Liver abscesses caused by Candida species are mainly found in immunocompromised hosts, associated with conditions (such as neutropenia and mucositis) that facilitate the spreading of microorganisms from the gastrointestinal tract.
Case reportWe present the case of a non-immunocompromised 72-year-old woman with a liver abscess caused by Candida haemulonii var. vulnera, in whom potential associated conditions could be polycystic kidney disease and renal replacement therapy. The patient experienced clinical resolution after percutaneous drainage and treatment with caspofungin.
ConclusionsTo our knowledge, this is the first case reported in Peru of a liver abscess due to Candida haemulonii var. vulnera, a clinical presentation that has not been described previously. This finding should prompt us to establish active surveillance of causal agents of systemic candidiasis.
Los abscesos hepáticos por especies de Candida están principalmente descritos en huéspedes inmunodeficientes debido a condiciones del propio paciente, como neutropenia y mucositis, que facilitan la diseminación de las levaduras desde el tracto gastrointestinal.
Caso clínicoPresentamos el caso de una mujer de 72 años no inmunocomprometida con un absceso hepático por Candida haemulonii var. vulnera, y con enfermedad poliquística renal y terapia de reemplazo renal como potenciales condiciones predisponentes. La evolución fue favorable tras un drenaje percutáneo y tratamiento con caspofungina.
ConclusionesEste es el primer caso de absceso hepático por Candida haemulonii var. vulnera en Perú; esta presentación clínica no se ha descrito previamente en la literatura. Este hallazgo nos demuestra que debe realizarse una vigilancia activa de los agentes etiológicos de las candidiasis sistémicas.
Intra-abdominal candidiasis is the most common form of deep-seated candidiasis. The clinical significance of isolating Candida from intra-abdominal samples is controversial because mixed infections are frequent and the benefits of an antifungal treatment in these scenarios are inconclusive.17 The clinical spectrum of intra-abdominal candidiasis includes liver abscess, mainly in onco-hematological patients. Other associated conditions are primary sclerosing cholangitis, chronic granulomatous disease, being a newborn, HIV infection and liver transplant. It is unusual in immunocompetent hosts.6
Candida haemulonii complex is an emerging pathogen in Latin America with multidrug resistance profile characterized by azoles co-resistance and variable susceptibility to polyenes and echinocandins.1,7,13 Here we describe the case of a patient with a liver abscess due to C. haemulonii var. vulnera with a successful outcome.
Clinical caseA 72-year-old woman, with a history of congenital polycystic kidney and liver disease and end-stage renal disease (ESRD) on hemodialysis, presented with diffuse, cramping abdominal pain and intermittent fever for two weeks. She was treated empirically with ciprofloxacin on suspicion of urinary tract infection. No improvement was noticed, and the woman developed nausea and vomiting. Clinical evaluation revealed fever (38.5°C) and right hypochondrial pain without signs of peritonitis. Laboratory results on admission were: 24,170white cells/mm3, PMNs 74.8%, bands 20%, hemoglobin 8.4g/dl, 237,000platelets/mm3, creatinine 11.45mg/dl, urea 175.48mg/dl, C-reactive protein 255.9mg/l. Blood and urine cultures were negative.
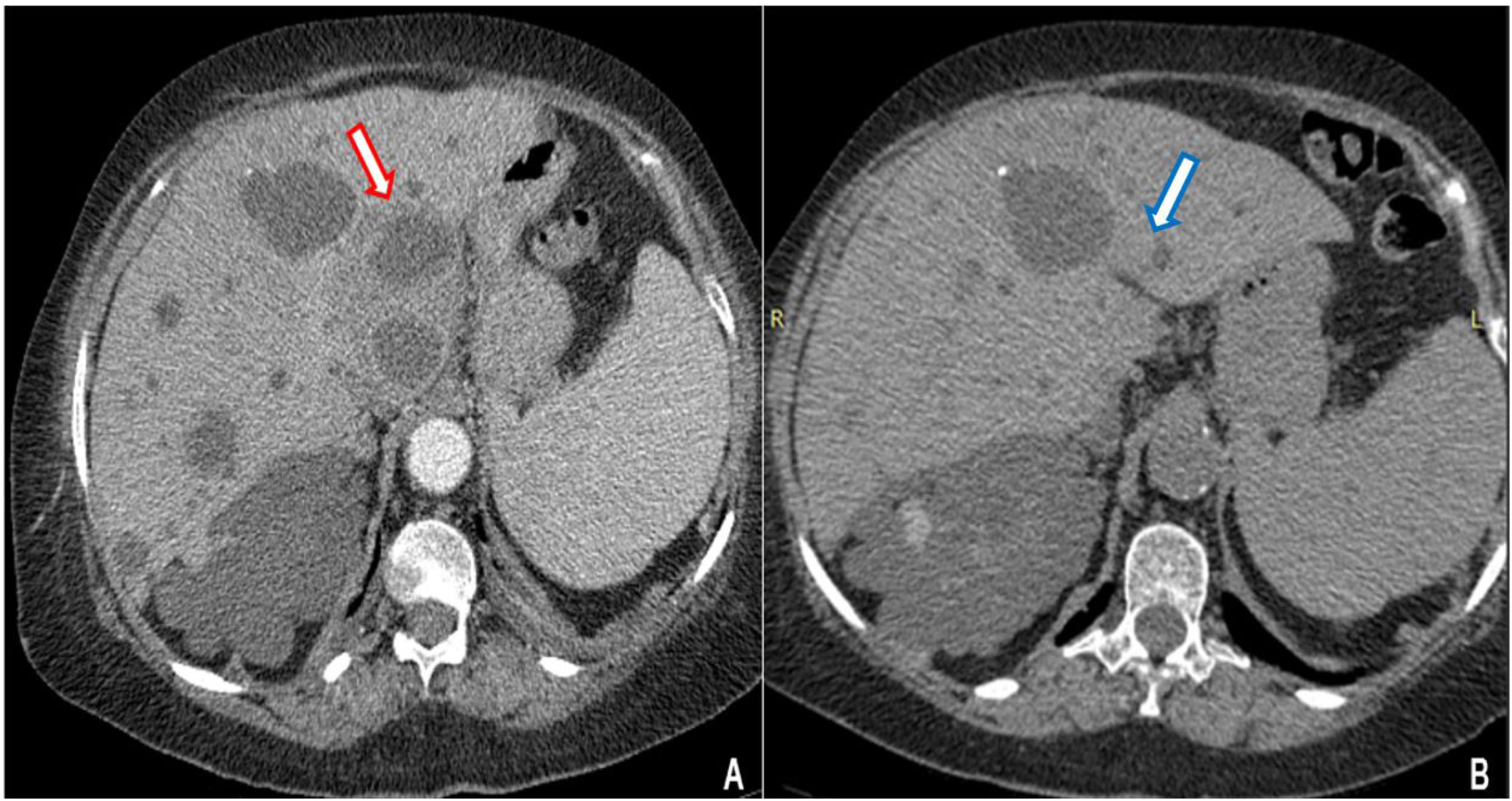
Contrast CT scan revealed multiple cystic images in the liver, one of which had edema and peripheral contrast enhancement with approximately 90cm3 of volume (Fig. 1A). The patient started a treatment with imipenem. Clinical and inflammatory parameters improved. After 14 days of antibiotics, a percutaneous drainage, which yielded 20ml of purulent fluid, was performed. Bacterial cultures were negative (no samples were sent for culturing anaerobic bacteria). After three weeks of the antibiotic treatment and drainage the patient was discharged. A week later she returned with abdominal pain. Coincidently, on the same day, our microbiology laboratory reported the isolation of a yeast from the purulent liver collection. It was identified as Candida haemulonii with the VITEK®2 system. Antifungal therapy was started upon readmission.
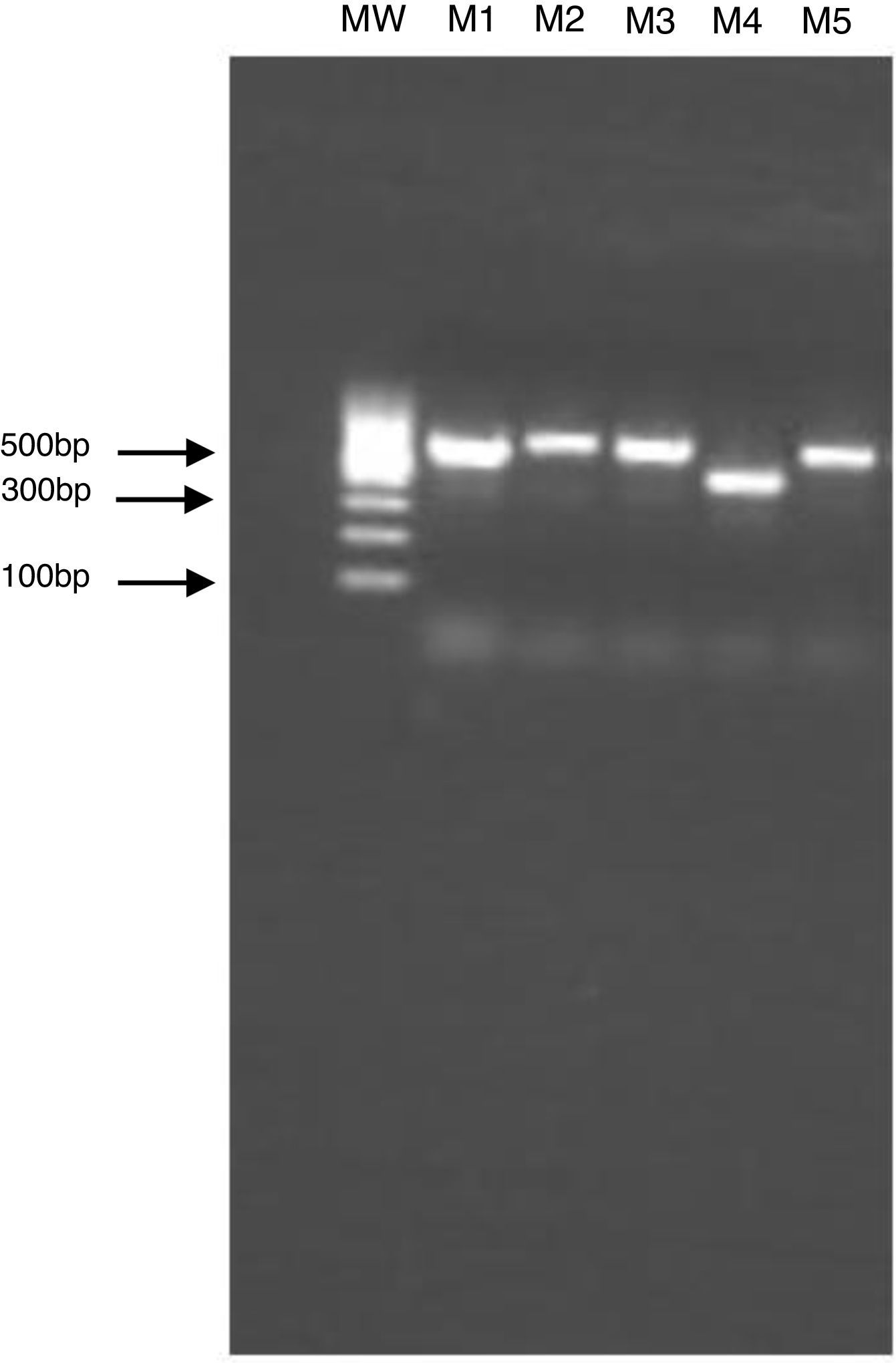
The clinical specimen was sent to the Laboratory of Clinical Mycology, at Alexander von Humboldt Institute of Tropical Medicine, for additional analysis. The molecular identification of the isolated strain was obtained by PCR amplification and sequencing of ITS1 and ITS2 regions of the ribosomal DNA. The size of the PCR products was confirmed by gel electrophoresis analysis (Fig. 2). The amplified products were sequenced by Macrogen Korea (https://dna.macrogen.com/) and analyzed using Sequencher 5.4.6 Software (Gen Codes Corporation). The results were compared with the nucleotide databases in the NCBI Genbank using BLAST tool, and 100% match with Candida haemulonii var. vulnera isolate sequences (GenBank code KP862812.1) was shown.
Electrophoresis of PCR products of the ITS region in 1.5% agarose gel. M1: Candida albicans, M2: Candida albicans, M3: Candida albicans, M4: Candida haemulonii, M5: Candida albicans, and MW: molecular weight marker. The gel electrophoresis was done to verify the size and quality of the PCR products prior to sequencing.
In vitro antifungal susceptibility testing was performed with a broth microdilution test according to the Clinical and Laboratory Standards Institute (CLSI) method, as outlined in document CLSI standard M27.3 The isolate was tested against amphotericin B, fluconazole, voriconazole and anidulafungin. We did not include caspofungin in this evaluation. A high interlaboratory variation in caspofungin minimum inhibitory concentration (MIC) values has been found when Candida species are evaluated by the CLSI reference broth microdilution method. For this reason, the use of anidulafungin has been proposed as a surrogate marker to predict susceptibility and resistance of Candida species to caspofungin.12 The MIC values were reported without a categorical interpretation of the results because neither a clinical breakpoint nor an epidemiological cut-off value (ECV) has been published for antifungal drugs against Candida haemulonii var. vulnera. MIC values were 1μg/ml for amphotericin B, >64μg/ml for fluconazole, and 0.06μg/ml for anidulafungin.
Caspofungin was administered to the patient at a dose of 70mg loading dose on day 1, followed by 50mg once daily for 14 days with full resolution of the abdominal pain and without evidence of any collection in the follow-up CT scan (Fig. 1B).
DiscussionThe C. haemulonii complex includes the following species: C. haemulonii, Candida duobushaemulonii and C. haemulonii var. vulnera. These yeasts are closely related to Candida pseudohaemulonii, Candida famata, Candida auris and Candida guilliermondii. They cannot be differentiated by commercial methods like VITEK 2, so it is recommended to use MALDI-TOF MS or molecular tests for its appropriate identification.2,4
Nucci et al, in a surveillance study of candidemia in seven Latin American countries, in which Peru was not included, found C. haemulonii causing less than 0.01% of all candidemia episodes.11 In our region, fungemia due to C. haemulonii has been described in Brazil, Honduras, Argentina, Colombia and Mexico.1,10,11,16 In Latin America, the isolation of species within the C. haemulonii complex from intra-abdominal samples is scarce,5 and they have only been recently described as a causal agent of peritoneal dialysis-related peritonitis in Colombia.1 In our literature review we found no previous reports of liver abscess caused by C. haemulonii var. vulnera or any previous identification of this organism from Peruvian patients.
Based on the clinical response shown by the patient, despite having no microbiological evidence of bacteria, we believe that she probably suffered a polymicrobial liver abscess that finally resolved with antibacterial and antifungal treatment. In a retrospective study of 163 patients with abdominal candidiasis, where over half of the cases were intra-abdominal abscesses, Vergidis et al. found bacterial co-infection in 67% of them.17 Mixed infections occur because of the dual blood supply of the liver, which increases the chances of developing multiple abscesses. However, in this case, the patient had a single abscess, and no candidemia or other additional sources of invasive candidiasis (peritonitis, cholangitis or intestinal leak) were identified.8 While patients with ESRD on hemodialysis have a high incidence of pyogenic liver abscesses, fungal abscesses are rare. Polycystic kidney disease was another risk factor described by Hong et al.9
C. haemulonii can form biofilms on medical devices and may cause recurrence or therapeutic failure in intra-abdominal infections. It is important then to remove devices as early as possible.14,15 Abscess drainage is also a priority. In our case, drainage was delayed due to an initially favorable response. The finding of C. haemulonii in the abscess culture prompted a discussion regarding the clinical interpretation of this isolate; at the end, the team decided to treat the patient with an antifungal. Recurring symptoms could be an indicator of the necessity for antifungal treatment.
Although C. haemulonii var. vulnera has not been reported as cause of liver abscess, it is important to correctly identify this yeast because of its susceptibility profile, ability to produce biofilms and the possibility of misidentification with other species, such as C. auris, when the molecular identification is not available.
We thank Rosario Velando and Susy Aranibar for their support with the in vitro susceptibility testing, and Alberto Díaz from the Guillermo Almenara National Hospital for his assistance with the microbiological evaluations.