Scedosporium species are considered emerging pathogens causing illness in immunocompetent and immunocompromised hosts.
Case reportA case of non-invasive pulmonary (fungal ball) infection by Scedosporium apiospermum complex in a 49-year-old female with bronchiectasis and cavities secondary to tuberculosis is described. The patient had a history of three years of cough and hemoptysis. A computed tomography scan of the thorax revealed the presence of a cavity in the lower lobe of the right lung, associated with bronchiectasis. A combination of surgical debridement and antifungal therapy (voriconazole) was the treatment of choice. Pulmonary resection (right lower lobectomy) was performed, and samples were sent for microbiological culture and histopathological examination; by means of the latter technique, hyphae were shown. The identification of Scedosporium angustum, a phylogenetic species of the S. apiospermum complex, was obtained by amplifying and sequencing the β-tubulin locus. Voriconazole therapy was started at a loading dose of 800mg/12h for the first 24h, followed by 200mg/12h for 6 months. The patient responded favorably to the treatment and remained asymptomatic.
ConclusionsThis case emphasizes the importance of considering Scedosporium species in the differential diagnosis of fungal balls by Aspergillus.
.
Las especies de Scedosporium se consideran patógenos emergentes que pueden causar enfermedades en hospedadores inmunocompetentes e inmunodeprimidos.
Caso clínicoSe describe un caso de infección pulmonar no invasiva (bola fúngica) por una especie del complejo Scedosporium apiospermum en una mujer de 49 años con bronquiectasias y cavitaciones secundarias a tuberculosis. La paciente tenía antecedentes de tos y hemoptisis de tres años de evolución. La tomografía computarizada de tórax reveló, como secuela, la presencia de una cavidad en el lóbulo inferior del pulmón derecho, asociada a bronquiectasias. El tratamiento de elección fue la combinación de desbridamiento quirúrgico y tratamiento antimicótico (voriconazol). Se realizó resección pulmonar (lobectomía inferior derecha), y se enviaron muestras para cultivo microbiológico y examen histopatológico. En el examen del tejido se observaron hifas. La identificación de Scedosporium angustum, una especie filogenética del complejo Scedosporium apiospermum, se obtuvo mediante amplificación y secuenciación del locus de la β-tubulina. Se inició tratamiento con voriconazol con una dosis de carga de 800mg/12h durante las primeras 24h, seguida de 200mg/12h durante 6 meses. La paciente respondió bien al tratamiento y permaneció asintomática.
ConclusionesEste caso enfatiza la importancia de considerar las especies de Scedosporium en el diagnóstico diferencial de bolas fúngicas en pulmón por Aspergillus.
Scedosporium species are ubiquitous fungi found in several substrates, such as compost, soil, sewage, and water.16 Among pathogenic fungi, Scedosporium, including Lomentospora prolificans,12 can cause infections in both immunocompetent and immunocompromised hosts.6,9,16,18 These species cause a broad range of clinical manifestations, from colonization of the respiratory tract, superficial infections and allergic reactions, to severe invasive localized or disseminated mycoses. In non-immunosuppressed hosts, Scedosporium strains classically cause traumatic infections leading to eumycetoma and pulmonary colonization, often in preformed cavities, eventually leading to allergic bronchopulmonary mycosis.9,16,17,20
Due to its intrinsic resistance to all current antifungal agents, treatment of Scedosporium infections still remains a great challenge. For the treatment of Scedosporium/Lomentospora infections, the European guidelines recommend voriconazole as the first-line treatment, together with surgical debridement when possible.6,19,23
The nomenclature of the genus Scedosporium/Pseudallescheria has suffered significant changes over the last decade due to molecular phylogenetic analyses.1,12,16,17 Nucleotide sequencing involving several genetic loci, such as β-tubulin (BT2), calmodulin, the second-largest subunit of RNA polymerase II, and the internal transcribed spacer is the global standard for precise identification at the species level within the Scedosporium genus.3,4,11,16
We are reporting here a case of a localized lung infection by Scedosporium in an immunocompetent patient with a suggestive image of pulmonary fungal ball obtained by means of computed tomography (CT) scan.
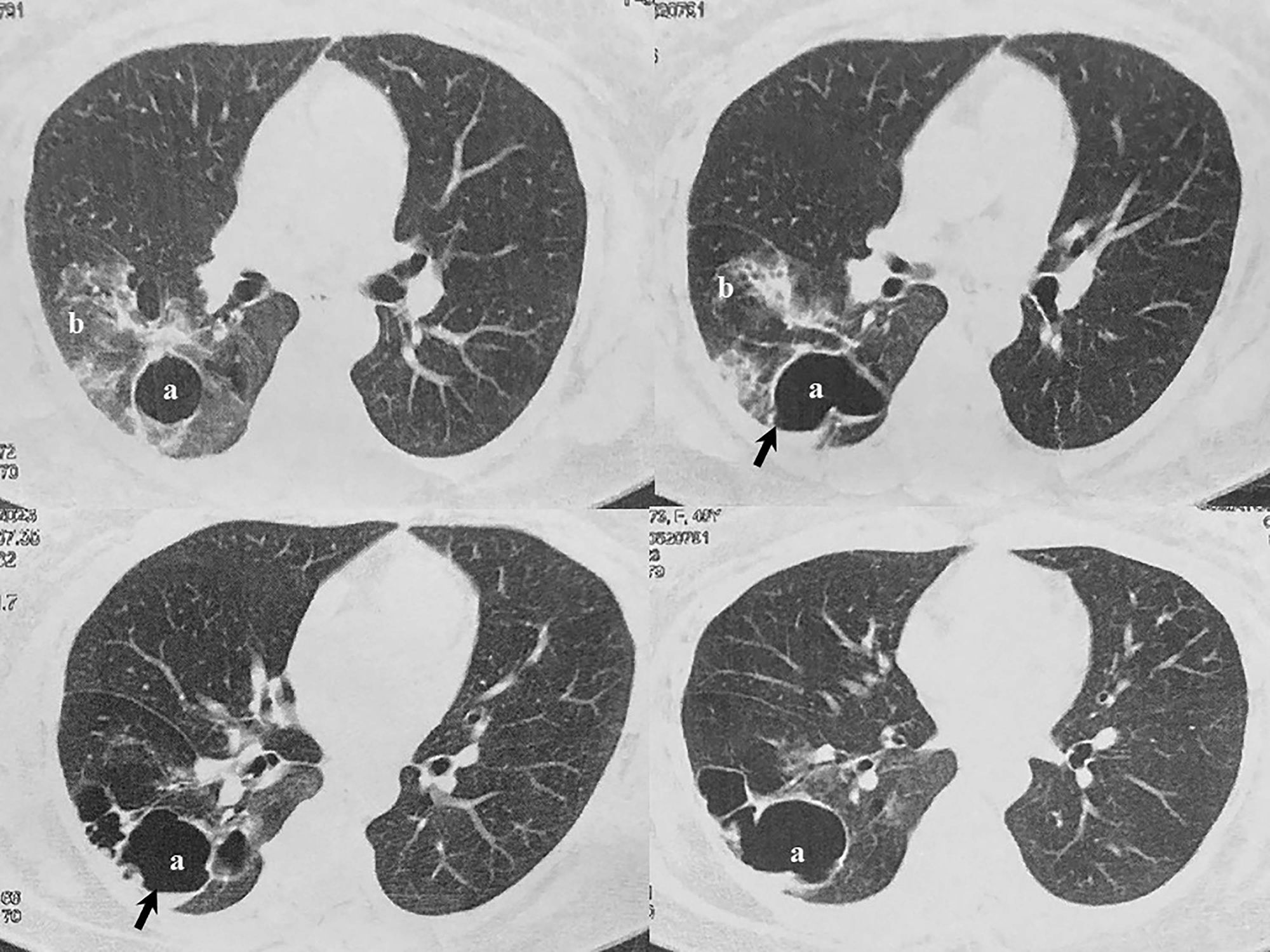
Case reportA 49-year-old female with bronchiectasis and a cavity secondary to tuberculosis suffered from multiple episodes (total of six) of pneumonia and hemoptysis between 2020 and 2022, which had not a clear diagnosis before her admission to the Hospital Naval Dr. Pedro Mallo. Mycobacterium tuberculosis disease occurred when she was 5 years old. Diagnostic tests for hepatitis B and C were negative. Likewise, human immunodeficiency virus test was non-reactive. In January 2023, a CT scan of the thorax revealed, as a sequela, the presence of a 40×71mm cavity in the lower lobe of the right lung that contained a small volume of fluid associated with bronchiectasis (Fig. 1). As the spirometry result was within normal parameters, a pulmonary lobectomy was performed. The tissue removed was sent for histopathological study and microbiological culture. Once the microorganism was properly identified, the patient was treated with voriconazole, with a loading dose of 800mg twice a day the first day and, subsequently, 200mg every 12h for 6 months. The patient responded well to therapy and remained asymptomatic. Control CT scans were done and, as expected, post-surgical changes with minimum fluid in the fissure adjacent to the surgical line were observed.
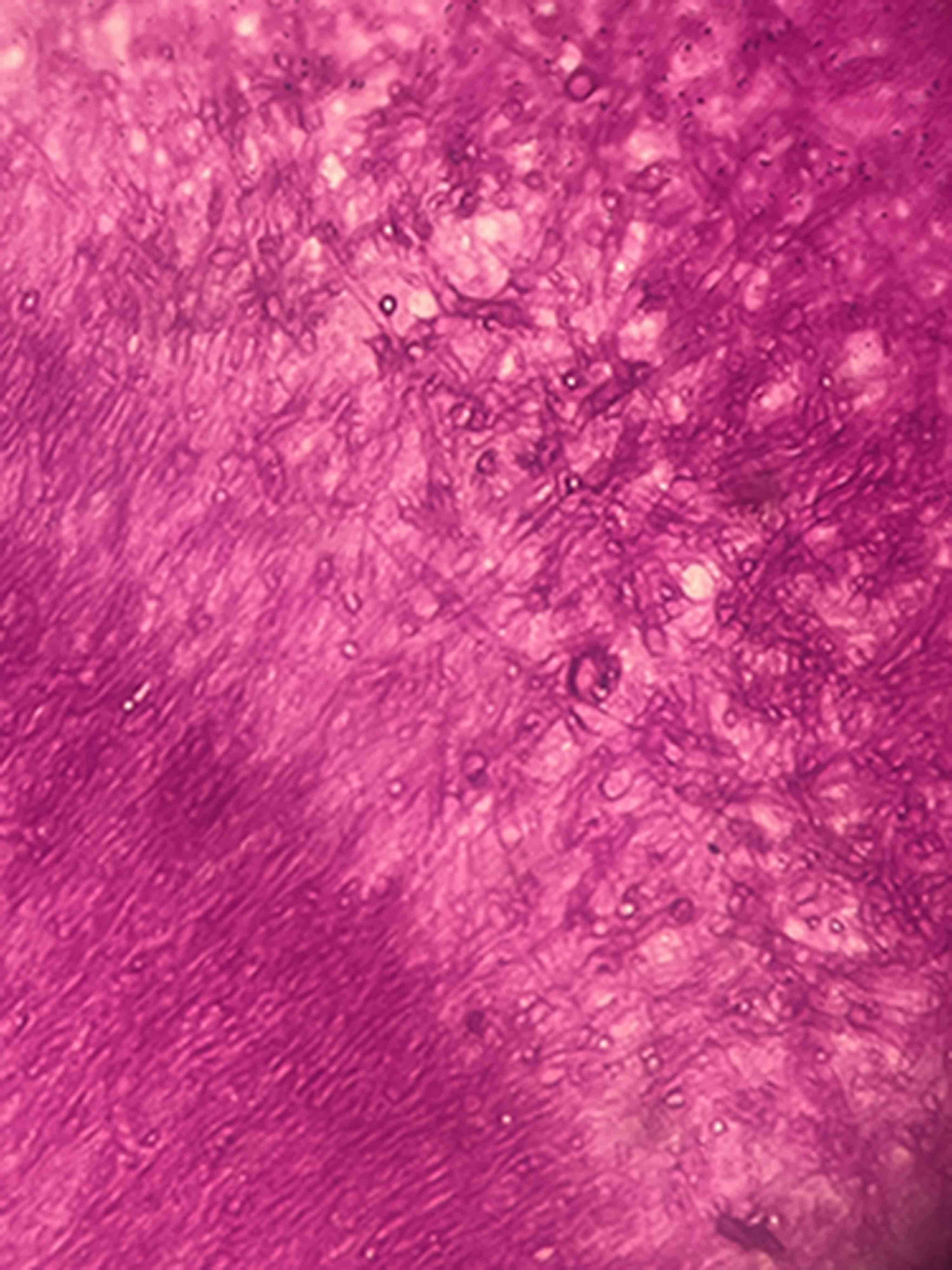
The analysis of the excised tissue revealed a prominent chronic inflammation with neutrophil foci in the lung parenchyma. However, fungal hyphae were not seen. Nodular, lumpy, brownish formations measuring up to 0.8cm were observed in the cavity. The cavity had a rigid wall and an extensive chronic inflammatory process covered by histiocytes. Histological examination in PAS stain (periodic acid-Schiff) of the excised tissue revealed septate hyphae with an irregular branching pattern grouped into a compact mass (Fig. 2). The tissue was cultured (Sabouraud dextrose agar, Lactrimel and Brain Heart Infusion agar with chloramphenicol) at 28°C and 37°C. Pure colonies grew rapidly, gray-white color and downy, with a gray-black reverse side; microscopic morphology was characterized by septate hyaline cylindrical hyphae from which conidiogenous cells emerged. Anellidic conidiogenesis producing oval, brown, sticky conidia was observed. The strain had the typical Scedosporium-like asexual morph, and it was identified as Scedosporium apiospermum complex. Besides, neither bacteria nor acid-fast bacteria were observed in Gram and Ziehl–Neelsen staining. There was no bacterial or Mycobacteria growth.
Antifungal susceptibility testing to voriconazole and itraconazole was performed by gradient diffusion strips using Etest (bioMérieux, France; Liofilchem S.r.I., Italy).19 MICs for voriconazole and itraconazole were 0.045μg/ml and 1μg/ml, respectively.
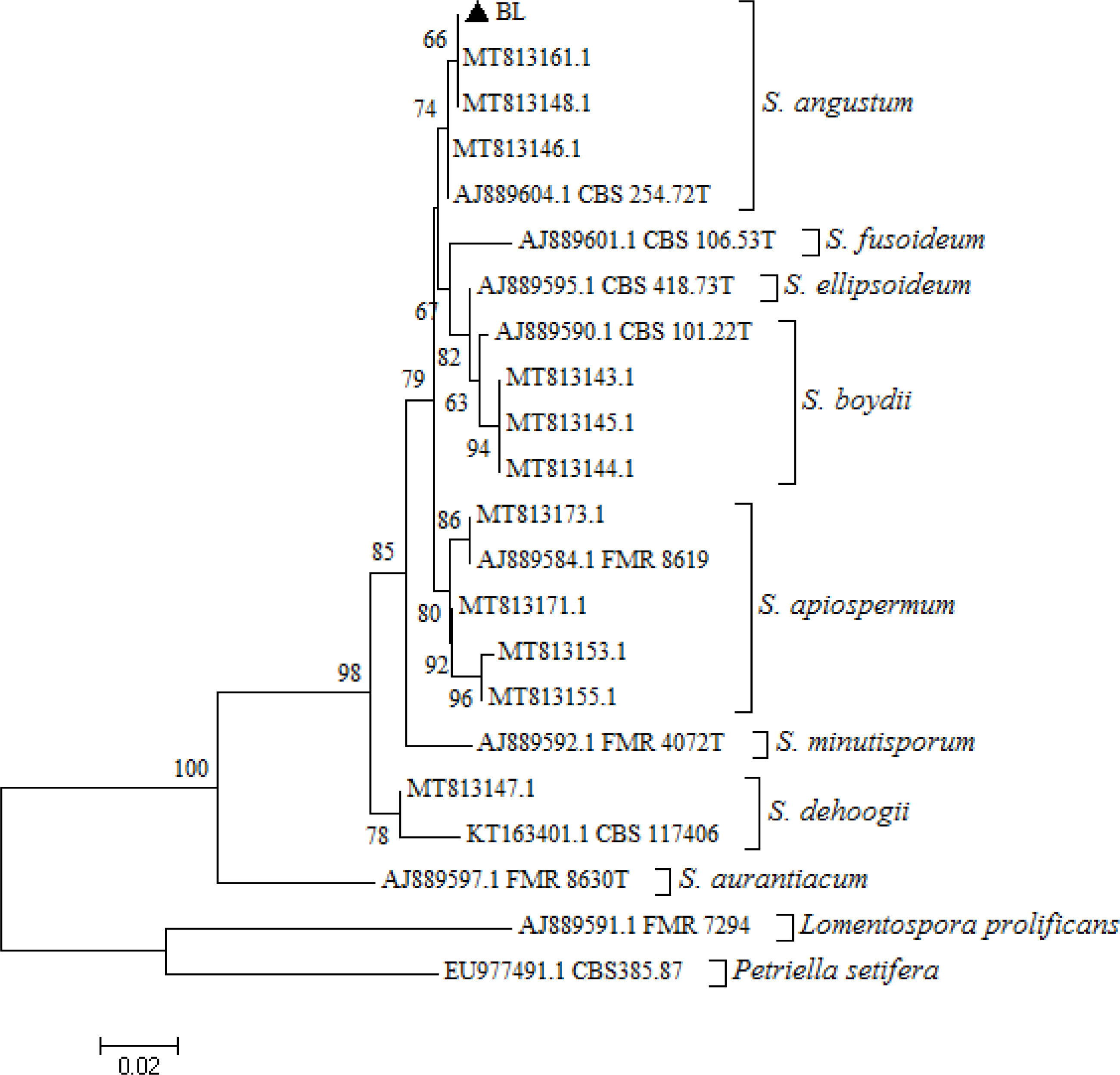
Molecular identification of the isolate was based on PCR amplification and sequencing of β-tubulin gene. DNA extraction was performed14 and the BT2 gene was partially amplified by PCR and sequenced.2 The amplification resulted in a fragment of 476 bp. The nucleotide sequence was compared with those available in the GenBank database using BLASTN National Center for Biotechnology Information (NCBI). The sequence of the β-tubulin region was submitted to the GenBank database, and assigned the following accession no.: PP706313. The Scedosporium culture was deposited at the Mycology Center in the University of Buenos Aires, School of Medicine. The β-tubulin 476 bp sequence showed a 99.79% similarity with Scedosporium/Pseudallescheria angustum (accession no. MT813161.1). Multiple alignments were performed using the Clustal W software, and phylogenetic analyses were conducted using the neighbour-joining method using the MEGA program package, version 5.05.21 The similarity of the nucleotide sequence of our strain with those in the GenBank database1 confirmed with high bootstrap support that the isolate was included in the Scedosporium angustum cluster (Fig. 3).
Neighbor-joining phylogenetic tree, developed with MEGA version 5.05, of the partial β-tubulin gene from reference strains belonging to the species Scedosporium angustum, Scedosporium fusoideum, Scedosporium ellipsoidea, Scedosporium apiospermum, Scedosporium aurantiacum, Scedosporium boydii, Scedosporium minutispora, Scedosporium dehoogii, Lomentospora prolificans, and Petriella setifera, as well as the strain isolated from the patient (▲ BL). The reference strains used (codes starting with MT, AJ, KT, EU) were retrieved from GenBank. Type strains have a “T” at the end of their codes. Bootstrap values of >50% with 1000 replicates used are written in each branch node. The bar indicates the number of substitutions per site.
Scedosporium is widely distributed in environmental sources.16 In the present report, the fungal infection was localized in the lung.17 This single finding was in line with the results obtained by CT scans of the thorax. In pulmonary colonization, the predisposing condition is usually the existence of a preformed bronchiectasis, cavity or cyst, most of which are caused by species of the genus Aspergillus, especially Aspergillus fumigatus. However, other genera such as Fusarium, Acremonium or Scedosporium are frequently isolated.5,9,15,16,20 Kantarcioglu et al. highlight the existence of post-tuberculosis cavitation as a major risk factor for acquiring pulmonary infections by the S. apiospermum complex.9 In histopathological observations of pulmonary colonization, Aspergillus species display a regular, dichotomous branching pattern, while Scedosporium strains exhibit a more irregular branching pattern (Fig. 2).10 However, different authors argue that S. apiospermum and Aspergillus look the same in histopathological analyses.7–9 Overall, it is always difficult to distinguish between them when examining the tissues.6
Clinical and imaging features of pulmonary S. apiospermum infection are similar to those observed in infections caused by Aspergillus, but it is essential to distinguish between them in order to administer the appropriate treatment.23 As in the present case, hemoptysis is reported as a common and potentially life-threatening symptom of pulmonary fungal balls by Aspergillus and S. apiospermum.9,16 Like in Aspergillus infections, surgical treatment should be considered for curative purposes if the general condition and the lung function allow it as S. apiospermum infection is associated with high mortality (7–23%) and morbidity rates.9,16,22
Abrantes et al.1 found that the most frequently recovered species of Scedosporium from clinical specimens in Argentina belonged to the S. apiospermum complex, which is clearly monophyletic and has an intraspecific genetic variability. S. angustum comprised a cluster with a high bootstrap branch support that was recognized in BT2 locus.1,4,17 Lackner et al. defined antifungal susceptibility ranges to voriconazole, amphotericin B, posaconazole, anidulafungin and micafungin for Scedosporium; according to them, our strain of S. angustum had a low MIC for voriconazole.13
Accurate identification of the Scedosporium species, surgical resection and high-dose voriconazole treatment have been associated with favorable outcomes in most reported cases caused by the S. apiospermum complex.23 In conclusion, this case emphasizes the importance of considering Scedosporium species in the differential diagnosis with Aspergillus when fungal balls are developed.
Ethical approvalAll data were gathered as part of routine work at the Hospital Naval Dr. Pedro Mallo and the Mycology Center in the University of Buenos Aires, School of Medicine.
FundingUniversidad de Buenos Aires, Facultad de Medicina, Argentina.
Conflicts of interestThere are no conflicts of interest.