The incidence and prevalence of invasive fungal infections have increased since the 1980s, especially in the large population of immunocompromised patients and/or those hospitalized with serious underlying diseases7,24. In addition, the mortality and morbidity of these infections is quite substantial. The most common fungal pathogens continue to be the species of Candida and Aspergillus7,54,86,91. Parallel to the increase in fungal infections, two triazoles (voriconazole and posaconazole) and three echinocandins (anidulafungin, caspofungin and micafungin) have been licensed for the treatment and prevention of these infections4-6.
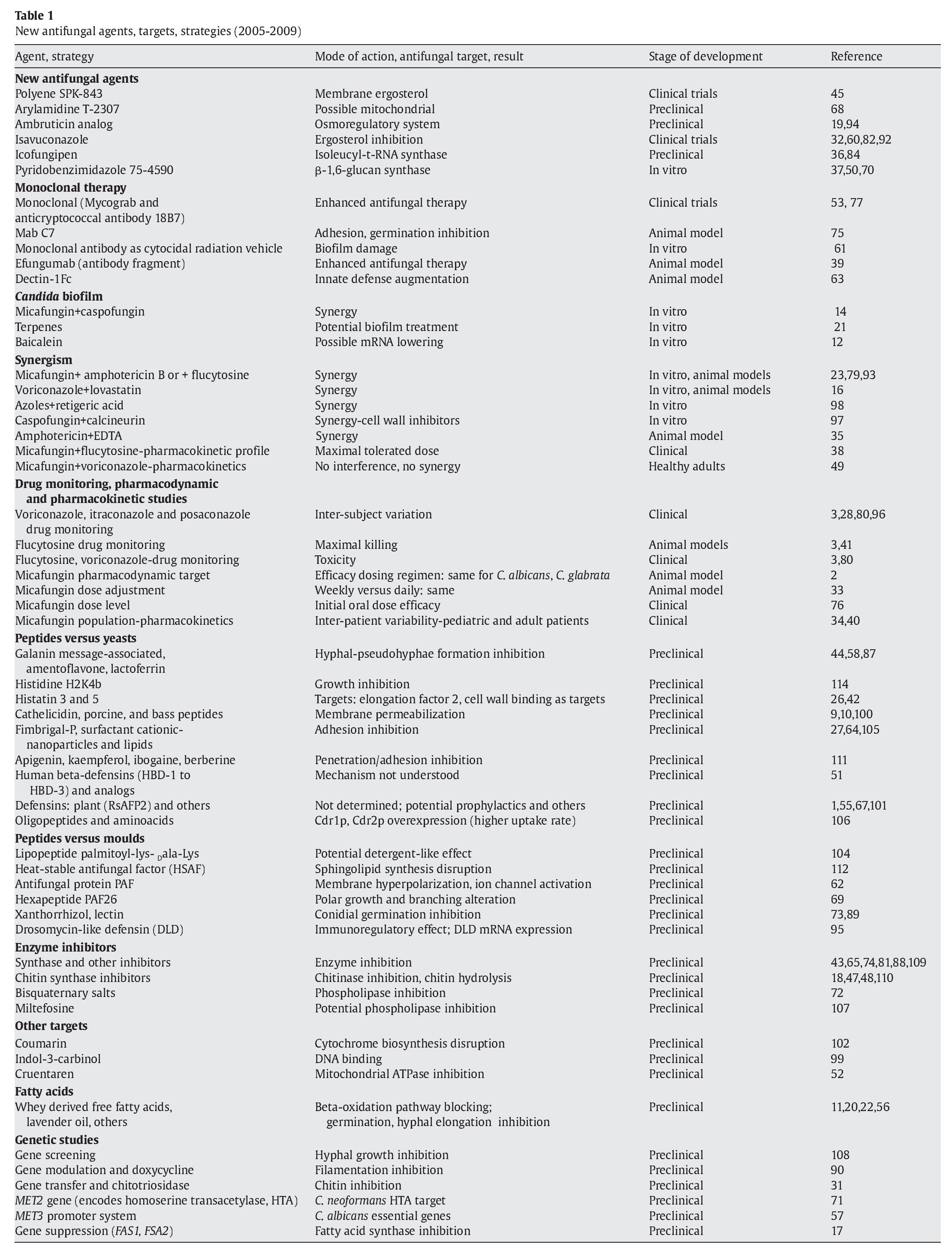
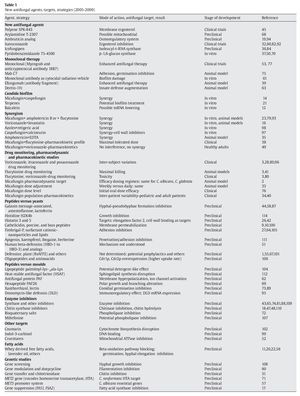
The echinocandins have a unique mechanism of action (inhibition of β-1,3-D-glucan synthase) and a broad and similar spectrum of in vitro activity against Candida spp. and Aspergillus spp.25,66,85. During the last few years, mechanisms of resistance to most licensed agents in Candida spp., and to a certain point in Aspergillus spp., have been elucidated25,46,83. Although resistance of common Candida spp. and Aspergillus spp. to echinocandins and azoles is rare, it has been documented and continues to be reported8,25,29,46,83. The mortality rates associated with invasive candidiasis are approximately 0.4 deaths per 100,000 population/year while there was a decrease with aspergillosis from 0.42 per 100,000 in 1997 to 0.25 per 100,000 in 2003 in the United States86. Although it is hoped that the introduction of these new agents will improve these rates, the mortality rate in most aspergillosis studies is about 50%. Therefore, there is a need for new targets or strategies in antifungal therapy. This review summarizes some of the new developments and/or discoveries found in the literature since 2005 (Table 1).
Antifungal agents under development
New polyene and other agents
The lipopeptide micafungin (8, FK463)103, like the other echinocandins, has fewer side effects than amphotericin B and other agents, but the echinocandins have not been approved as the first line therapy for invasive aspergillosis. The novel polyene SPK-843 showed less renal toxicity than both amphotericin B or liposomal amphotericin B and also better activity than micafungin and both established polyenes in a murine model of pulmonary aspergillosis45. Clinical trials are presently being conducted. Preclinical in vitro and in vivoevaluations of the novel arylamidine T-2307 indicate that this agent has potential for the treatment of candidiasis, cryptococcosis and aspergillosis. The mechanism of action of T-2307 is not yet established, but it has been suggested that it is associated with the mitochondrial function of the fungal cell68. These preliminary results support the continued development of these compounds.
An ambruticin analog (a cyclopropyl-pyran acid which interferes with the osmoregulatory system) was effective in both murine models of coccidioidomycosis94 and pulmonary aspergillosis19, but no further information was found in the literature since 2006.
New triazole
Voriconazole has no activity against the mucoraceous. The new triazole isavuconazole, (BAL4815) in late state clinical development for the treatment of aspergillosis, appears to have in vitro activity against the zygomycetes (MIC50 and MIC100 of 1 and 2 μg/ml, respectively) versus voriconazole MICs of ≥ 16 μg/ml32; also, its activity was superior to that of both itraconazole and voriconazole against Candida spp.92. However, contradictory results have been documented for the zygomycetes in other studies (MICs50 of > 6 μg/ml)60,82.
Icofungipen
Icofungipen (PLD-118, BAY 10-8888) is a derivative of cispentacin. It is a beta amino acid that targets isoleucyl-t-RNA synthetase; intracellular inhibitory concentrations at the target site are achieved by its active accumulation in susceptible fungal cells. Although its in vitro activity against Candida albicans is poor, it has shown strong in vivo activity in a neutropenic rabbit model for disseminated candidiasis, including the treatment of central nervous system infection36,84. It has dose-dependent pharmacokinetics and it shows potential for the treatment of invasive candidiasis.
Inhibitor ofβ-1,6-glucan synthesis
75-4590, a pyridobenzimidazole, is a specific inhibitor of β-1,6-glucan synthase; it has shown activity against Candida spp. and appears to inhibit hyphal elongation of C. albicans50. Genetic analysis of a resistant mutant of Saccharomyces cerevisiae indicated that its primary target was Kre6p (a β-1,6-glucan synthase)70. Its growth inhibition is dose-dependent; since Kre6p homologous have been found in Aspergillus fumigatus, partial silencing of KRE6P expression makes A. fumigatus more susceptible to Congo red which appears to indicate the role of Kre6p in cell wall construction37.
Monoclonal antibody therapy
Patient therapy
Casadevall13 considers serum therapy the third age of antimicrobial therapy. In 2006, Pachl et al77 reported the results of the combination of amphotericin B and Mycograb (Neutec Pharma), a human recombinant monoclonal antibody as an inhibitor of heat shock protein 90, in patients with invasive candidiasis. An 84% overall response was observed by day 10 in the combined therapy versus 48% in patients treated with amphotericin B alone; clinical and mycological response, Candida-attributable mortality and rate of culture-confirmed sterilization were also superior with the combined therapy. The first application of monoclonal antibody therapy for a fungal disease in humans was the evaluation of the murine-derived anticryptococcal antibody 18B7 for cryptococcal meningitis by Larsen et al53. Their promising results support further evaluation of 18B7.
Animal models in vitro
The monoclonal antibody Mab C7 has been shown to inhibit the adhesion and germination of C. albicans and has direct candidacidal activity75. The use of microbe-specific monoclonal antibodies as delivery vehicles for targeting biofilms with cytocidal radiation was successfully evaluated by Martinez et al61; they found that Cryptococcus neoformans biofilms were susceptible to this treatment, which could be a novel option for either the prevention or treatment of bio-films. More recently, the combination of caspofungin and efungumab, a human antibody fragment, was used against the heat shock protein 90, a target of the human response in invasive candidiasis39; these preliminary results indicate that efungumab enhanced the activity of caspofungin in the animal model. Similar results were obtained by Mattila et al63 in an immunosuppressed murine model of invasive pulmonary A. fumigatus infection when animals were treated with Dectin-1 Fc via beta-glucan recognition and opsonic elimination; the conclusion was that Dectin-1 Fc could serve as a prophylactic treatment of this infection.
Strategies for the treatment of biofilms
C. albicans biofilms are intrinsically resistant to most antifungal agents. The optimal efficacies of caspofungin and micafungin were evaluated using anin vitro model of C. albicans biofilm14. Caspofungin (2 mg/ml) and micafungin (5 mg/ml) could be good candidates for the reduction or control of fungal biofilms associated with silicone medical devices, as part of the antifungal lock. Both echinocandins were able to significantly and persistently reduce the yeast metabolic activity of intermediate and mature biofilms, 12 h and 5 days old, respectively, when used as catheter lock solutions. The in vitroactivity of terpenes21 and baicalein12 has also been evaluated against C. albicans biofilms and they appear to be promising candidates to either treat or reduce the incidence of device-associated infections. The cells treated with baicalein expressed lower levels of mRNA than the cells grown in its absence12.
Synergism
Antifungal drug-drug combinations
The echinocandins do not have any activity against C. neoformans. The in vitro interactions of micafungin with either amphotericin B, fluconazole, itraconazole or voriconazole were evaluated for different Cryptococcus spp.; no antagonism was observed and synergy was frequently observed with the combination of micafungin and amphotericin B93; similar results were observed in experimental aspergillosis23 with the same combination and more recently, against simulated Candida endocarditis vegetations with the combination of micafungin and flucytosine79. More research is needed regarding the combinations of echinocandins with triazoles and lipid formulations in randomized clinical trials. Although the combination of caspofungin with these latter agents has provided mostly favorable results, they were not obtained in randomized clinical trials113.
Antifungal drug combination with other agents
The combination of the statin lovastatin and voriconazole was synergistic both in vitro and in vivo in a fly Drosophila melanogastermodel of zygomycosis16. More recently, favorable in vitro data has been reported for retigeric acid either alone or in combination with azoles against C. albicans98. Steinbach et al97 demonstrated, using a calcineurin A mutant (cnaA), that calcineurin is critical for A. fumigatus hyphal growth, tissue invasion and pathogenicity and enhanced the antifungal activity of cell wall inhibitors such as caspofungin or nikkomycin. EDTA, a lead poisoning chelator therapeutic that appears to have antifungal activity, was shown to have synergistic activity in combination with amphotericin B lipid complex in a rat model of immunosuppressed A. fumigatus invasive pulmonary aspergillosis35. The clinical significance of these observations is yet to be determined.
Pharmacokinetic studies
A pharmacokinetic study was conducted to determine the maximal tolerated dose of micafungin, and especially the pharmacokinetic profile when micafungin was combined with fluconazole in cancer patients undergoing either bone marrow or peripheral stem cell transplants38. This combination was found to be safe and the maximal tolerated dose of micafungin was not reached at 200 mg/ day for four weeks. Keirns et al49 reported that voriconazole did not affect the pharmacokinetics of micafungin; however, an absence of drug interaction was observed in healthy adults. These are promising results, but data from patients are needed.
Drug monitoring, pharmacodynamics and pharmacokinetic strategies
Drug monitoring
Therapeutic monitoring is essential to ensure drug exposure (dosage increase when it is possible) or to avoid toxicity (administer lower doses) during the antifungal treatment of invasive mycoses. Monitoring of voriconazole serum concentrations is important due to the frequent inter-subject variability (trough concentrations of <0.1 to about 10 μg/ml from patients taking 200 mg twice a day)96. There was a 90% response to voriconazole therapy when serum levels were >1 μg/ml, but only 54% when the serum concentrations were lower in patients with invasive candidiasis or aspergillosis80. Based on those results, the paucity of voriconazole MIC data for Histoplasma capsulatum, the lack of prospective trials to establish the effectiveness of this agent for histoplasmosis treatment and the wide range of voriconazole serum concentrations (<2.05 to<0.125 μg/ml) also found in their study, Freifeld et al28 have recommended measuring trough levels in patients receiving voriconazole for histoplasmosis. As there is also inter-subjective variability of itraconazole and posaconazole serum concentrations, drug monitoring of these triazoles also could be useful3. Maximal organism killing has correlated with flucytosine concentrations above the MIC in animal models3,41. In addition, high flucytosine levels have correlated with toxicity and elevated voriconazole concentrations with encephalopathy3,80.
Pharmacodynamics and pharmacokinetics
Pharmacodynamic results indicated that the current clinical dosing regimens of micafungin were appropriate for the treatment of infections caused by both C. albicans and Candida glabrata; micafungin exposures needed for efficacy were similar2. Relating the results in the murine neutropenic candidiasis model to human micafungin pharmacokinetics for the 100 mg/day dosing regimen would predict an inhibitory pharmacodynamic target against both species with MICs up to 0.06 μg/ml. In addition, the free drug micafungin exposures required to produce stasis and killing endpoints were similar to those reported for anidulafungin against C. albicans and C. glabrata2. Other strategies regarding dosing regimen adjustment to improve micafungin efficacy also have been examined in a murine neutropenic model of candidiasis33 and in patients76. Furthermore, population studies have provided real inter-patient (pediatric and adult) pharmacokinetic variability34,40.
Serum effect on antifungal activity
Serum-MICs of both caspofungin and micafungin for C. albicans were better predictors of in vivo potency than conventional MICs (hyphal growth inhibition or C. albicans kidney burden measurement)59. These results were confirmed recently by the reports of the influence of serum in drug protein binding. Using in vitro growth assays, it has been reported that protein binding shifted the antifungal activity of echinocandins against Aspergillus spp. and Candida spp. resulting in nearly equivalent MICs or MECs78; serum decreased the sensitivity of glucan synthase to echinocandins. Because of that, it has been suggested that the susceptible breakpoint established by the Clinical and Laboratory Standards Institute of ≥ 2 μg/ml does not apply to the three echinocandins, but only to caspofungin. Using fks1 mutants, Garcia-Effron et al30 have demonstrated that serum MICs captured all (100%) fks1 mutants above the MIC breakpoint, but this breakpoint was less applicable for anidulafungin and micafungin. Micafungin or anidulafungin MICs it should be equal or greater than 0.5 μg/ml provided similar results (95% of the mutant isolates were captured). Their recommendation was to either lower the breakpoint or to use caspofungin in vitro data as a surrogate marker to identify echinocandin resistance, since the three echinocandins have similar activity target, resistance mechanisms, spectrum and in vitro potency; the use of surrogates has been previously suggested for the triazoles, where fluconazole breakpoints can be used to assess patterns of susceptibility of other triazoles.
Peptides
Further of research has been dedicated to the investigation of the antifungal activity of a variety of peptides mostly against C. albicans, A. fumigatus and C. neoformans. Although they are promising leads for the development of new agents, a great deal of investigation is needed to determine their clinical usefulness. Some of the developments in this area are summarized below.
Activity against C. albicansand C. neoformans
Inhibition of the transformation from budding to hyphal or pseudohyphae formation, an important virulent factor in C. albicans, has been observed with the galanin message-associated peptide (GMAP)87, amentoflavone44 and a lactoferrin-derived peptide58; lactoferrin activity was dose-dependent and it was effective in disseminated murine candidiasis
The histatins have potential as antifungal agents since they are the first line of defense against infection with oral candidiasis. Zhu et al114 synthesized a four-branched histidine (H2K4b) that affected the growth of several species of Candida by pH buffering followed by endosomal-disruption. Since this molecule accumulated efficiently in C. albicans, it may indicate its ability to transport other antifungal agents. Histatin resistant derivatives of C. albicans had the same killing mechanism as the parent strain, but they had different proteins than those found in the parent cell; the most important of those differences was the absence in the resistant derivatives of the elongation factor 2 (Ef2), a specific target for the antifungal sordarin. There was also a decrease in the transcript level of the potassium transporter encoded by TRK1, a critical mediator of histatin killing. These results indicate that there may be several intracellular targets for histatin 3 in C. albicans26. Among at least 50 histatin peptides derived from posttranslational proteolytic processing, histatin 5 (Hst 5) has shown the highest level of activity against C. albicans. Its mechanism of action involved, first binding to the cell wall protein Ssa2 of C. albicans, followed by translocation to intracellular targets. Jang et al42 demonstrated that binding and transportation were independent events and that the P-113 fragment of Hst 5 required a specific peptide sequence for translocation.
Cathelicidin peptides were shown to have killing activity against C. albicans and C. neoformans that was associated with membrane permeabilization, but they had little activity against moulds9. However, the porcine 1905-Da cationic proline-rich peptide (SP-B) has shown activity against both yeast species and also A.fumigatus10. Recently, it was demonstrated that the fungicidal activity of the bass peptide derivative piscidin 2 (P2) was based on the formation of pores in the fungal membrane100.
Several investigators have focused their research on the adhesion and penetration of C. albicans in tissues. Foldvari et al27 demonstrated in a rat model of oral candidiasis that Fimbrigal-P (an antiadhesion synthetic carbohydrate) reduced fungal burden and was a promising antifungal agent for the prevention and treatment of infections when the target was β-GaINac(1-4)-β-alactosidase disaccharide. The surfactant-coated cationic nanoparticles and lipids are potential prophylactics that act by priming the buccal epithelial cells against fungal adhesion and infection64,105. The activity of two flavonoid compounds (apigenin and kaempferol), the indole alkaloids ibogaine and berberine were evaluated as potential inhibitors of the virulent factors responsible for the penetration of C. albicans into human cells; they appeared to inhibit adherence and had aspartyl proteinase activity. The application of these compounds in cutaneous infection was shown to suppress symptoms and accelerated the elimination of the pathogen from the infection site111.
The human beta-defensins HBD-1 to HBD-3 and their analogs phd1 to phd-3 have shown fungicidal activity against C. albicans. Although the mechanism of action is not yet understood (the initial site of action is the fungal membrane), both analogs may be potential antifungal therapeutic agents51. A plant defensin (RsAFP2), not toxic to mammalian cells, has been found to be prophylactically effective against murine candidiasis101. Active research continues regarding the characterization of other defensins as possible antifungal agents1,55,67.
It was demonstrated that the susceptibility to oligopeptides and amino acids was enhanced in C. albicans over expressing Cdr1p and Cdr2p, which resulted in higher uptake rates of these peptides via oligopeptide permeases106.
Activity against A. fumigatusand other moulds
Vallon-Eberhard et al104 have described that the ultra-short lipopeptide, palmitoyl-lys-ala-Dala-Lys (linked to fatty acids) was superior to amphotericin B in an immunosuppressed murine model of invasive pulmonary aspergillosis by A. fumigatus, which highlighted the potential of this family of lipopeptides as antifungal agents. Although enough data are not available regarding its mechanisms of action, it was suggested that the activity is membranolytic (detergent-like effect), similar to that of other enzyme inhibitors, e.g., echinocandins. Yu et al112 reported the antifungal activity of a heat-stable antifungal factor (HSAF) against a variety of fungal pathogens; its target is the disruption of the biosynthesis of sphingolipids, essential but different components of fungal and mammal cells. ThePenicillium chrysogenum antifungal protein (PAF) elicited hyperpolarization of the plasma membrane and the activation of ion channels62. The small hexapeptide PAF26 altered hyphal morphology (polar growth and branching), chitin deposition and caused other detrimental effects69; this peptide had preferential activity against moulds. Conidial germination of Aspergillus spp. and other moulds was shown to be inhibited by xanthorrhizol89 and lectin73.
A drosomycin-like defensin (DLD), a human homologue of drosomycin from the fly D. melanogaster, showed specific antifungal activity against filamentous fungi. Both an immunoregulatory effect on Aspergillus-stimulated cytokine production and the expression of DLD mRNA in mostly skin human tissues were observed, which is consistent with its putative role as a defensin against invading microorganisms95.
Enzyme inhibitors
Synthases and other enzymatic targets
Other possible antifungal agents are the synthase inhibitors such as pleofungins (inositol phosphorylceramide)109, N-alkyl derivatives that inhibit glucosamine-6p synthase65, elastase inhibitor from A. flavus (AFLEI) in combination with other existent licensed agents74, the GMP synthase inhibitors in C. albicans and A. fumigatus88 and the inhibition of mRNA polyadenosine polymerase43,81 by the natural products parnafungins; these inhibitors deserve further investigation for potential clinical use.
Chitin synthase inhibitors
The cell wall components chitinases are essential for cell wall plasticity during growth. Recently, the in vitro antifungal activity of the acidic mammalian chitinase against C. albicans and A. fumigatuswas demonstrated; efficient hydrolysis of chitin was observed18. These results confirmed earlier observations regarding the antifungal in vitro activity against a variety of fungal pathogens of other natural chitin synthase inhibitors such as sesquiterpene furan compound C-J-01110, O-methyl pisiferic acid and 8,20-dihydroxy-9(11),13-abietadien-12-one and 2'-benzoyloxycinnamaldehyde47,48.
Phospholipases
Other possible targets for drug development are the phospholipase inhibitors; inhibition of C. neoformans by bisquaternary ammonium salts correlated with the inhibition of cryptococcal phospholipase B1 (PLB1, a newly identified virulent factor); C. albicans was also inhibited72. On the other hand, Widmer et al107 found that miltefosine delayed C. neoformans infection and mortality and reduced brain burden in a murine model of cryptococcosis; however, the relatively low inhibitory effect on the phospholipase B1 enzyme at concentrations exceeding the MIC by 2 to 20 times suggested that there was another mechanism involved in addition to phospholipase inhibition.
Other targets
Disruption of cytochrome biosynthesis which could induce apoptosis by coumarin derivatives in C. albicans102 and the candidacidal activity of Indol-3-carbinol by binding fungal DNA99 are two different mechanisms of action. Cruentaren has shown an inhibitory effect on mitochondrial ATPase activity as well as the growth of some yeasts and moulds52. Chamilos et al15 have shown that caspofungin MICs were lower when the C. parapsilosis mitochondrial respiratory pathway was inhibited; therefore this pathway could be responsible for the decreased susceptibility of this species to caspofungin and other echinocandins.
Fatty acids
The antifungal activity of fatty acids has been recognized for years. Although some of them are used as topical over-the-counter formulations, several fatty acids were evaluated between 2006 and 2008 for either topical use (6-acetylenic acids)56 or for the treatment of more invasive mycoses, e.g., (+/-)-2-methoxy-4-thiatetradecanoic and (+/-)-2- hydroxyl-4-thiatetradecanoic acids blocked the beta-oxidation pathway of C. albicans andC. neoformans11, and whey-derived free fatty acids20 and lavender oil22 inhibited germination or hyphal elongation of C. albicans.
Discovery of antifungal targets by genetic studies
The application of chemically induced haplo-insufficiency (growth phenotypes associated with the loss or deletion of function) has been used to screen for genes involved in the hyphal growth of C. albicans108, as well as to investigate fungal viability and virulence of other species; this type of research has led to the discovery of many putative antifungal targets.
Saville et al90 genetically engineered a C. albicans tet-NRG1 strain in which they could modulate filamentation and virulence by the presence or absence of doxycycline. They were able to confirm that this species can only cause disease when filamentation was induced with doxycycline. Doxycycline removal led to increased survival; mortality rates also increased markedly the longer the intervention was delayed. It was concluded that filamentation inhibition could be targeted to treat disseminated candidiasis.
Chitotriosidase, which is secreted by human macrophages, has been associated with the defense against chitin-bearing pathogens. The engineered cells (gene transfer of the chitotriosidase gene into Chinese hamster ovary cells) inhibited growth in vitro of Aspergillus niger, C. albicans and C. neoformans and increased longevity in a murine model of C. neoformans31. This effect was possible by the prolonged delivery of recombinant chitotriosidase.
Nazi et al71 identified the MET2gene (required for virulence) of C. neoformans H99 that encoded HTA (homoserine transacetylase) by complementation of an Escherichia colimetA mutant that lacks the gene encoding homoserine trans-succinylase (HTS). By screening a 1,000-compound library for HTA inhibitors, the first antifungal inhibitor of HTA was identified; this identification validated the use of fungal HTA as a potential target of new antifungal agents.
Using a genome comparison tool, Liu et al57 identified 240 conserved genes as possible antifungal targets in ten fungal genomes; essential genes in C. albicans were then identified by a repressible MET3 promoter system. When the expression of the C. albicansERG-1target was reduced via down-regulation of the MET3 promoter, the mutant became hypersensitive to its terbinafine inhibitor. Antifungal target candidates can be screened by this process.
It has been reported that fluconazole potency against C. neoformans was enhanced and became fungicidal when the expression of the genes (FAS1 or FAS2) that encoded C. neoformans fatty acid synthase was suppressed17; these observations indicated that fatty acids were essential for C. neoformans in vitro and in vivo growth. Therefore, FAS1 and FAS2 can potentially be fungicidal targets for C. neoformanseither alone or combined with azoles. Again further development is needed.
Conclusions
Although much progress has been accomplished towards the identification and understanding of putative targets or mechanisms of action that could lead to the development of new and improved antifungal agents, the usefulness of these compounds can only be assessed in randomized clinical trials.
Author's disclosure
The author has nothing to declare.
Correo electrónico: avingrof@vcu.edu; avingrof@verizon.net
Historia del artículo:Recibido el 5 de febrero de 2009
Aceptado el 11 de febrero de 2009