Candida guilliermondii has been recognized as an emerging pathogen showing a decreased susceptibility to fluconazole and considerably high echinocandin MICs.
AimsEvaluate the in vitro activity of anidulafungin in comparison to amphotericin B and fluconazole against different isolates of C. guilliermondii, and their efficacy in an immunosuppressed murine model of disseminated infection.
MethodsThe in vitro susceptibility of four strains against amphotericin B, fluconazole and anidulafungin was performed by using a reference broth microdilution method and time-kill curves. The in vivo efficacy was evaluated by determination of fungal load reduction in kidneys of infected animals receiving deoxycholate AMB at 0,8mg/kg i.v., liposomal amphotericin B at 10mg/kg i.v., fluconazole at 50mg/kg, or anidulafungin at 10mg/kg.
ResultsAmphotericin B and anidulafungin showed fungicidal activity, while fluconazole was fungistatic for all the strains. In the murine model, liposomal amphotericin B at 10mg/kg/day was effective in reducing the tissue burden in kidneys of mice infected with any of the tested strains. However, amphotericin B, anidulafungin and fluconazole were only effective against those strains showing low MIC values.
ConclusionsLiposomal amphotericin B showed the higher activity and efficacy against the two strains of C. guilliermondii, in contrast to the poor effect of fluconazole and anidulafungin. Further studies with more isolates of C. guilliermondii representing a wider range of MICs should be carried out to assess whether there is any relationship between MIC values and anidulafungin efficacy.
Candida guilliermondii es un patógeno emergente, con reducida sensibilidad al fluconazol y a las equinocandinas.
ObjetivosEvaluar la actividad in vitro de la anidulafungina, en comparación con la de la anfotericina B y el fluconazol, frente a C. guilliermondii y su eficacia en un modelo animal de infección diseminada.
MétodosLa sensibilidad in vitro se valoró mediante microdilución en caldo y curvas de mortalidad. La eficacia in vivo se evaluó mediante la determinación de la carga fúngica en riñón de ratones inmunosuprimidos con infección diseminada por C. guilliermondii tratados con anfotericina B desoxicolato (0.8mg/kg i.v.), anfotericina B liposomal (10mg/kg i.v.), fluconazol (50mg/kg) o anidulafungina (10mg/kg).
ResultadosLa anfotericina B y la anidulafungina mostraron actividad fungicida, mientras que el fluconazol fue fungistático frente a todas las cepas. En el modelo murino, la anfotericina B liposomal redujo para todas las cepas la carga fúngica en riñones, mientras que la anfotericina B desoxicolato, la anidulafungina y el fluconazol fueron efectivas solo en aquellos animales infectados con las cepas de menor valor de concentración mínima inhibitoria (CMI).
ConclusionesLa anfotericina B liposomal mostró la mayor actividad y eficacia frente a C. guilliermondii, en contraste con el limitado efecto del fluconazol y de la anidulafungina. Se necesitan estudios que incluyan cepas con un rango más amplio de CMI que permitan determinar la relación entre la actividad in vitro y la eficacia de la anidulafungina.
The fungus Candida guilliermondii is widely distributed in nature, including the human microbiota of the skin and mucosal surfaces.22 Although this species shows a reduced virulence in comparison to other Candida species,3 it is currently considered an emerging pathogen, with a major incidence in Latin America.18C. guilliermondii has been recognized as the etiologic agent of a wide variety of clinical infections, including disseminated ones mainly in immunocompromised patients,22 and nosocomial outbreaks in surgical patients with intravascular devices.13 Currently, the recommended treatment for invasive candidiasis in neutropenic patients includes caspofungin (CFG) or micafungin (MFG) as first-line therapies, liposomal amphotericin B (LAMB) and anidulafungin (AFG) being alternatives, while fluconazole (FLC) is recommended only when susceptibility to this drug is confirmed.23 However, several studies have shown that C. guilliermondii has a decreased susceptibility to FLC8,15,19, and therapeutic failures associated with isolates with high amphotericin B (AMB) minimal inhibitory concentrations (MICs) have been reported.9,12,24 Although nearly 90% of isolates shows echinocandins MICs equal or lower than clinical breakpoints (CBP) of susceptibility (2μg/ml),17 similar to other species of Candida, such as C. parapsilosis, some isolates of C. guilliermondii show MICs considerably high.8,15 Available data concerning the AFG efficacy in invasive candidiasis are limited and the potential role of that drug in the clinical practice is poorly known.23 In this context, animal studies can play an important role for a better understanding of the in vitro–in vivo correlation.11 Therefore, our main objective was to evaluate the in vitro and in vivo activities of AFG against different isolates of C. guilliermondii, comparing the results with those of AMB and FLC.
Materials and methodsFungal isolatesFour clinical isolates of C. guilliermondii (UTHSC 11-142, UTHSC 10-499, UTHSC 11-685 and UTHSC 10-3207) were used in the in vitro study and two of them (UTHSC 11-685 and UTHSC 11-142) were selected for the murine model on the basis of their different in vitro susceptibilities. The isolates were identified by sequencing the internal transcribed spacer (ITS) region and the D1–D2 domains of the rRNA, comparing the sequences with those of the type strain of this species.
In vitro studiesThe in vitro susceptibility of the four strains to AMB, FLC and AFG was evaluated using a reference broth microdilution method,6Candida parapsilosis ATCC 22019 and Candida krusei ATCC 6258 being included as quality controls.
Time-kill curves were developed for all the strains according to previous studies.5,20 In brief, a stock solution of each antifungal was prepared, AMB (Sigma–Aldrich Co., St. Louis, USA) and AFG (Pfizer Inc., Madrid, Spain) were dissolved in dimethyl sulfoxide and FLC (Pfizer Inc., Madrid, Spain) in distilled water. Further, drug dilutions were prepared in 9ml of standard RPMI 1640 medium to obtain concentrations of 0.03, 0.12, 0.5, 1, 2, 8 and 32μg/ml of each drug. The isolates were subcultured at 35°C for 24h on potato dextrose agar (PDA) plates. Cultures of C. guilliermondii were suspended in sterile saline and the resulting suspensions were adjusted at 5×106 colony forming units (CFU)/ml by haemocytometer counts and by serial plating onto PDA to confirm viability. Dilutions and controls (drug-free) were inoculated with 1ml of the fungal suspensions, resulting in a starting inoculum of 5×105CFU/ml, and incubated at 35°C. An aliquot of 100μl from each tube was collected at 0, 2, 4, 6, 8, 24, and 48h after inoculation and diluted in distilled water; 30 μl of them were cultured onto PDA plates and incubated at 35°C for 48h for CFU/ml determination. A CFU decrease of ≥99.9% or 3 log10 unit compared to starting inoculum was considered fungicidal, while a reduction of <99.9% or <3log10 unit, was considered fungistatic. The limit of detection was 50CFU/ml. All time-kill curve studies were performed in duplicate.
In vivo studiesMale OF-1 mice (Charles River; Criffa SA, Barcelona, Spain) with a mean weight of 30g were used in the experiment. Mice were housed in standard boxes with free access to food and water. All animal procedures were supervised and approved by the Universitat Rovira i Virgili Animal Welfare and Ethics Committee.
Mice were rendered neutropenic one day prior to infection by an intraperitoneal (i.p.) injection of 200mg/kg of cyclophosphamide (Genoxal; Laboratorios Funk SA, Barcelona, Spain) plus an intravenous (i.v.) injection of 5-fluorouracil (Fluorouracilo; Ferrer Farma SA, Barcelona, Spain) at 150mg/kg.10,14 The day of infection, mice were challenged i.v. with 1×108CFU/animal of each of the two strains of C. guilliermondii, UTHSC 11-685 and UTHSC 11-142, in 0.2ml of sterile saline into the lateral tail vein.3,4
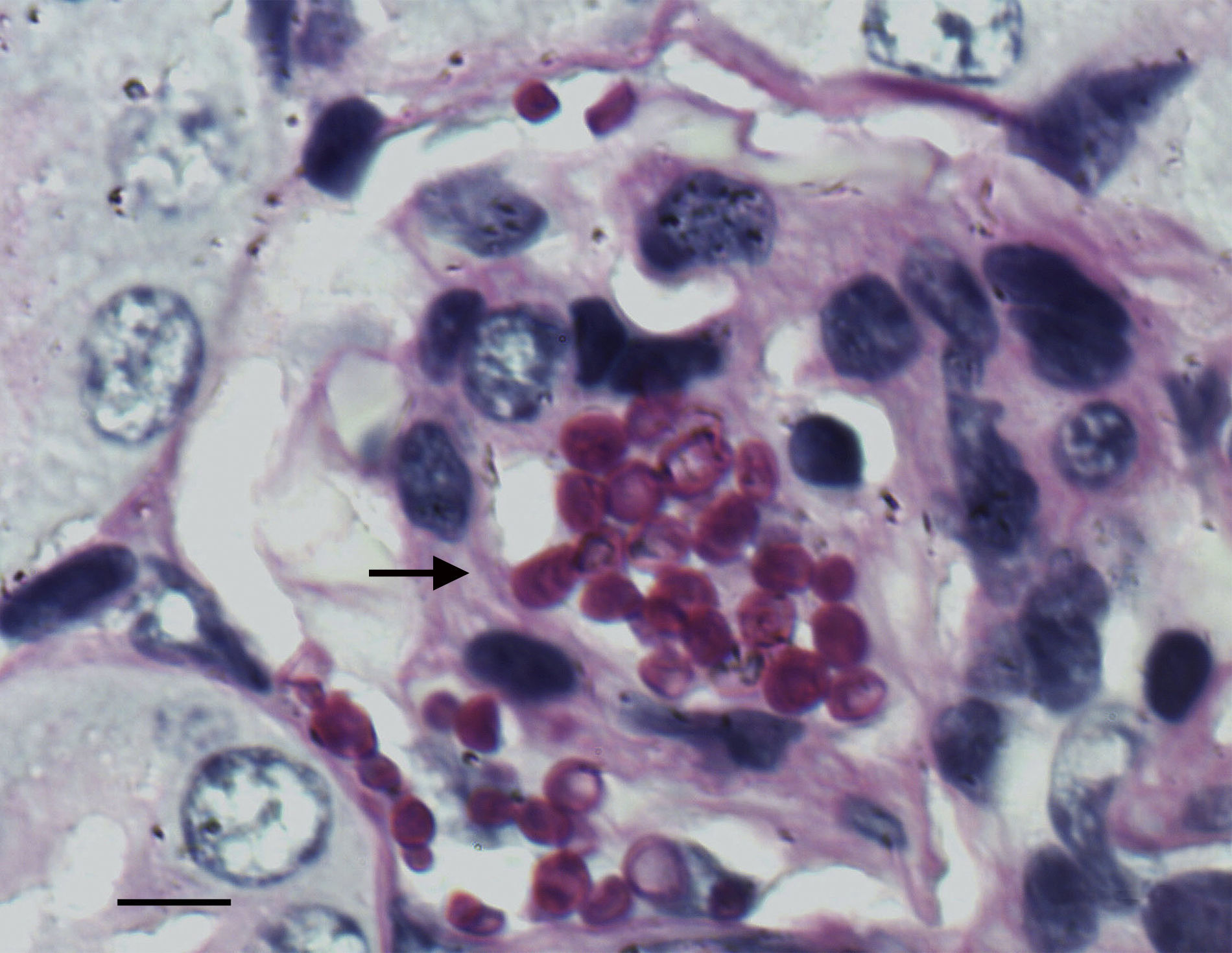
Groups of eight animals were randomly established for each strain and drug. The groups were treated as follows: amphotericin B deoxycholate (AMBd) (Xalabarder Pharmacy, Barcelona, Spain) at doses of 0.8mg/kg i.v. once a day (QD); liposomal amphotericin B (LAMB) (Gilead Sciences S.A., Madrid, Spain) at 10mg/kg i.v., QD; FLC (Pfizer Inc., Madrid, Spain) at 25mg/kg orally (p.o.) by gavage, twice daily (BID); and AFG (Ecalta; Pfizer Ltd., Sandwich, Kent, United Kingdom) at 10mg/kg of body weight/dose i.p., QD. All treatments began 24h after challenge, and lasted for 7 days. Controls received no treatment. To prevent bacterial infections, all mice received 5mg/kg day ceftazidime subcutaneously from days 1 to 7 after infection. Mice were checked daily and were euthanized on day 8 post-infection by CO2 anoxia. The efficacy of each drug was evaluated by tissue burden reduction and histopathological studies. Kidneys were aseptically removed, and one of them was weighed and homogenized in 2ml of sterile saline. Serial 10-fold dilutions of the homogenates were plated onto PDA and incubated for 48h at 35°C for CFU/g calculation. For the histopathology study the remaining kidney was fixed with 10% buffered formalin, dehydrated, paraffin embedded, and sliced into 2μm sections, which were stained with hematoxylin–eosin (H-E) and periodic acid-Schiff (PAS) stain for examination by light microscopy.
StatisticsColony counts from tissue were analyzed using the Mann–Whitney U-test, using Graph Pad Prism 4.0 for Windows (GraphPad Software, San Diego, CA, USA). When P values were below 0.05 the differences were considered statistically significant.
ResultsIn vitro studiesMICs of AMB were 0.25–1μg/ml, 0.06–0.25μg/ml for AFG and 0.5–1μg/ml for FLC. Following the cut-offs of susceptibility for AMB, FLC and AFG against C. guilliermondii,16 all isolates were susceptible to the three drugs. Quality control strains susceptibilities were within the accepted ranges.6
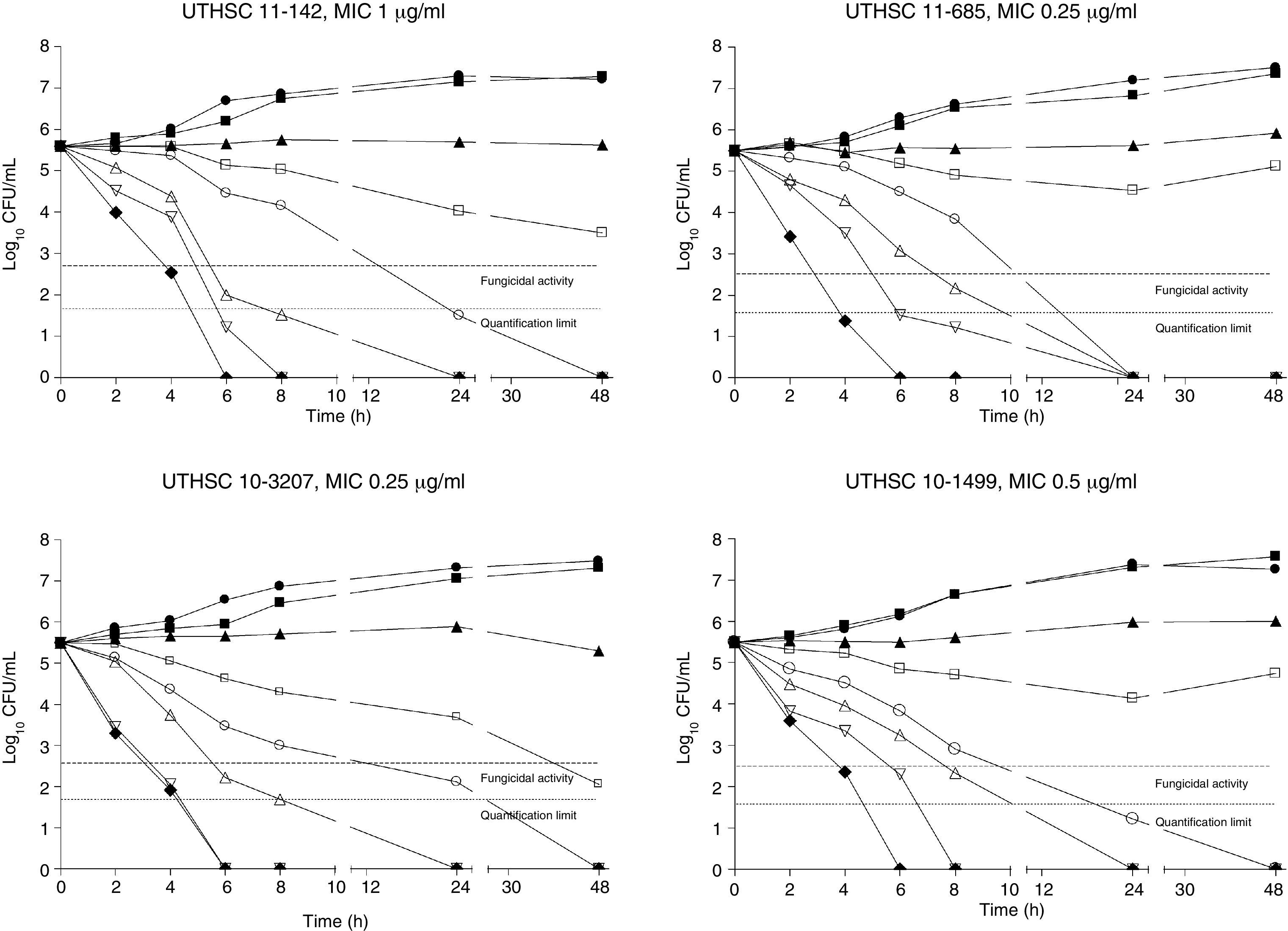
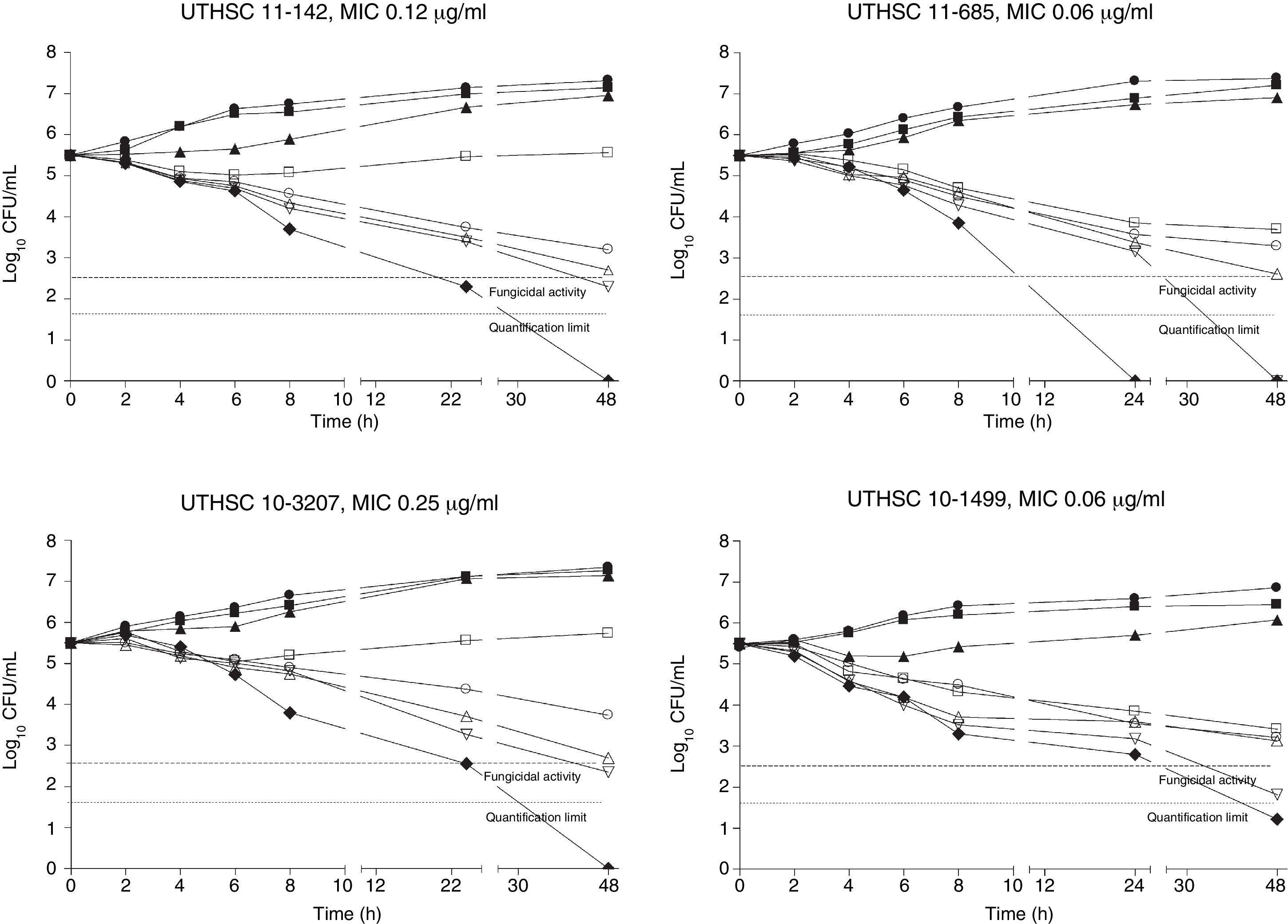
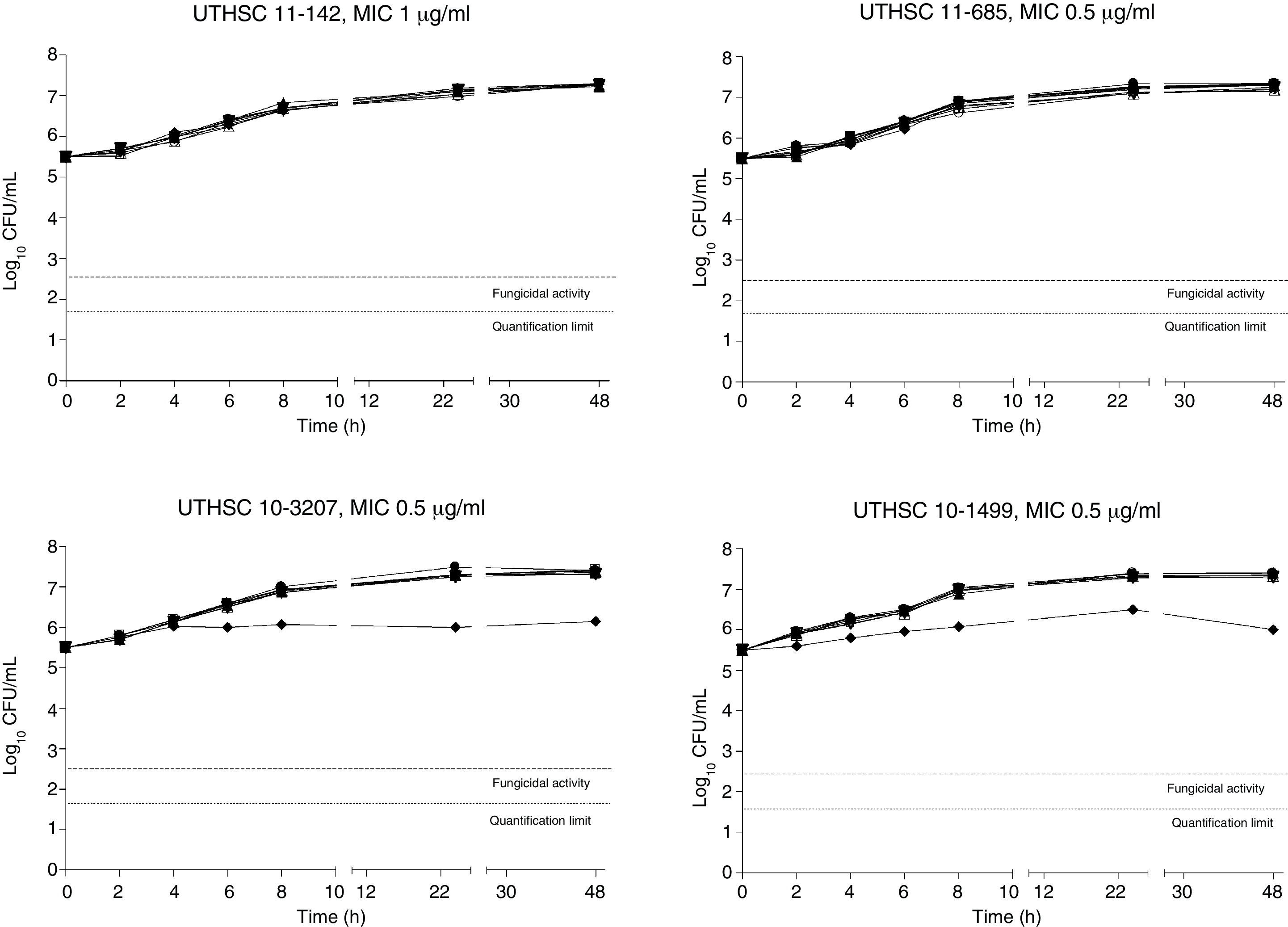
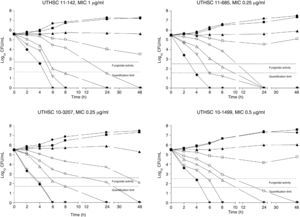
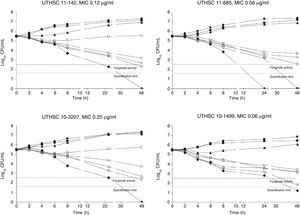
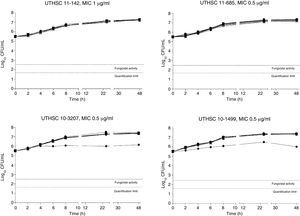
The killing kinetics of AMB showed a fast fungicidal activity that increased with drug concentration. At concentrations equivalent to the MIC, that drug showed a fungicidal effect against three of the four isolates tested (Fig. 1). This activity started immediately after inoculation at concentrations over 1μg/ml, the fungicidal endpoint being reached after 4h at 32μg/ml. AFG at concentrations above 0.5μg/ml showed fungicidal activity starting after 4h of incubation. The fungicidal endpoint was reached at 12–24h of incubation at 32μg/ml (Fig. 2). FLC showed fungistatic activity against all four isolates (Fig. 3).
Time-killing kinetics assays of AMB against four strains of C. guilliermondii. (■) 0.03μg/ml, (▴) 0.12μg/ml, (□) 0.5μg/ml, (○) 1μg/ml, (Δ) 2μg/ml, (▿) 8μg/ml, (♦) 32μg/ml, (●) control. Dashed lines represent a CFU decrease of 3log10 units in growth compared with the initial inoculum (fungicidal activity), dotted lines indicate the quantification limit of the test.
Time-killing kinetics assays of AFG against four strains of C. guilliermondii. (■) 0.03μg/ml, (▴) 0.12μg/ml, (□) 0.5μg/ml, (○) 1μg/ml, (Δ) 2μg/ml, (▿) 8μg/ml, (♦) 32μg/ml, (●) control. Dashed lines represent a CFU decrease of 3 log10 units in growth compared with the initial inoculum (fungicidal activity), dotted lines indicate the quantification limit of the test.
Time-killing kinetics assays of FLC against four strains of C. guilliermondii. (■) 0.03μg/mL, (▴) 0.12μg/ml, (□) 0.5μg/ml, (●) 1μg/ml, (Δ) 2μg/ml, (▿)8μg/ml, (♦) 32μg/ml, (●) control. Dashed lines represent a CFU decrease of 3log10 units in growth compared with the initial inoculum (fungicidal activity), dotted lines indicate the quantification limit of the test.
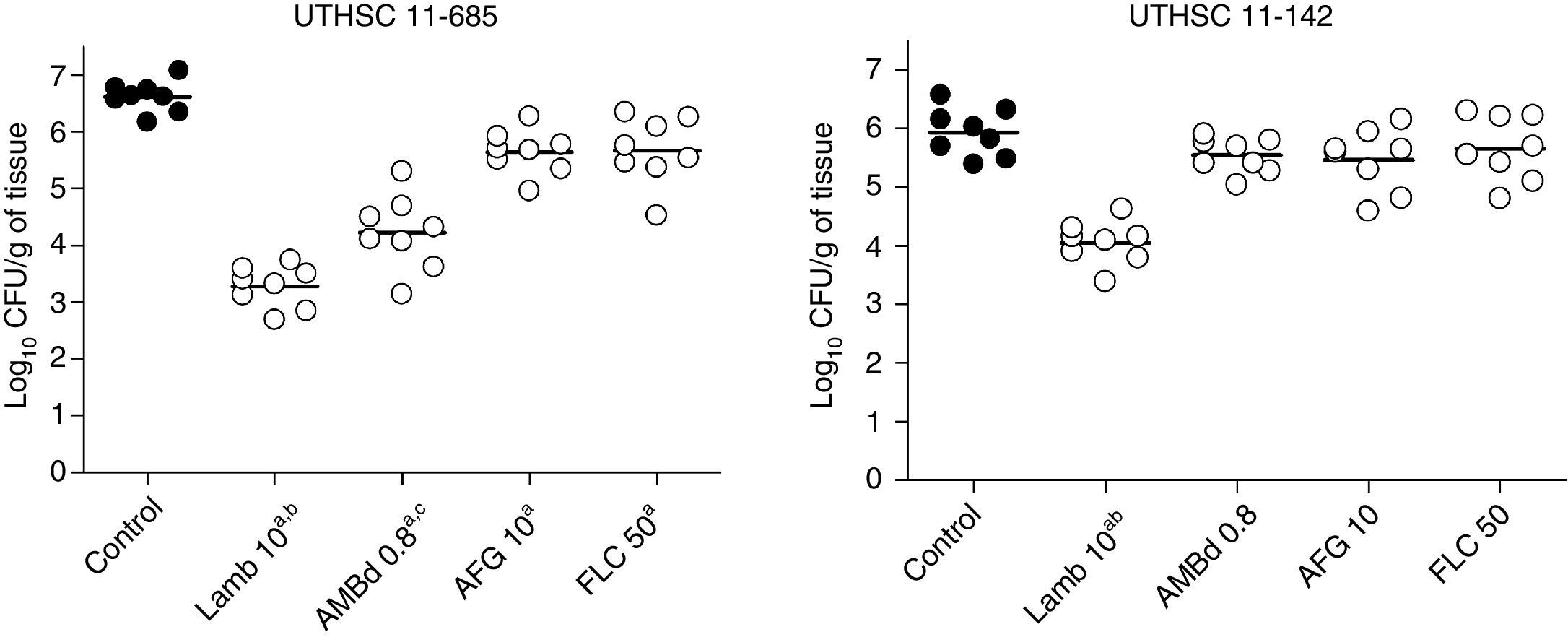
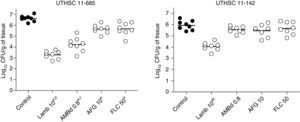
LAMB at 10mg/kg was the only drug able to reduce the fungal load in kidneys of mice infected with each of the two strains, being the reduction significantly higher than that of the other therapies (P≤0.04). AMBd and FLC were only able to reduce the tissue burden in mice infected with the strain that showed the lowest MICs for these two drugs, i.e., 0.25μg/ml for AMB and 0.5μg/ml for FLC (P≤0.008). In the case of AFG the fungal load reduction was modest and lower than that for AMBd, and it significantly reduced the tissue burden in kidney only with respect to control group for strain UTHSC 11-685 (P=0.002) (Fig. 4).
Effects of antifungal treatment on colony counts of C. guilliermondii in kidney of neutropenic mice, 8 days post infection. LAMB 10, liposomal amphotericin B at 10mg/kg QD; AMBd 0.8, amphotericin B deoxycholate at 0.8mg/kg QD; AFG 10, anidulafungin at 10mg/kg QD. aP<0.05 versus control; bP<0.05 versus AMBd 0.8, AFG 10 and FLC 50; cP<0.05 versus AFG 10 and FLC 50.
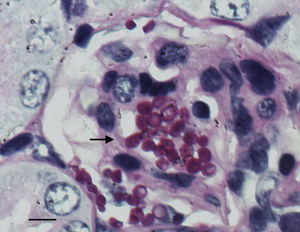
The histological study showed focal infiltration of fungal cells in kidneys of untreated animals and in mice treated with AMBd, FLC or AFG. Kidneys of mice treated with LAMB showed only a mild fungal invasion. Signs of necrosis, inflammatory response or parenchyma alterations were nor observed in controls neither in treated animals (Fig. 5).
DiscussionThe in vitro studies did not reveal decreased susceptibility of C. guilliermondii isolates to FLC or AFG. In agreement with previous studies, time-kill curves of AMB showed a concentration-dependent fungicidal activity against all the isolates,4,5,7 and FLC showed a fungistatic effect regardless of the concentration tested.7 It is known that AMBd exhibits a higher efficacy than its lipidic formulation, especially in kidney, when administered both at the same doses.1 However, pharmacokinetic studies showed that after the administration of 0.75mg/kg of AMBd the Cmax of AMB attained in mice serum was 0.30μg/ml.25 However, the AMB MIC of one of the two isolates tested is higher than this value; therefore, we used a high dose of LAMB in order to reach higher concentrations.1 Indeed, the administration of LAMB at 10mg/kg was effective in reducing the fungal load of both strains. This fact correlated with killing curves, where AMB achieved its fungicidal activity against the two isolates tested in vivo, at concentrations of 1μg/ml. To our knowledge, this is the first study that tried to establish a relationship between the killing kinetics and the in vivo experimental efficacy of AFG and FLC against clinical isolates of C. guilliermondii. Only a previous study on echinocandins exists, particularly on caspofungin (CFG) in disseminated infection by C. guilliermondii. CFG at 1mg/kg was effective in reducing the kidney fungal load in mice infected with one strain of C. guilliermondii with a MIC of 8μg/ml, while time killing revealed that no fungicidal activity was achieved at concentrations of 64μg/ml.4 Conversely, our study showed a concentration-dependent activity of AFG, which at 32μg/ml exerted a fungicidal activity, as previously reported,17 at 24h and at 8μg/ml. Previous studies reported AFG concentrations in serum and kidney of approximately 13μg/ml after 7 days of treatment at doses of 10mg/kg.21 Here, AFG was able to reduce only modestly the fungal burden in kidneys of neutropenic mice infected with one of the two strains tested, which does not seem to be related with the low AFG MICs difference between the two strains tested (1 dilution), suggesting that the response to AFG treatment is strain dependent. Similarly, FLC was also only able to reduce slightly the fungal burden in kidney of mice challenged with one of the two strains in spite of the dose administrated which reach serum concentrations above the MICs,2 which was also not surprising due to its fungistatic activity.
In conclusion, our study showed the higher activity and efficacy of LAMB against the two strains of C. guilliermondii, in contrast to the poor effect of FLC and AFG. However, further studies with more isolates of C. guilliermondii representing a wider range of AFG MICs should be carried out to assess if any relationship between MIC values and AFG efficacy exists.
Conflict of interestNone to declare.