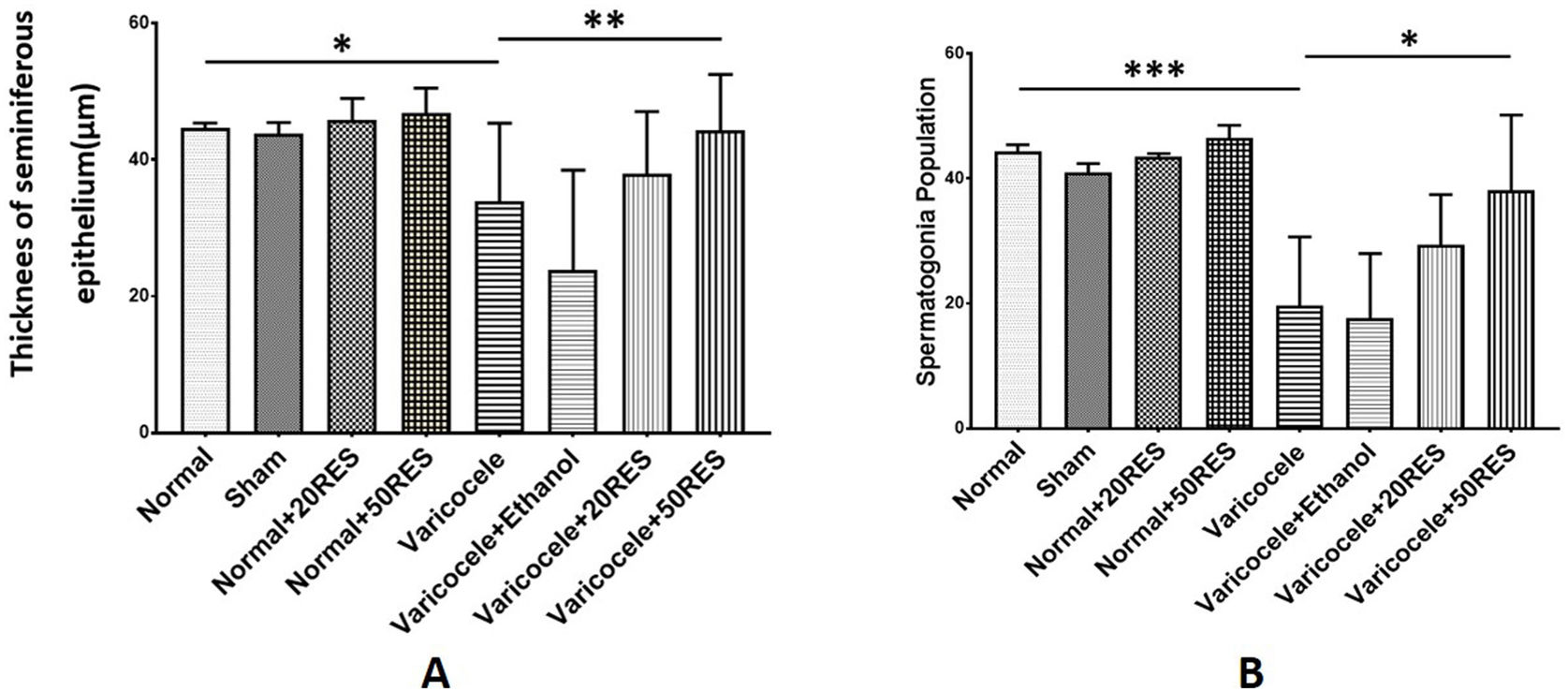
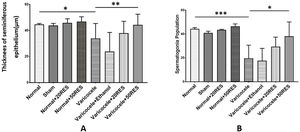
According to histhopathology analysis, we showed a significant reduction in seminiferous epithelium thickness and spermatogonia number following varicocele induction compared to the control group (p≤0.05), while resveratrol administration (50mg/kg) significantly improved these two indices (p≤0.05) (Fig. 3A and B).
Testis histopathology evaluation in different groups. The thickness of seminiferous epithelium (A) and spermatogonia population (B) reduced significantly in following varicocele, and treatment with resveratrol in 50mg/kg concentration improved testicular damage induced by varicocele. *: p≤0.05, **: p≤0.01, ***: p≤0.001.
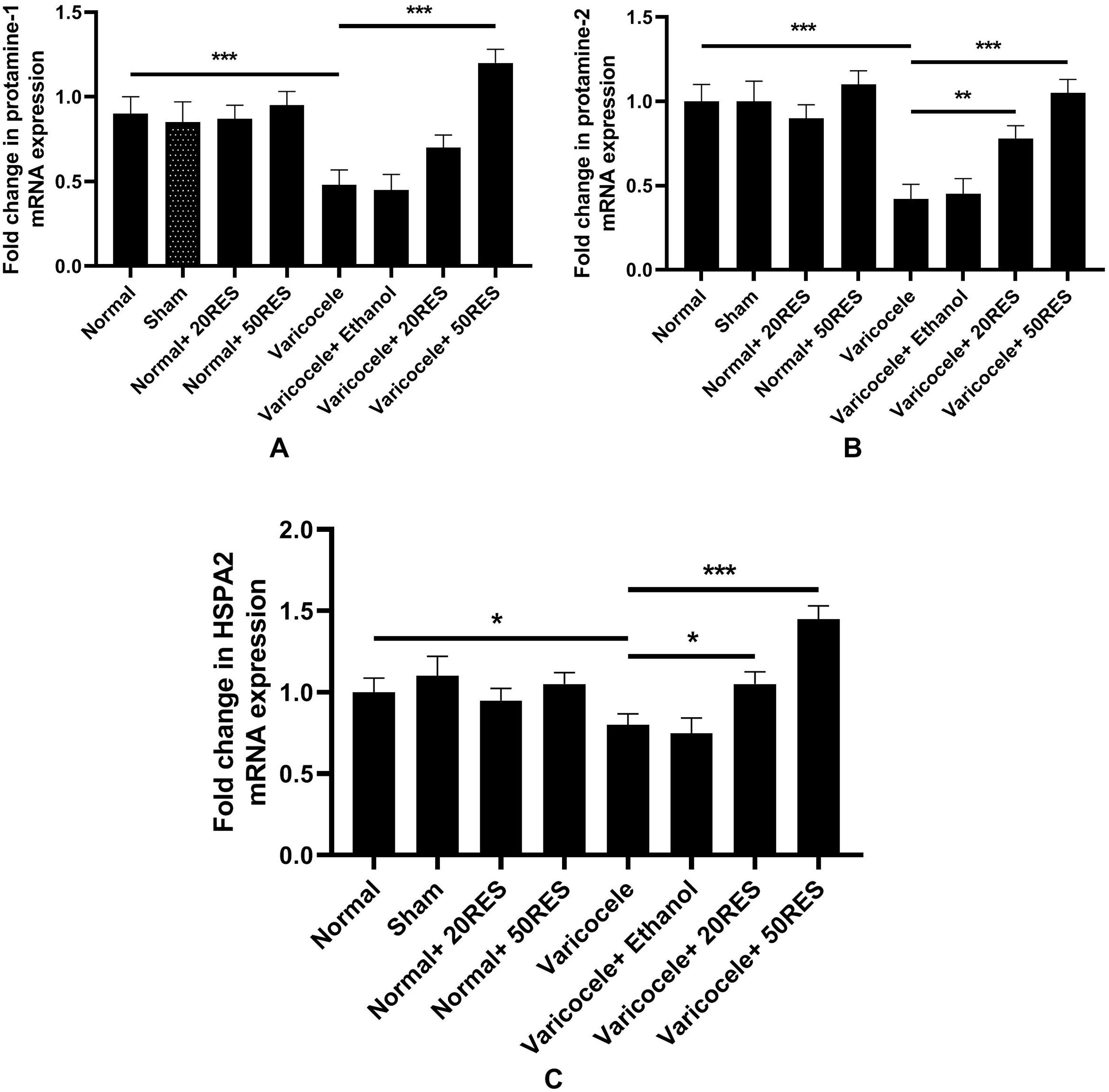
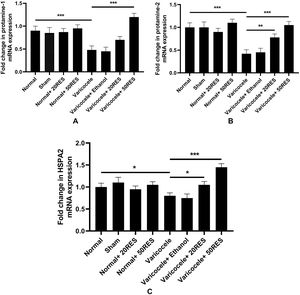
In this study, we evaluated HSPA2, protamine 1, and 2 expressions. According to our results, the expression level of these genes significantly reduced in the varicocele group compared to the control group (p<0.05). Two months resveratrol treatment (50mg/kg) markedly returned the protamine 1 mRNA levels to the normal level, but 20mg/kg resveratrol administration could not significantly increase the expression level of this gene (p<0.001) (Fig. 4A). Protamine 2 (Fig. 4B) and HSPA2 (Fig. 4C) genes expression increased in both resveratrol doses. Interestingly, the HSPA2 mRNA level increased in the varicocele+50mg/kg group compared to the control group. There were no significant differences between normal resveratrol administered groups compared to the control group.
DiscussionIn this study, we demonstrated that varicocele induction has adverse effects on sperm parameters and testis histopathology, whereas RES administration, especially in high doses (100mg/kg), could eliminate these destructive features. Spermatogonia stem cell (SSC), germ cell in seminiferous tubules, provides a constant testicular cell pool by its continuous division and needs low temperature for normal functioning.24 Hypoxia, increasing the testicular temperature, ROS production and hormonal dysfunction occur following varicocele. These pathological conditions lead to activation of a cascade of molecular alteration which induces apoptosis17 and inflammation in testis tissue. In response to apoptosis in testis tissue, the number of sperms and spermatogonia reduce.9 In our previous work, we showed that the genes expression of CatSper 1, and 2 which related to the sperm motility decreased following varicocele induction.21 In this study, we administered resveratrol for two months to decrease the deleterious effects of varicocele. RES has many therapeutic benefits such as antioxidant properties (through inhibition of lipid peroxidation), anti-inflammatory, vasodilator, anti-cancer, phytoestrogen, and anti-apoptotic features.25 RES could overcome the destructive effects of varicocele by down-regulating inflammasome complex and genes related to apoptosis.17 According to our results, RES improved sperm parameters and testis histopathology and decreased DNA damage and abnormal chromatin condensation. In addition, RES could up-regulate HSPA2, protamine 1 and, 2 expressions, which reduced during varicocele. HSPA2 is preserved in various cells from bacteria to humans and its amino acidic sequences have no changes during evolution.26 Redgrove et al. (2012) showed that HSPA2 has a key role in sperm-zona interaction. In non-capacitated sperms HSPA2 together with arylsulfatase A (ARSA) are present on inside of sperm membrane while sperm adhesion molecule 1 (SPAM1) is expressed on the surface of cell membrane. After capacitation, the position of ARSA and SPAM1 change but HSPA2 remains unchanged. Any depletion in the expression of HSPA2 is associated with the failure of sperm-zona pluceida binding.27 The absence or mutation of HSPA2 gene can potentially arrest spermatogenesis in meiosis I leading to male infertility.26 On the other hand, low expression of HSPA2 is associated with oligospermia, sperm DNA damage, sperm apoptosis, abnormal sperm morphology, cytoplasmic residues, and histone-protamine non-replacement.28 Lima et al. (2006) showed that HSPA2 gene expression was lower in adults with varicocele and oligospermia.29 For increasing the low HSPA2 expression in varicocele, we utilized RES and noticed this antioxidant could raise HSPA2 gene expression and protein level did not assess in this study. Moteiei et al. (2013) revealed that there is a positive correlation with HSPA2 expression and sperm count and morphology.30 RES exerts its protective effect on cardiac cells against stress response by activating heat shock protein.31
Protamine 1 and 2 genes involved in normal spermiogenesis whose expression is almost equal. During spermiogenesis, somatic histones are replaced by protamines in gradual process. Protamine genes are expressed in round spermatids immediately after meiosis is completed almost at equal (1:1) ratio. This ratio increases significantly in varicocele patients.32 Protamine deficiency in sperm nucleus or defects protamine–histone replacement may reduce chromatin density and consequently causes sperm DNA damage.33 Microarray analysis of testicular biopsies from varicocele patients showed protamine 2 gene expression reduction.34 Our results demonstrated that RES treatment could alter protamine 1, and 2 expression to the normal levels. Nashtaie et al. (2018) incubated normal sperms with RES (15μM) one hour before cryopreservation and they believed that RES could protect frozen–thawed sperms by activating the AMPK pathway, which has an important role in cellular energy homeostasis.35 The other possible mechanism for RES efficacy on male fertility is activating sirtuin-1 as an essential factor in controlling cell metabolism and inflammation.13 Our previous study showed that RES can reduce inflammatory mechanisms in varicocele testis by down-regulating the NLRP3 complex.17 Therefore, it seems that RES could protect spermatogenesis against varicocele disruption via different mechanisms.
ConclusionOur study showed that RES could overcome the deleterious effects of varicocele by up-regulating genes which are essential in spermiogenesis. These data, along with those reported previously, suggest that RES administration has beneficial effects on male fertility, and it might be used as adjuvant therapy for improving fertility in patients suffering from varicocele.
Ethical disclosuresProtection of human and animal subjectsThe authors declare that the procedures followed were in accordance with the regulations of the relevant clinical research ethics committee and with those of the Code of Ethics of the World Medical Association (Declaration of Helsinki).
Confidentiality of dataThe authors declare that no patient data appear in this article.
Right to privacy and informed consentThe authors declare that no patient data appear in this article.
FundingThis study was granted by the council of Arak University of Medical Sciences (grant number: 1159).
Conflict of interestThe authors declare no conflict of interest.