Malassezia species normally colonize the skin but they can change their saprophytic state and invade the stratum corneum as pathogens.
AimsTo determine the prevalence of Malassezia species isolated from patients with pityriasis versicolor (PV) and to analyse their distribution according to the location of the lesion on the body.
MethodsThis study included 218 patients with PV and positive Malassezia cultures who resided in the city of Resistencia, a subtropical area located in northeast Argentina. Age, gender, and the body site of lesions were recorded. Strains were identified by PCR-RFLP.
ResultsMalassezia sympodialis (37.7%) and Malassezia globosa (37.2%) were the most prevalent species isolated alone or in association with other Malassezia species in 82% of the patients. Malassezia furfur (21.3%) was the third most common species, followed by Malassezia slooffiae (1.7%), and Malassezia restricta (1.3%), which was found only in combination with M. globosa and M. sympodialis. Malassezia dermatis (0.4%) and Malassezia pachydermatis (0.4%) were each isolated once. None of the species affected a body site with statistical significance. Significant difference between genders according to age was found only in the 31–40-year-age group.
ConclusionsThis study suggests that M. sympodialis and M. globosa represent the main species implicated in the pathogenicity of PV. M. furfur appears to be the third agent of importance in this geographical area. Statistical analyses showed none of the species was particularly associated with any one of the body sites.
Las especies de Malassezia colonizan normalmente la piel, pero ante ciertas condiciones pueden cambiar su estado saprofítico y transformarse en patógenas.
ObjetivosEstudiar la prevalencia de especies de Malassezia aisladas de pacientes con pitiriasis versicolor (PV) y su distribución de acuerdo al sitio anatómico de las lesiones.
MétodosEste trabajo se realizó en la ciudad de Resistencia, ubicada en una región subtropical del nordeste de la Argentina. Se incluyeron 218 pacientes con PV y cultivo positivo para Malassezia. Edad, género y sitios anatómicos de las lesiones fueron registrados. Las cepas fueron identificadas por PCR-RFLP.
ResultadosMalassezia sympodialis (37,7%) y Malassezia globosa (37,2%) fueron las especies con mayor prevalencia, aisladas en el 82% de los pacientes, bien como agente único o en asociación con otras especies. Malassezia furfur (21,3%) se encontró en tercer lugar, seguida por Malassezia slooffiae (1,7%). Malassezia restricta (1,3%) se aisló solo en asociación con M. globosa y M. sympodialis. Malassezia dermitis (0,4%) y Malassezia pachydermatis (0,4%) fueron aisladas una sola vez. No se encontró una relación significativa entre las especies aisladas y los lugares anatómicos. Únicamente se encontraron diferencias significativas entre sexos (masculino y femenino) en el grupo de 31–40 años de edad.
ConclusionesEstos resultados sugieren que M. sympodialis y M. globosa son las principales especies implicadas en la patogenicidad de la PV, sin predominio de ninguna de ellas. M. furfur aparece como el tercer agente en importancia en esta área geográfica. No se encuentra una relación estadísticamente significativa entre una determinada especie y algún sitio anatómico en particular.
Malassezia species normally colonize the skin. For reasons currently unknown, these yeasts can change their saprophytic state and invade the stratum corneum as pathogens.5,11,14,17,22
Pityriasis versicolor (PV) is a chronic, benign skin disease that is generally asymptomatic. It occurs worldwide and is very common in tropical and temperate regions, where the major frequency of recurrence is observed.14,16 It predominantly affects young adults of both genders, and manifests characteristic clinical lesions as slightly scaly macules that vary in colour from hypopigmented (white) to hyperpigmented (pink, tan to brown).2,5,11,14,15
The fungal nature of PV was recognized by Eichstedt in 1846 and in 1874 the round and oval budding yeast cells of the organisms were described by Louis Malassez.15 Some years later, the genus Malassezia was created by Baillon with Malassezia furfur as the generic type species.11,15,16 The variable and unstable morphology, and the strictly lipophilic nature that requires specialized media for growth, have complicated the study of this genus.2,13,16,20
Distinctive morphologic and physiologic features allow differentiating Malassezia from other yeast genera. However, as Malassezia species share several of these characteristics, a rapid and simple method to identify them does not exist. Identification schemes based on morphologic, physiologic, and biochemical features do not address the ambiguity between some species, nor do they permit differentiation of all currently described species.1,3,9,18 In an effort to overcome the disadvantages of conventional methods, different molecular techniques have been developed. In the last decade, several PCR-based techniques for Malassezia species discrimination have been proposed, such as PCR with enzymatic restriction, randomly amplified polymorphic DNA (RADP), and pulsed field gel electrophoresis (PFGE).1,10,18,20 Nevertheless, a specific, reliable, but also simple and inexpensive method for genotypic differentiation of all currently known Malassezia species is still not available.
Today, on the basis of morphology, ultrastructure, physiology, and molecular biology studies the genus Malassezia includes the following species: M. furfur, Malassezia sympodialis, Malassezia globosa, Malassezia restricta, Malassezia obtusa, Malassezia slooffiae, Malassezia pachydermatis, Malassezia yamatoensis, Malassezia dermatis, Malassezia nana, and Malassezia japonica.12,19,23,25–27 A further species, Malassezia equi, has been described and tentatively named, but not fully characterized.1 In 2007 Malassezia caprae and Malassezia equina, isolated from animals, were postulated as new species.4
The revision of the genus Malassezia opened up new questions about ecology and pathogenicity of Malassezia species. Many studies have looked at the geographical variation in species distribution; in addition, there are indications that the dominant species may vary at different body sites and in different conditions.1,8,22 The aims of this study were to determine the prevalence of Malassezia species isolated from patients with PV and to analyse their distribution according to the skin lesion body sites.
Materials and methodsBetween May 2006 and May 2008, skin samples obtained from patients with PV were studied at the Mycology Department of Instituto de Medicina Regional, Universidad Nacional del Nordeste, Resistencia, Argentina. All the patients included live in the city of Resistencia. Located in the northeast border of Argentina (27°55′S–58°22′O), it has a subtropical climate.24
Samples were scraped off with a sterile blade and subsequently were inoculated on modified Dixon's medium at 32°C for 7 days.13 In case of multiple lesions, all lesions were sampled and a record of the body site was made. If the same Malassezia species was isolated from different anatomical regions it was considered as a single isolate.
DNA extraction: Cellular lysis was performed by a boiling–thermic shock combination. One loopful of yeast was suspended in 80μl of distilled water and boiled for 5min at 100°C, then frozen for 10min at −70°C, and finally boiled again for 5min at 100°C. The crude DNA suspension (extract) was stored at −20°C until its analysis.
Strains were identified by PCR-RFLP. This technique allows the identification and differentiation of M. furfur, M. sympodialis, M. globosa, M. restricta, M. obtusa, M. slooffiae, M. pachydermatis, M. yamatoensis, M. dermatis, M. nana, and M. japonica.21 Amplification was performed using generic primers, forward 5′-TAA CAA GGA TTC CCC TAG TA -3′ and reverse 5′-ATT ACG CCA GCA TCC TAA G -3′. The primers successfully amplified the target part of 26S rDNA from all tested Malassezia strains, providing a single PCR product of the expected size (approximately 580bp).21
Amplified DNA products were subjected to further restriction fragment length polymorphism (RFLP) using separately Cfo I and Mbo I. Cfo I does not allow differentiation of M. sympodialis from M. dermatis. In order to determine the best specific digestion patterns, different restriction enzymes were analysed by CLC Free Workbench software (version 3.0.2 CLC bio A/S) for the high conserved 26S rDNA sequences of known Malassezia species (accession numbers: AJ249955, AJ249956, AJ249954, AB105862, AB105199, AJ249952, AJ249951, AB070365, AJ249953, AB125263, AJ249950 and CBS 9432). Based on this analysis, enzyme Mbo I was selected.
Amplified DNA products were subjected to restriction fragment length polymorphism (RFLP) using Cfo I (Promega) in a first identification step. Whenever the restriction pattern produced by M. sympodialis or M. dermatis appeared, additional Mbo I (Fermentas) was used for discrimination between these species.
CBS 7886 M. globosa, CBS 7019 M. furfur, CBS 7222 M. sympodialis, CBS 9169 M. dermatis, CBS 9432 M. japonica, CBS 10533 M. pachydermatis, CBS 7991M. restricta, CBS 9725 M. yamatoensis, CBS 7876 M. obtuse, and CBS 7956 M. slooffiae were included as reference strains.
Using the Chi-square test, PV relationships with age and gender were analysed. The Chi-square test was also used for all statistical analyses, and a P value of <0.05 indicated statistical significance.
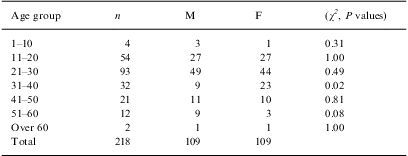
ResultsSpecimens were obtained from 218PV patients including 109 males (age range: 7–61 years; median Z: 23 years) and 109 females (age range: 10–65 years; median Z: 23 years). The patients were categorized into 7 age groups (Table 1).
Number of patients and their distribution by age and gender.
| Age group | n | M | F | (χ2, P values) |
| 1–10 | 4 | 3 | 1 | 0.31 |
| 11–20 | 54 | 27 | 27 | 1.00 |
| 21–30 | 93 | 49 | 44 | 0.49 |
| 31–40 | 32 | 9 | 23 | 0.02 |
| 41–50 | 21 | 11 | 10 | 0.81 |
| 51–60 | 12 | 9 | 3 | 0.08 |
| Over 60 | 2 | 1 | 1 | 1.00 |
| Total | 218 | 109 | 109 |
F: female; M: male.
The highest prevalence of PV was observed between 11 and 40 years old (median Z: 24 years), but 42.6% of the cases were observed in the 21–30-year-old group (median Z: 26 years; Table 1).
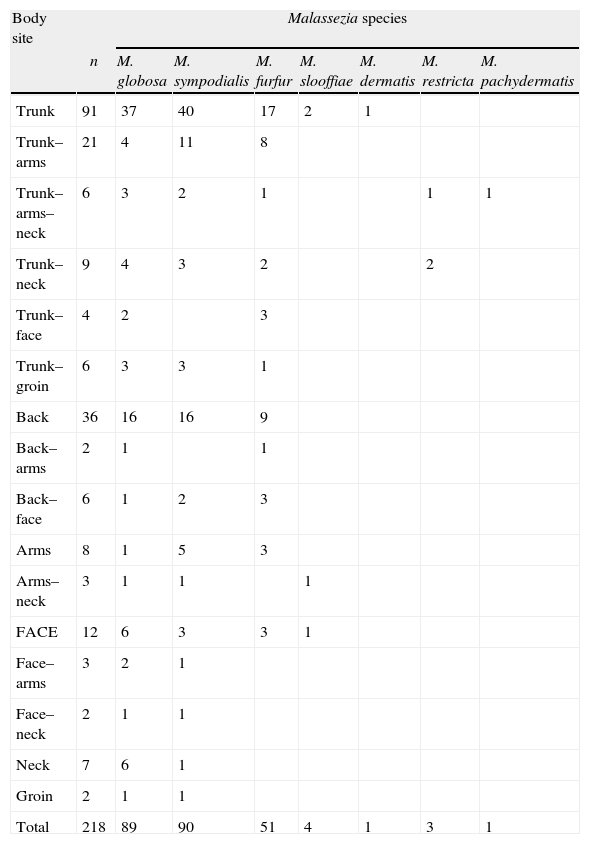
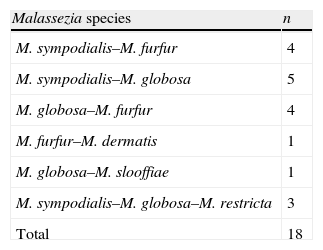
Among the 218 patients, 56 had lesions in two different body sites and 6 patients in three (Table 2). A single agent was isolated in all cases of patients with multiple lesions. From these 218 patients, 239 strains were obtained with a distribution as follows: 200/218 (91.74%) PV patients had a single Malassezia species in their lesions, 15/218 (6.88%) had 2 species, and 3/218 (1.38%) had 3 species. Species that were isolated together in the same patient are displayed in Table 3.
Distribution of Malassezia species isolates according to body sites.
| Body site | Malassezia species | |||||||
| n | M. globosa | M. sympodialis | M. furfur | M. slooffiae | M. dermatis | M. restricta | M. pachydermatis | |
| Trunk | 91 | 37 | 40 | 17 | 2 | 1 | ||
| Trunk–arms | 21 | 4 | 11 | 8 | ||||
| Trunk–arms–neck | 6 | 3 | 2 | 1 | 1 | 1 | ||
| Trunk–neck | 9 | 4 | 3 | 2 | 2 | |||
| Trunk–face | 4 | 2 | 3 | |||||
| Trunk–groin | 6 | 3 | 3 | 1 | ||||
| Back | 36 | 16 | 16 | 9 | ||||
| Back–arms | 2 | 1 | 1 | |||||
| Back–face | 6 | 1 | 2 | 3 | ||||
| Arms | 8 | 1 | 5 | 3 | ||||
| Arms–neck | 3 | 1 | 1 | 1 | ||||
| FACE | 12 | 6 | 3 | 3 | 1 | |||
| Face–arms | 3 | 2 | 1 | |||||
| Face–neck | 2 | 1 | 1 | |||||
| Neck | 7 | 6 | 1 | |||||
| Groin | 2 | 1 | 1 | |||||
| Total | 218 | 89 | 90 | 51 | 4 | 1 | 3 | 1 |
Trunk: chest, back, and abdomen.
The most frequent isolate was M. sympodialis [90/239 (37.7%)], followed by M. globosa [89/239 (37.2%)], M. furfur [51/239 (21.3%)], M. slooffiae [4/239 (1.7%)], M. restricta [3/239 (1.3%)], and finally M. dermatis [1/239 (0.4%)] and M. pachydermatis [1/239 (0.4%)] (Table 2).
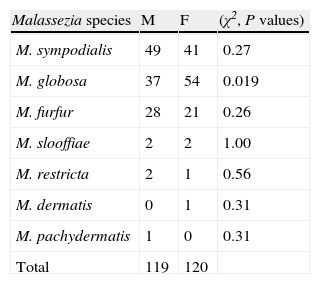
Chi-square analysis for M. globosa, M. sympodialis, and M. furfur indicated that none of them were significantly associated with any of the body sites we distinguished, namely trunk, arms, neck, face, and groin (all P values >0.05). The distributions of Malassezia species and gender relationships are presented in Table 4.
DiscussionThe fungal origin of PV was recognized in the 19th century. The pathogenesis of this skin condition and the association of the new species with PV lesions are not yet well established.
In this study, M. sympodialis and M. globosa as agents of PV were significantly more common than other species; moreover, they were isolated in equal rates. In contrast with these results, others studies report one of these species as predominant in different geographical areas. Some of them report M. globosa as the predominant species related to PV6,8,22 and others report M. sympodialis as the species with the highest rates of isolation from PV lesions.16,17
M. slooffiae and M. restricta were less isolated as previously reported.7,16,22 It is interesting that M. dermatis was isolated from one patient and this is the first report of the isolation of this species in our region. This may be because it is the first time that a molecular method has been applied to Malassezia species identification in Argentina.
The isolation of M. pachydermatis from PV lesions is also reported for the first time in our region. As this patient had extensive lesions on trunk, arms, and neck, and M. pachydermatis was isolated from all of them, it is highly probable that it was the causative agent. This patient indicated he had close contact with his dogs, which lived inside his house. Up to now M. pachydermatis has been considered only as a transient member of the human cutaneous biota1; therefore its association with PV deserves further investigation.
Anatomic sites from which more than one species was recovered included the back, trunk, neck, and groin. The PCR-RFLP methodology employed allowed the detection and characterization of Malassezia species mixtures, which otherwise are difficult to discriminate. Detection of mixtures would be important because Malassezia species could have different responses to antifungal agents.
As Mirhendi et al. indicated, Cfo I allows a clear discrimination of 9 Malassezia species but is not useful to differentiate M. dermatis and M. sympodialis. To differentiate these species they used BstF5121; however this enzyme is not available in our country. We were able, however, to distinguish these species using Mbo I.
PV lesions are more commonly seen on trunk and arms in a bilateral and asymmetrical distribution.2,11,14,17,22 We observed a high frequency of patients with multiple lesions (28.4%) and lesions in unusual locations, such as neck, face, and especially the groin. Probably the high ambient temperature and humidity of our region encourage PV spread.8
Epidemiological studies point out a prevalence of some species in certain body sites and also their relationship with some pathology.14,16,17,22 We did not observe differences in Malassezia species distribution according to the body sites. This might be related to the frequent presence of patients with extensive PV lesions. M. restricta is often associated with head, including scalp, neck, and face areas.3,16 In this study, the three M. restricta isolations were always found in trunk and neck lesions in association with the more frequent species.
PV is a disease more commonly found among teenagers and young adults of both genders. Among children it is generally rare, and unusual in older adults, although cases are more common in tropical zones.2,5,11,14,16,22 This agrees with the prevalence observed in this work, where 16% of cases were found in patients older than 41 years. It may be that subtropical climate factors of this region favour a broader age range of incidence for PV.
Significant difference between genders according to the patient's age group was found only in the 31–40-year-old group, with a higher frequency for female patients. This difference may be related to a higher frequency of women seeking treatment for aesthetic reasons.
The Chi-square test showed significant differences in species distribution according to sex only for M. globosa, which was more frequent in female patients. No special association with gender was obtained for the other species isolated.
Our results add to previously published epidemiological studies, all of which show geographical variations in Malassezia species distribution. This cannot be explained solely by differences in sampling techniques, culture medium, or identification procedures. Ethnic origin or/and geographical factors could contribute towards these differences.1,8
Since the taxonomic revision carried out by Guého et al.12, a number of studies have evolved with the aim of elucidating the ecology and role of the different species in the pathological disorders associated with this group of yeasts. This work is a contribution to the knowledge of Malassezia genus ecology. Further studies will be necessary in order to explain the relationships of these species with the environment and with the associated pathologies.
We gratefully acknowledge Dr. Aristea Velegraki and Dr. George Gaitanis, Mycology Laboratory, Medical School, University of Athens, Greece, for advice and valuable suggestions in revising the manuscript.