To evaluate the prevalence of tinea capitis, tinea pedis, and tinea unguium in children from several schools of Barcelona city.
MethodsDuring the period of 2003–2004, a prospective cross-sectional study was carried out in 1,305 children (9% immigrant population) between the ages 3 and 15 in 17 schools in Barcelona. A systematic examination of the feet, (including nails and scalp), was performed to identify lesions compatible with tinea. Cultures of scalp and feet samples were done and analysis of environmental samples was performed for dermatophyte isolation.
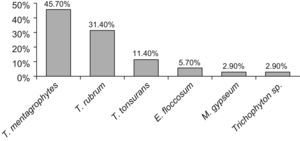
ResultsDermatophytes were isolated in 2.9% of the samples with a prevalence of 2.5% in feet, 0.23% in scalp, and 0.15% in nails of the feet. The predominant etiologic agents in feet were Trichophyton mentagrophytes in 45.7% of the cases and Trichophyton rubrum in 31.4%. In the nails, T. rubrum and Trichophyton tonsurans were isolated, while T. mentagrophytes (2 cases) and Trichophyton violaceum (1 case) were identified in scalp samples. Forty-five per cent of dermatophytes were isolated from healthy feet, the majority of cases in children 13–15 years old (p<0.05). Microsporum gypseum was the only agent identified in the environmental samples, and was also found in one of the cases of tinea pedis.
ConclusionThe results of this study demonstrate a low prevalence of tinea capitis and tinea unguium in school children of Barcelona. On the contrary, high prevalence of dermatophytes in feet was found. It highlights the high prevalence of healthy carriers of dermatophytes in feet.
Evaluar la prevalencia de tinea capitis, tinea pedis y tinea unguium en niños de diferentes escuelas de la ciudad de Barcelona.
MétodoDurante el periodo 2003–2004 se efectuó un estudio prospectivo en 1.305 niños (9% de inmigrantes) de edades comprendidas entre los 3 y los 15 años, en 17 escuelas de dos distritos de la ciudad de Barcelona. Se realizó un examen sistemático de los pies, uñas de los pies y cuero cabelludo de todos los niños, seguido de la toma y posterior cultivo de muestras del cuero cabelludo y pies, así como de muestras ambientales para aislar e identificar la presencia de hongos dermatofitos.
ResultadosSe aisló un 2,9% de dermatofitos de las muestras clínicas, correspondiéndose con una prevalencia del 2,5% en pies, 0,23% en cuero cabelludo y 0,15% en uñas de pies. Las especies predominantes fueron Trichophyton mentagrophytes en el 45% de los casos y Trichophyton rubrum en el 31,4%. En uñas se cultivó T. rubrum y Trichophyton tonsurans, mientras que en cuero cabelludo se aisló T. mentagrophytes (dos casos) y Trichophyton violaceum (un caso). El 45% de los dermatofitos se aislaron de niños aparentemente sanos, la mayoría de los cuales se encontraban entre los 13 y los 15 años de edad (p<0,05). Microsporum gypseum fue el único dermatofito aislado del medio ambiente, encontrándose también en un caso de tinea pedis.
ConclusiónLos resultados de este estudio demuestran una baja prevalencia de tinea capitis y tinea unguium en escolares de Barcelona. Destaca la elevada prevalencia de aislamientos de dermatofitos en pies, con gran proporción de portadores sanos.
Tinea is a mycosis caused by dermatophytes, filamentous fungi that invade the keratinized tissues, including the corneous layer of the skin, nails, and hair. The most frequent agents are antropophylic or zoophylic species like Microsporum canis, Trichophyton mentagrophytes, Trichophyton rubrum, Trichophyton tonsurans, and Epidermophyton floccosum.14
Tinea capitis has decreased in developed countries, while it presents a high prevalence in developing countries. On the contrary, tinea of the feet or tinea pedis seems to be one of the most common clinical forms of mycosis in adult and young population, usually associated with the use of sport footwear and sports.6
In Spain, limited information is available on the prevalence of these two mycoses in the infantile population. Two previous publications have confirmed that tinea of the scalp occurs rarely in the school population in Madrid (0.64%) and Barcelona (0.23%).7,32 Cuetara et al.7 have concluded that the prevalence of tinea of the scalp in Madrid's schools was significantly higher in immigrant population, while Triviño-Duran et al.32 in Barcelona showed the lack of statistic significance when compared with autochthonous population. In this study, prevalence of tinea pedis was also determined, reaching 10-fold times the scalp infection. The population analyzed by Triviño-Duran et al. corresponded to a unique school district with a high presence of non-autochthonous population (34.7%).
The main goal of the present study was to compare these results with the ones obtained in another district of the same city with smaller number of foreign population (8.3%) and higher socioeconomic level.8,9 Associated with this objective, an epidemiological survey of environmental samples of the public and sport spaces used by students was done.
Material and methodsPopulationFrom November 2003 to June 2004, a total of 1305 children from 17 schools of the district of Sant Martí (the second district in effective extension and population from Barcelona), were involved in the study. The children were divided into three age groups: 3–5-year-old group—522 children (40%); 10–12-year-old group—303 children (23.2%); and 13–15-year-old group, with 480 children (36.8%). From the total, 50.1% were boys (654 children) and 49.9% were girls (651 children).
The origin of these children was determined: 87.4% (n=1140) had been born in Spain, 9.9% (n=129) had immigrated mainly from Latin America, 1.1% from Eastern European countries, and 0.8% from Maghreb.
Environmental studyEight areas (property of schools or municipal centers) where sport activities are held during the school schedule were analyzed for the presence of dermatophytes. Likewise, seven outside areas where the youngest children play were also studied.
Data collectionThe same nurse from the Community Health of the Public Health Agency of Barcelona revised and collected the samples from all the children involved in the study during the school schedule. Previously, a description of the study was provided to the children's parents and their consent was obtained together with the response of a written epidemiological questionnaire. Personal data were compiled: age and sex of the child, geographical origin, as well as his/her parents, and child's extracurricular activities in public pools and gyms, as well as contact with pets with hairy coat.
Clinical samplesEach child was visually examined, searching for lesions compatible with tinea on the head (alopecia and/or desquamation, inflammation, and capillary fragility), feet, and feet nails (desquamation and/or scaling, plantar fissures, change in color, groove). All the observations were registered in a personal record.
The scalp of each child was brushed with a sterile dental brush by applying a soft massage and saving the brush in a sterile bag. Subsequently, a Rodac contact plate with DTM culture medium (Dermatophyte Test Medium) was applied directly to the scalp in the biparietal and occipital areas.4
Samples from the feet were collected using a sterile swab that was applied to interdigitale surfaces and then preserved in transport medium.
All samples were stored at 6–8°C until their processing in the laboratory.
Environmental samplesTwo or more samples were taken from floors of eight gyms using Rodac plates with Sabouraud agar medium supplemented with chloramphenicol and cycloheximide (bioMérieux, France). The locker rooms, classrooms, the gym equipment, and floor and walls of the showers were sampled.
Twenty-one samples from the sandboxes were also collected into sterile bags and tested for keratinized fungus and dermatophytes.11
Processing of samplesThe samples collected from the children were processed in no later than 24h. Each dental brush was sowed by direct contact on a Sabouraud agar plate supplemented with chloramphenicol and cycloheximide, while the swabs were seeded on Sabouraud agar without cycloheximide.
All plates were incubated at 30°C during a maximum of 1 month and were inspected twice a week. The colonies of fungi compatible with dermatophytes were isolated in potato–glucose agar for their identification. The identification was based on the morphological study of the colonies and, if necessary, a urease test was carried out using Christensen Urea agar.4,5,16,30
Sand samples were frozen at −4°C for 1h to eliminate arthropods and helminthes, filling two sterile Petri dishes for each sample. Sterile hairs collected from a healthy young child were deposited on the sand surface, and maintained in humidified conditions with distilled water supplemented with 4% gentamycin.33 For quality control, plates with sterile sand were inoculated with McFarland 1 suspension of T. mentagrophytes, T. rubrum, Microsporum gypseum, and M. canis. Afterwards, several sterile hairs were deposited onto the sand surface.
The samples were examined weekly with a magnifying glass during the following 6 weeks. The hairs showing developed fungal colonies were seeded in potato-agar. The identification was made following the described methodology.
Case confirmationIndependently of the presence of lesions, the pediatrician of the team verified the diagnosis of tinea of school children from whom dermatophytes were isolated and collected new samples.
Awaiting the results, flutrimazol 1% gel (Micetal gel®, Lab Uriach, Spain) was applied for washing the scalp or feet.
Direct microscopic observation of the scales was performed using 30% KOH, and part of the samples were seeded into Sabouraud agar with chloramphenicol.
If the samples were negative in children without lesions, the treatment was interrupted; on the contrary the treatment of feet lesions was changed to a once-a-day application of 1% flutrimazol cream (Cream Micetal®). In the confirmed cases of tinea capitis or tinea unguium, terbinafine (5mg/kg weight/day p.o.) was prescribed. The check-ups of the affected children were performed every 4 weeks until the samples became negative.
The parents of the infected children were informed of the results. At the end of the study, the obtained results were reported to the authorities of the participating schools; also written guidelines of preventive measures were provided.
DefinitionsThose cases presenting cutaneous or nail alterations plus positive cultures for dermatophytes were defined as tinea; if no lesions were observed during the pediatric examination with the second culture positive for dermatophytes, those cases were defined as healthy carriers.
ResultsPrevalenceOf the 1,305 students in the study, from ages 3 to 15, 38 dermatophyte positive cultures were obtained from 34 children, indicating an overall prevalence of dermatophytes of 2.9%. (Table 1)
In two cases a culture was positive from feet and nails, and in one from scalp and foot.
Three cases of tinea capitis (0.23%), 2 cases of tinea unguium, and 23 of tinea pedis (in 2 cases coexisting with nail infection) were diagnosed. Healthy carriers were found in 8 cases; dermatophytes were isolated from feet and in one from scalp.
In 32 children, 34 samples from feet yielded a positive culture to dermatophytes (2.53%); in one of them two dermatophyte species, T. mentagrophytes and M. gypseum, were simultaneously isolated.
In two 13-year-old children, several nails of either foot were affected of tinea pedis. In both cases, the cultures of the nails showed the same dermatophytes isolated from the feet: T. rubrum and T. tonsurans (Fig. 1).
In one case, T. mentagrophytes was isolated simultaneously from the scalp and feet.
In eight of the nine children without lesions, repeated culturing of the samples gave rise to the same dermatophyte as that in the initial sample.
Socio-demographical dataOf the three cases of tinea capitis, two were 15-year-old girls (both at the same school class); the third was a 4-year-old boy.
In tinea pedis, 34.4% were girls and 65.6% boys, with a prevalence of 1.7% in girls and 3.2% in boys (p=0.76).
Respecting age, 47% belonged to the 13–15-year-old group, 37.5% to the 10–12-year-old, and 15.5% to the youngest group.
Etiologic agentT. mentagrophytes was the most frequent species, being isolated in two cases of tinea capitis and in 16 feet samples (45.7%). T. rubrum was isolated from the feet of 10 children (31.4%); T. tonsurans was identified in 4 cases (11.4%), and E. floccosum in 2 cases (5.7%). M. gypseum was isolated in association with T. mentagrophytes from one of the samples of feet in a healthy carrier, and Trichophyton violaceum in one case of tinea capitis in a child coming from Ethiopia. One isolate belonging to Trichophyton genus could not be identified to the species level.
In the two cases of nail abnormalities associated with tinea pedis, T. rubrum and T. tonsurans were identified as causative agents.
Distribution of agents by the body site in healthy carriers and tinea infection is presented in Table 2.
Species of dermatophytes isolated in 34 school children according its body site and clinical signification.
In 97.4% of those interviewed, the country of origin was determined. Dermatophytes were isolated in 28 of 1140 children born in Spain (94.56% of them in Catalonia) and in 7 of 129 children born abroad, from whom 4 of 73 were from Latin America, 1 of 8 from Romania, 1 of 4 from China, and 1 of 2 from Ethiopia.
Environmental samplesM. gypseum was isolated in floor samples of the showers of two of the eight examined centers. In the remainder samples, as well as in sand, no other dermatophyte species were isolated; Geophylic fungi, including Chrysosporium sp. and Scopulariopsis sp., were identified. Fusarium, Aspergillus, Penicillium, Ochroconis, Alternaria, Mucor, Rhizopus, and Bipolaris genus were identified in environmental samples.
DiscussionAlthough the prevalence of tinea has decreased in industrialized countries, there is a marked difference depending on the region studied, along with the causative species. Thus, it is necessary to conduct geographically defined studies for the knowledge of the epidemiology of tinea.
The prevalence of tinea capitis (0.23%) is similar to that found in other studies carried out in Turkey,1,24 where the prevalence was 0.3%, which is inferior to 0.64% found in Madrid,7 2.5% found in London,15 or those more elevated such as Ivory Coast with 11.34%,21 and 13% in Cleveland.13
Simultaneous use of two culture techniques (brushing and direct contact) tend to increase the number of isolations, showing that these mycoses presented low or very low prevalence in the studied population. Combined use of diagnosing methods has been proposed.2
The prevalence of scalp tinea was the same found in a previous study, but in this case there was a bigger proportion of foreign children; the prevalence of feet tinea has been ten times greater than that of tinea capitis, slightly lower than the 2.8% obtained in schools with bigger immigrant population.32
Tinea of the nails was also demonstrated to occur with very low prevalence (0.15%), as was described in most publications on onychomycosis in pediatrics.19,28 The finding of two children with nail lesions of more than a year of evolution associated with tinea pedis, which have not been considered previously, implies the necessity of systematic examination of nails during regular medical revisions, especially in adolescent children.
No statistical differences in the prevalence of tinea pedis between the autochthonous and foreign population were found. This fact could be the result of the lesser number of immigrants.
The etiology of tinea pedis shows a similar pattern to the one found in France, Italy, and other countries, with two predominant agents: T. mentagrophytes and T .rubrum.3,14,18,20,23,28
The only uncommon species, T. violaceum, was isolated in an immigrant child from Ethiopia that had arrived recently to Barcelona as consequence of an adoption. Woldeamanuel et al.34 found that this species constitute 87–90% of all isolates from Ethiopian schoolchildren. Studies that were carried out in Granada (Spain) more than 30 years ago described this species as an important causative agent of tinea capitis.17 Currently, T. violaceum is very rarely the cause of tinea capitis in Spain.27 Some authors have observed the introduction of this agent into our environment by immigrants of Moroccan origin.29
An increase in the frequency of tinea of the feet was observed with age (p<0.05). Higher prevalence was observed in the 13–15-year-old group, most frequently in boys, coinciding with other studies.18,24,28,32
Healthy carriers were 25.7% of positive dermatophyte cultures. The difference among asymptomatic carriers varies highly between studies.3,25
In one of the cases, a non-school family member (not included), a sibling who plays water polo, was affected. Both tinea pedis and tinea unguium were caused by T. rubrum, suggesting transmission through direct contact. The same situation in two girl-classmates could explain the isolation from the scalp of the same agent, T. mentagrophytes.
Although infection by contact with infected pets is documented,12,31 statistical differences in the prevalence of tinea among the children in contact with cat and dogs and those who did not were not found.
The biggest incidence of tinea pedis has been related to the use of occlusive footwear and sport activities.6 In our study, no marked differences were detected in the prevalence of tinea among children who practiced sports, including swimming, and those who did not (3.5% and 2.4%, respectively).
Sport installations and auxiliary material as potential sources of transmission were analyzed, and M. gypseum was isolated from the floor of two public gymnasiums, being cultured from the feet of one healthy carrier in the same center. This species is frequently isolated in earth samples in the entire world.10,11,22,26,35
The high prevalence of tinea pedis in schoolchildren should be considered by pediatricians for including this pathology in the differential diagnosis of dermatitis.
It is important to promote preventive measures, thereby avoiding possible infections through interpersonal contact as well as using common sport spaces. Guidelines aimed at preventing the spread of dermatophytes should be available in schools.
This study provides remarks on the utility of educational health programs informing on the nature and ways of prevention of tinea pedis. It also stresses the importance of physical examination on the part of pediatricians to detect the infection at early stages.