The role of culture-independent techniques (galactomannan, (1-3)-β-d-glucan) in the early diagnosis of invasive fungal diseases (IFD) is well assessed in hematological patients, but there are no clear conclusions in patients with chronic obstructive pulmonary disease (COPD).
AimsTo study the usefulness of nonculture-based techniques in the diagnosis of IFD in COPD-patients at risk for IFD.
MethodsA prospective observational study based on monitoring COPD patients at risk for IFD during 2007–2010 was carried out. The presence of galactomannan, (1-3)-β-d-glucan and an indirect immunofluorescence of Candida albicans germ tube specific antibodies (CAGTA) were performed.
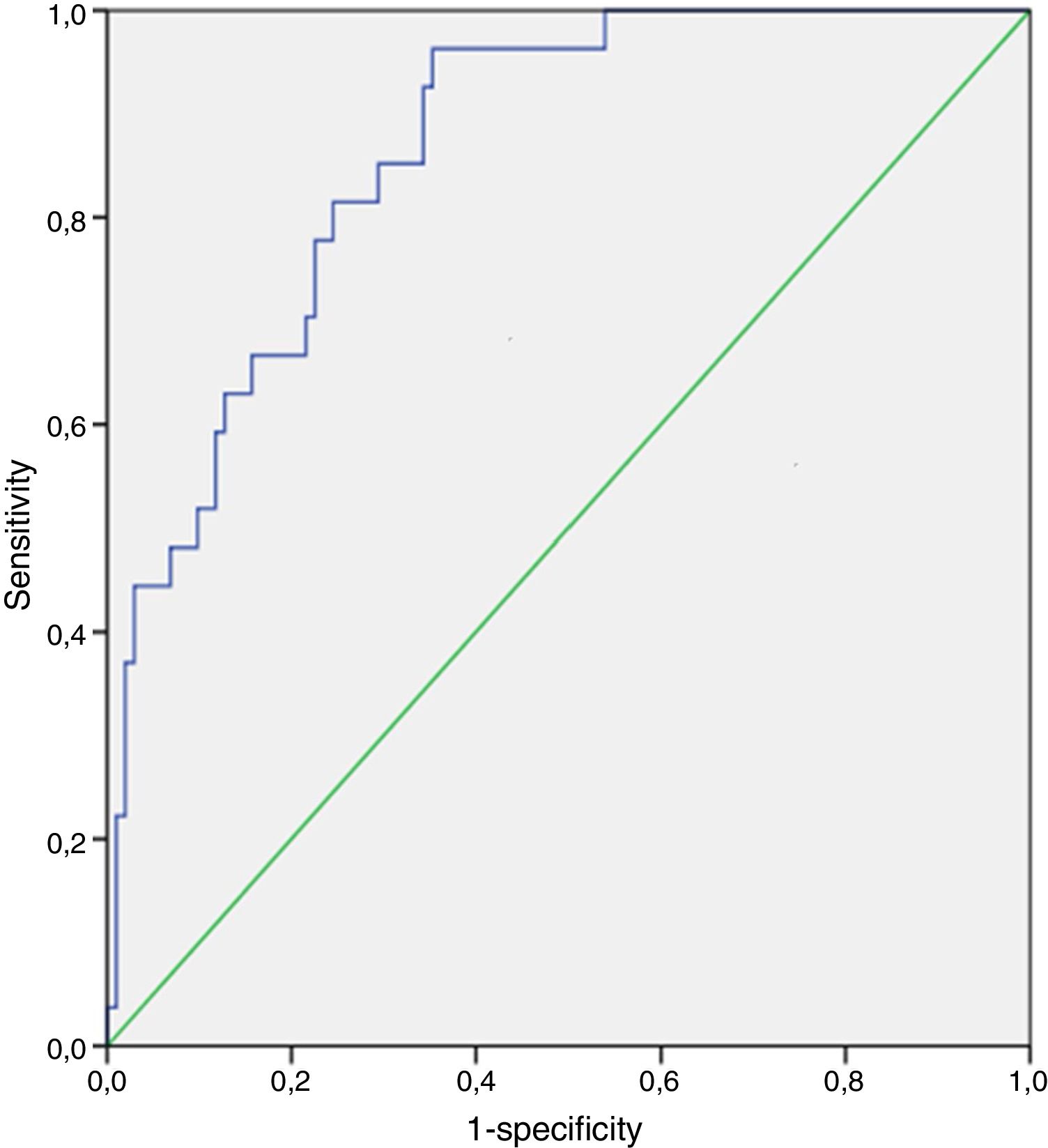
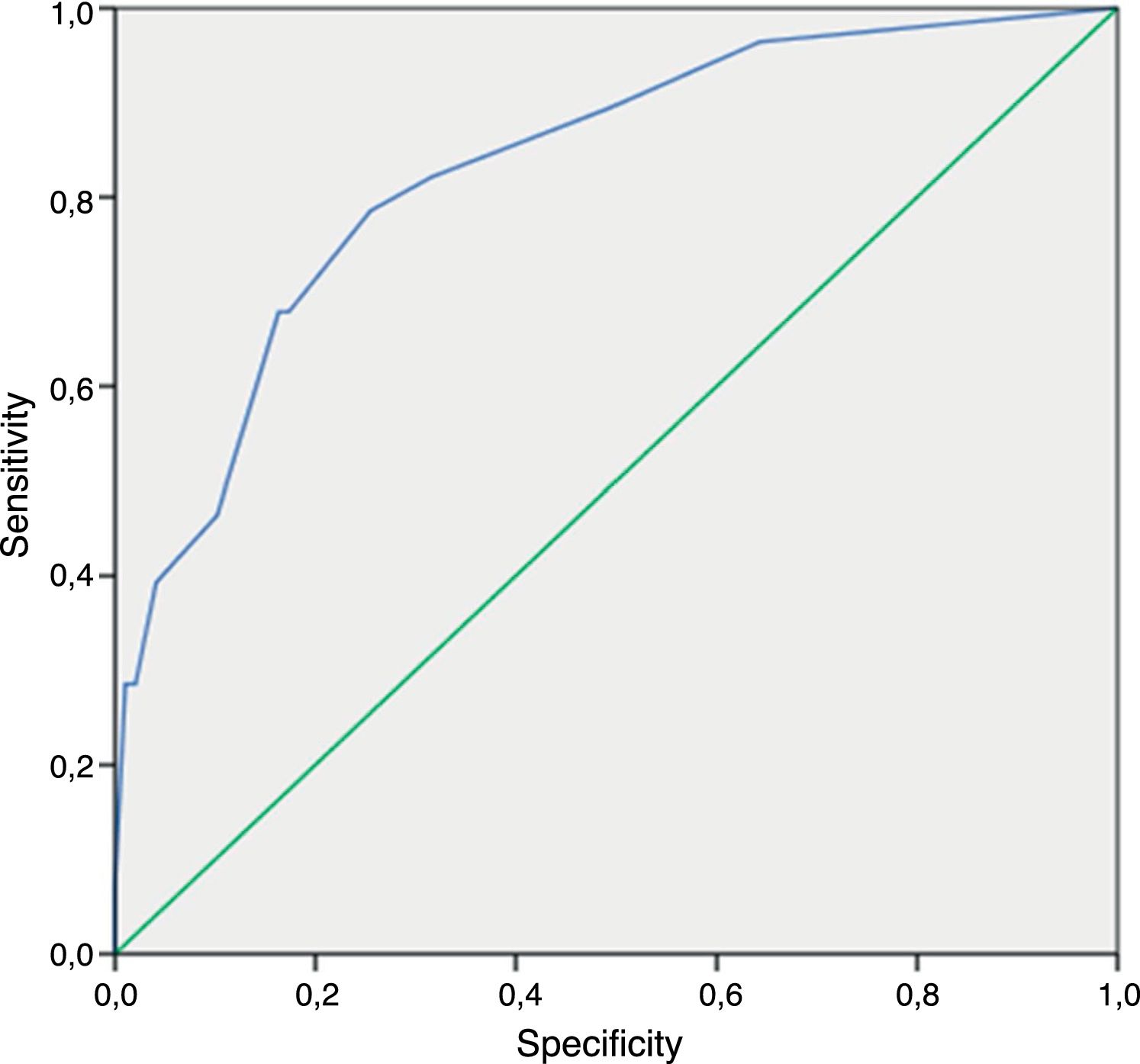
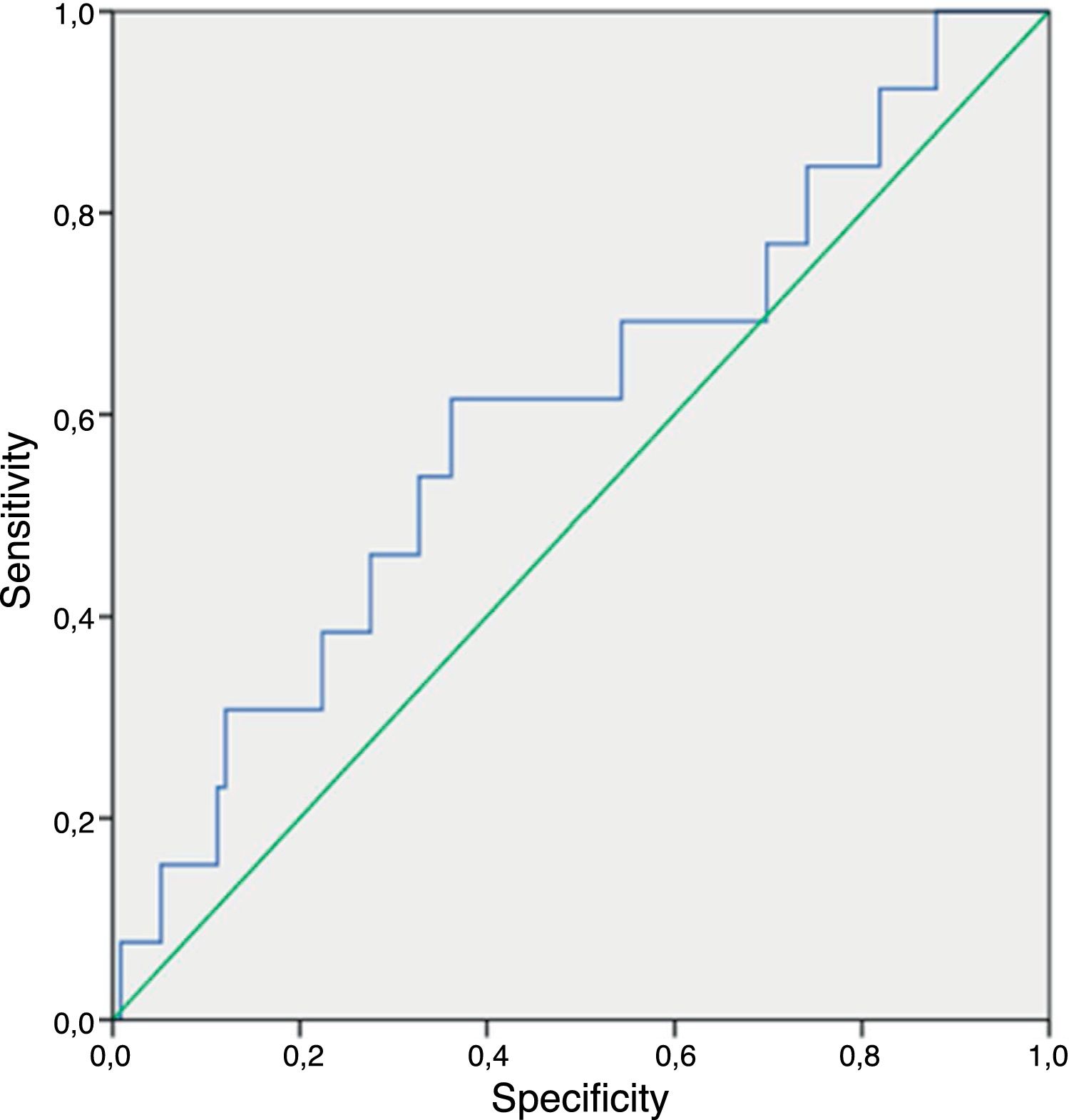
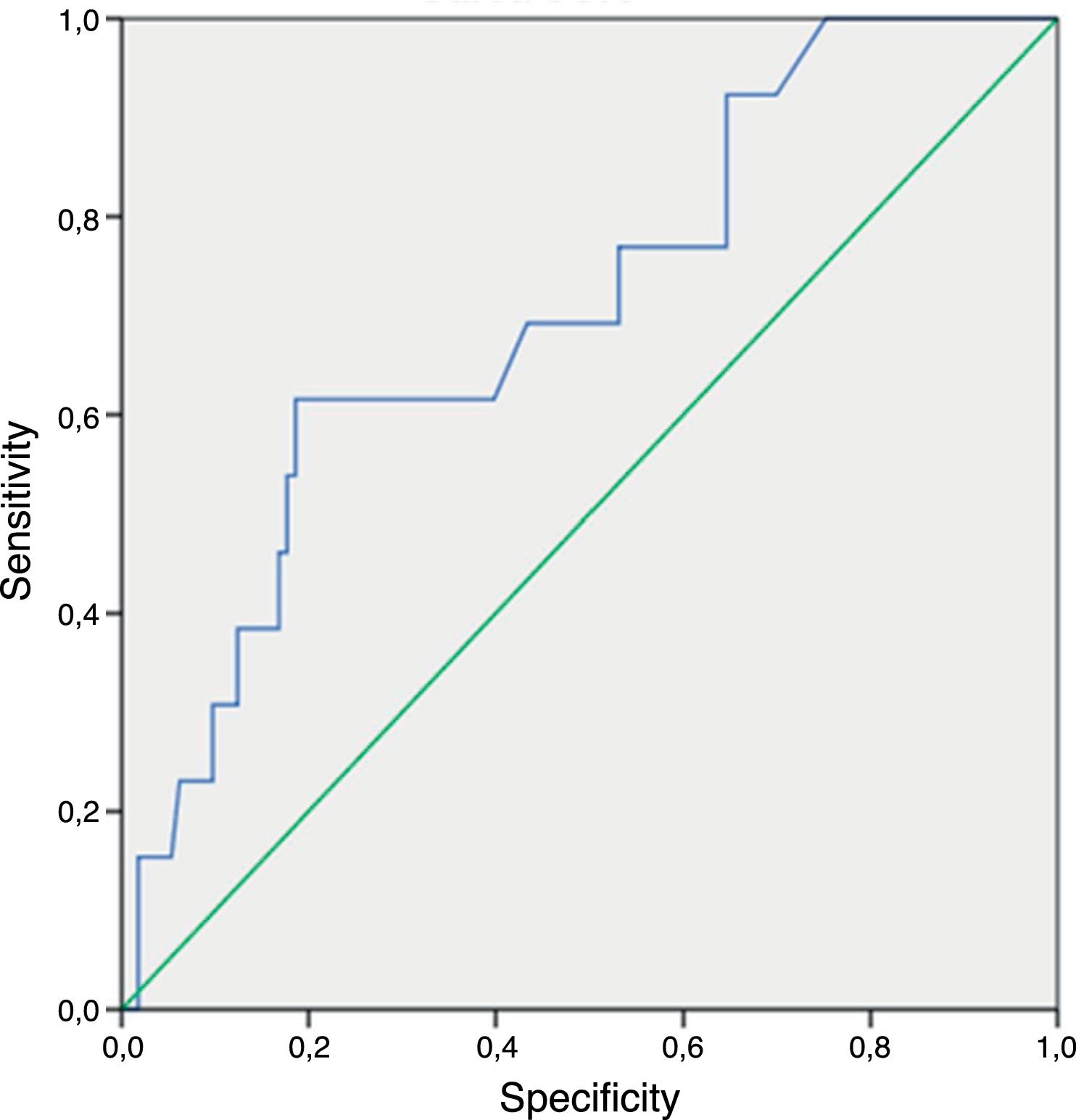
ResultsAmong 43 COPD patients, 16 (37.2%) were diagnosed with IFD: seven cases were proven IFD (five invasive candidemia – IC, one invasive aspergillosis – IA and a rhinocerebral zygomycosis) and nine probable IFD (seven IA and two IC). In the diagnosis of IC and IA, the negative predictive value (NPV) of (1-3)-β-d-glucan was 100%. Regarding CAGTA in IC, NPV was 96.2%. Finally, NPV of galactomannan in IA was 91.2%. The area under the ROC curve for (1-3)-β-d-glucan in IC and for the rest of the IFD cases was 0.86 (95% CI, 0.79–0.93) and 0.60 (95% CI, 0.43–0.77), for CAGTA in IC was 0.83 (95% CI, 0.74–0.91) and for galactomannan in IA was 0.71 (95% CI, 0.56–0.85). Positive (1-3)-β-d-glucan preceded the growth of Candida (average of 1.7 days) in blood culture.
ConclusionsIn COPD patients at risk for IFD the assayed techniques are especially useful to rule out the presence of IFD.
El papel de las técnicas independientes de cultivo [galactomanano, (1-3)-β-D-glucano] en el diagnóstico precoz de micosis invasoras (MI) está bien establecido en pacientes hematológicos, pero no existen conclusiones claras en pacientes con enfermedad pulmonar obstructiva crónica (EPOC).
ObjetivosEstudiar la utilidad de las técnicas independientes de cultivo en el diagnóstico de MI en pacientes con EPOC que corren el riesgo de contraer una MI.
MétodosSe llevó a cabo un estudio prospectivo y observacional en que se supervisaron pacientes con EPOC que corrían el riesgo de contraer una MI de 2007 a 2010. Para ello se estableció la existencia de galactomanano, (1-3)-β-D-glucano y se realizó el ensayo CAGTA (inmunofluorescencia indirecta para determinar la existencia de anticuerpos IgG frente a antígenos de la superficie de la fase micelial de Candida albicans).
ResultadosSe diagnosticaron 16MI en 43pacientes con EPOC (37,2%): siete fueron MI probadas (cinco candidemias invasoras [CI], una aspergilosis invasora [AI] y una cigomicosis rinocerebral) y nueve fueron MI probables (siete AI y dos CI). En el diagnóstico de CI y AI, el valor predictivo negativo (VPN) del (1-3)-β-D-glucano fue del 100%. En cuanto al CAGTA en CI, el VPN fue del 96,2%. Finalmente, el VPN del galactomanano en AI fue del 91,2%. El área bajo la curva ROC (receiver operating characteristic) del (1-3)-β-D-glucano en CI y en el resto de los casos de MI fue 0,86 (IC95%=0,79-0,93) y 0,60 (IC95%=0,43-0,77), para CAGTA en CI fue 0,83 (IC95%=0,74-0,91) y para galactomanano en AI fue 0,71 (IC95%=0,56-0,85). La positividad del (1-3)-β-D-glucano se anticipó como media 1,7días al crecimiento de Candida en el hemocultivo.
ConclusionesEn pacientes con EPOC que corren el riesgo de contraer MI, estas técnicas son muy útiles para descartar la existencia de MI.
Invasive fungal diseases (IFDs) are common infections in immunocompromised patients, not only in traditional risk populations (e.g. oncohematologic patients), but also in other at-risk populations such as critically ill patients or those with chronic obstructive pulmonary disease (COPD). In COPD patients, mucociliary activity impairment, immunosuppression due to the inhibition of alveolar macrophages and neutrophils by steroids, and treatment with broad-spectrum antibiotics play a role in the development of IFD.7
Candida is the most frequent fungal pathogen in IFD. Among the emerging invasive mycoses are those caused by filamentous fungi, such as Aspergillus, Fusarium, mucorales, and Scedosporium.22
IFD in patients under intensive care is among the most difficult infectious diseases to recognize clinically and its diagnosis is frequently missed.1,17,22 Nevertheless, the importance of early diagnosis and antifungal therapy for the prognosis of patients with IFD is clear. Because of this, diagnostic methods with appropriate sensitivity and specificity are needed.
Microbiological diagnostic techniques for IFD can be classified into:
- •
Conventional techniques: direct examination and culture.
- •
Unconventional techniques (culture-independent methods): detection of galactomannan (GM), (1-3)-β-d-glucan and antibodies, and molecular amplification techniques.
The origin of the samples for performing diagnostic techniques is very important. Sample availability, especially in selected patients whose clinical situation is very critical, is also a decisive factor when choosing the most appropriate diagnostic methods. For example, skin lesions, if present, are relatively easy to biopsy; corneal samples are useful in the diagnosis of keratitis produced by Aspergillus or Fusarium. Lung biopsy may be required if IFD with a pulmonary focus is suspected and the clinical situation of the patient is appropriate in order to tolerate the biopsy.
Culture allows us not only to identify the etiologic agent at the species level, but also to evaluate its antifungal susceptibility. However, specimen retrieval for culture may require invasive techniques, is laborious, and culture sensitivity is generally low (around 50% depending upon the patient, infectious focus and sample). Furthermore, the isolation of a certain etiological agent in a non-sterile sample does not exclude contamination or colonization.
Accordingly, the diagnosis of IFD often depends on a combination of clinical criteria and the presence of fungal etiologic agents in the most appropriate samples from the sites of infection. It is worth noting the high rate of confirmation of suspected IFDs in critically ill patients, especially if they have risk factors.11 Early diagnosis and treatment have been shown to improve patient outcomes, but an early diagnosis is still a challenge, and a wider variety of validated diagnostic techniques is needed. Recently, testing both galactomannan and (1-3)-β-d-glucan in serum and galactomannan in bronchoalveolar lavage has been accepted by the European Organization for the Research and Treatment of Cancer/Mycoses Study Group (EORTC/MSG) as complementary diagnostic techniques in the diagnosis of IFD.8 Monitoring of at-risk patients with galactomannan is recommended to establish an early diagnosis and check fungal disease progression.10 In contrast, PCR is still under investigation for the diagnosis of IFD. Lack of standardization of the various molecular assays has hindered their widespread acceptance in the diagnosis of IFD. Nevertheless, the close future is promising.14,15,26,27 The use of culture-independent techniques appears to be helpful for an early diagnosis of IFD. There is abundant literature offering useful conclusions for clinical practice concerning the role these techniques have in hematological patients. However, there are no clear conclusions in the case of other groups such as patients suffering from COPD.9,16,23
The overall objective of this work is to study the usefulness of nonculture-based diagnostic techniques for IFD diagnosis in non-neutropenic, hospitalized patients with COPD who are at risk for IFD.
Patients and methodsThe study was conducted at the Department of Clinical Microbiology and the Intensive Care Unit (ICU) of the University Hospital Severo Ochoa located in Madrid (Spain).
It was a prospective observational study carried out over a period of three years (January 2008 to December 2010) as part of a project (FIS 070134), and was approved by the Ethics Committee.
Selection of patientsInclusion criteria: COPD patients admitted to hospital with risk factors for developing IFD.20
Exclusion criteria:
- •
Community-acquired infection
- •
Estimated life expectancy of less than a week
- •
Patients under 18 years
- •
Neutropenia (neutrophil count<500neutrophils/mm3)
- •
Pregnant or lactating women
- •
Patients who refused to give informed consent
Data was collected sequentially over the entire project, with a follow up of the patients along the different episodes that they might suffer in the three years of observation.
Patients underwent clinical, microbiological and radiological monitoring. The data collection protocol consisted of the following variables:
- •
Personal variables (age and gender)
- •
Hospitalization time
- •
Diagnosis in admission
- •
Risk factors for IFD up to a month before and during admission according to Meersseman, 2007:18
- ∘
Simultaneous administration of two or more antibiotics, prescription of broad-spectrum antibiotics over a minimum of 5 days.
- ∘
High doses of corticosteroids or other immunosuppressive drugs.
- ∘
Chemotherapy and/or radiation therapy during the hospitalization.
- ∘
Parenteral nutrition and/or mechanical ventilation for 48h or more.
- ∘
Central venous catheter.
- ∘
Hospitalization in ICU for at least 7 days with a diagnosis of liver cirrhosis.
- ∘
Acute pancreatitis and abdominal surgery or other surgeries during the episode.
- •
Category of IFD (proven, probable, possible) according to De Pauw.8
- •
Clinical variables:
- ∘
Clinical severity according to the SOFA – Sequential Organ Failure Assessment – score.29
- ∘
Mucositis during the episode
- ∘
Fever during the episode
Microbiological variables:
- ∘
Sample type
- ∘
Date of sampling
- ∘
Results of microbiological tests, including those for nonculture-based diagnostic techniques –galactomannan, (1-3)-β-d-glucan and CAGTA.
- ∘
Known circumstances relating to false positive galactomannan (bacteremia, dialysis and fiber-rich diet).6
- ∘
Factors related to false positive (1-3)-β-d-glucan (hemodialysis equipment having cellulose membranes, treatment with albumin, immunoglobulins and some anticancer agents as lentinan or polysaccharide K, and sulfonamides).12,14
- •
Antifungal prophylaxis in the month prior to hospitalization (drug, dose and duration of treatment)
- •
Empirical antifungal treatment (drug, dose, and duration of treatment)
- •
High-resolution CT scan
- •
Histopathologic analysis
Galactomannan (Platelia Aspergillus, Bio-Rad Laboratories, Marnes La Coquette, France), (1-3)-β-d-glucan (Fungitell, Associates of Cape Cod, Falmouth, MA, USA) and CAGTA (Candida albicans IFA IgG, Vircell, Spain) were performed according to the manufacturer's recommendations for testing serum samples. They were performed twice a week. The optical density value of the serum galactomannan cut-off was 0.5. A galactomannan result was considered positive when two sequential determinations had a value of 0.5 or greater. Serum concentrations of (1-3)-β-d-glucan are interpreted as negative (<60pg/ml), indeterminate (60–79pg/ml) or positive (>80pg/ml). A CAGTA result was considered positive with a serum titer ≥1:160 in at least one sample.
Blood and sterile sites cultures were processed with automated systems (Bactec 9240, Becton Dickinson Diagnostic Instruments System, Sparks, MD, USA). Yeast species were identified with the API ID32C (bioMérieux, Madrid, Spain). Additional studies of fungal colonization were performed on rectum, oropharynx, pericatheter area and urine.
Data analysisAll quantitative data are presented as mean±SD. Sensitivity (Se), specificity (Sp) and predictive values (PPV and NPV) of IFD culture-independent diagnostic markers were calculated according to Altman and Bland.4,5 The criteria that define proven or probable IFD according to De Pauw8 were considered the gold standard. The corresponding ROC curves were also calculated. The highest measurements obtained from each patient's values were considered to calculate receiver operating characteristic (ROC) curves. Data analysis was performed using IBM SPSS Statistics for Windows, Version 20.0. Armonk, NY: IBM Corp.
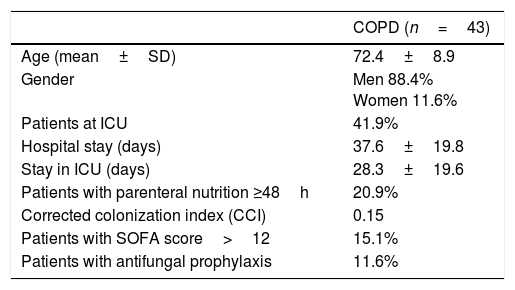
ResultsThe characteristics of the 43 patients with COPD and risk factors for developing IFD are shown in Table 1. The underlying diseases of these patients were the following: type 2 diabetes mellitus (18.6%), surgical history in the year before (14%), cancer history in the last 9 months (16.3%), non-invasive fungal infection history (4.7%), and transplant (2.3%). No COPD patient was found with positive serology for HIV. In terms of frequency, respiratory infection as a cause of hospitalization (83.7%) was followed by bacteremia/sepsis (9.3%). Other causes of hospitalization were abdominal infection (7%), abdominal surgery (4.7%), suspected IFD (2.3%), study of pulmonary nodules (2.3%), and palliative care (2.3%). Eighteen patients (41.9%) were hospitalized in ICU.
Characteristics of the study population.
| COPD (n=43) | |
|---|---|
| Age (mean±SD) | 72.4±8.9 |
| Gender | Men 88.4% Women 11.6% |
| Patients at ICU | 41.9% |
| Hospital stay (days) | 37.6±19.8 |
| Stay in ICU (days) | 28.3±19.6 |
| Patients with parenteral nutrition ≥48h | 20.9% |
| Corrected colonization index (CCI) | 0.15 |
| Patients with SOFA score>12 | 15.1% |
| Patients with antifungal prophylaxis | 11.6% |
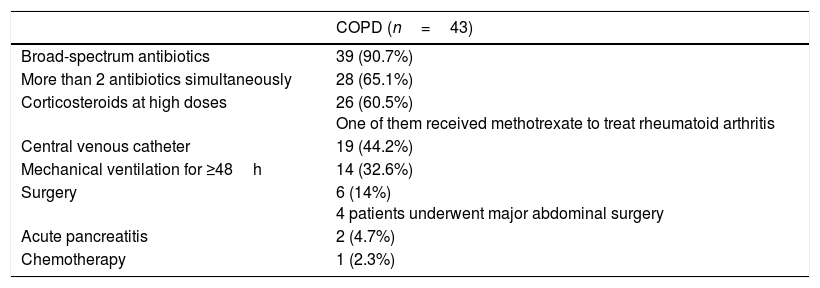
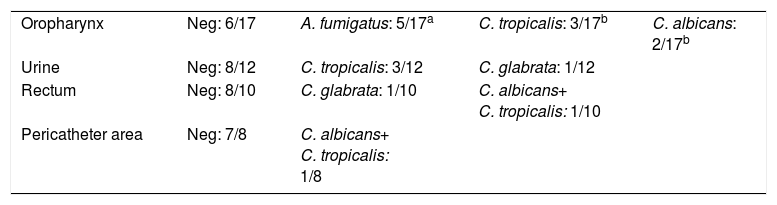
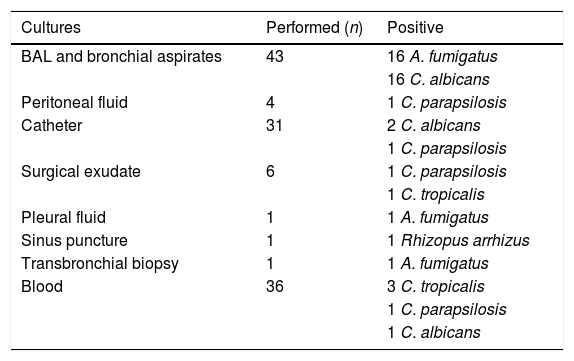
Risk factors for IFD in COPD patients are specified in Table 2. The most frequent was the use of broad-spectrum antibiotics (90.7%). The results of colonization studies are detailed in Table 3. Multifocal colonization was present in 15% of patients. The average corrected colonization index (CCI) in these patients was 0.15±0.2 (0–1), and 16.1% showed a CCI above 0.4. Sixteen IFD cases (37.2%) were diagnosed among 43 patients (Table 4). The results of the microbiological cultures associated with the diagnosis of IFD are shown in Table 4.
Risk factors for IFD.
| COPD (n=43) | |
|---|---|
| Broad-spectrum antibiotics | 39 (90.7%) |
| More than 2 antibiotics simultaneously | 28 (65.1%) |
| Corticosteroids at high doses | 26 (60.5%) One of them received methotrexate to treat rheumatoid arthritis |
| Central venous catheter | 19 (44.2%) |
| Mechanical ventilation for ≥48h | 14 (32.6%) |
| Surgery | 6 (14%) 4 patients underwent major abdominal surgery |
| Acute pancreatitis | 2 (4.7%) |
| Chemotherapy | 1 (2.3%) |
Results of fungal colonization studies.
Microbiological cultures and diagnosed IFD in the study population (43 patients).
| Cultures | Performed (n) | Positive |
|---|---|---|
| BAL and bronchial aspirates | 43 | 16 A. fumigatus |
| 16 C. albicans | ||
| Peritoneal fluid | 4 | 1 C. parapsilosis |
| Catheter | 31 | 2 C. albicans |
| 1 C. parapsilosis | ||
| Surgical exudate | 6 | 1 C. parapsilosis |
| 1 C. tropicalis | ||
| Pleural fluid | 1 | 1 A. fumigatus |
| Sinus puncture | 1 | 1 Rhizopus arrhizus |
| Transbronchial biopsy | 1 | 1 A. fumigatus |
| Blood | 36 | 3 C. tropicalis |
| 1 C. parapsilosis | ||
| 1 C. albicans |
| IFD | n | |
|---|---|---|
| Proven IFD | Candidemia | 5 |
| Invasive aspergillosis | 1 | |
| Rhinocerebral zygomycosis | 1 | |
| Probable IFD | Invasive candidemia | 2 |
| Invasive aspergillosis | 7 | |
| Possible IFD | – | 0 |
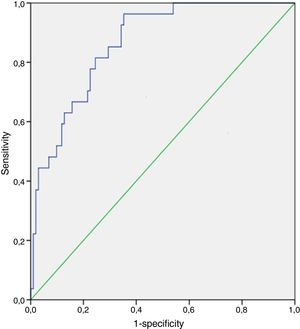
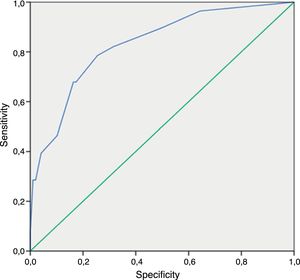
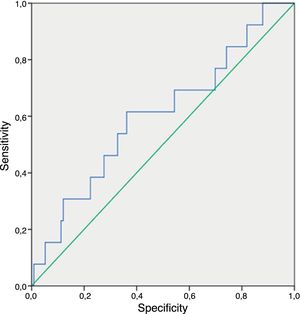
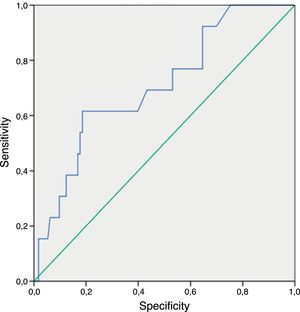
Regarding the nonculture-based diagnostic techniques, the results of sensitivity, specificity, predictive values and ROC curves are shown in Tables 5 and 6, and in Figs. 1–4. Positive (1-3)-β-d-glucan preceded in 1.7 days on average the growth of Candida in blood culture. The tables only refer to invasive candidemia and invasive aspergillosis. With respect to other types of fungal disease, only one rhinocerebral zygomycosis was diagnosed (probable invasive aspergillosis was also diagnosed in the same patient). In this patient, both the (1-3)-β-d-glucan and galactomannan were positive. The false positive rates obtained with galactomannan, (1-3)-β-d-glucan and CAGTA were 2.4%, 4.5% and 3.1% respectively.
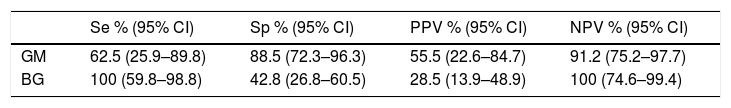
Sensitivity (Se), specificity (Sp) and predictive values (positive – PPV – and negative – NPV –) of galactomannan and (1-3)-β-d-glucan in the diagnosis of proven or probable IA in COPD patients.
| Se % (95% CI) | Sp % (95% CI) | PPV % (95% CI) | NPV % (95% CI) | |
|---|---|---|---|---|
| GM | 62.5 (25.9–89.8) | 88.5 (72.3–96.3) | 55.5 (22.6–84.7) | 91.2 (75.2–97.7) |
| BG | 100 (59.8–98.8) | 42.8 (26.8–60.5) | 28.5 (13.9–48.9) | 100 (74.6–99.4) |
Sensitivity (Se), specificity (Sp), PPV and NPV of CAGTA and (1-3)-β-d-glucan in the diagnosis of proven IC – including candidemias – or probable IC in COPD patients.
| Se % (95% CI) | Sp % (95% CI) | PPV % (95% CI) | NPV % (95% CI) | |
|---|---|---|---|---|
| CAGTA | 85.7 (42–99.2) | 72.2 (54.6–85.2) | 37.5 (6.3–64.1) | 96.2 (79.1–99.8) |
| BG | 100 (56.1–98.7) | 41.6 (25.9–59.1) | 25 (11.4–45.2) | 100 (74.6–99.4) |
According to the literature, the incidence of IFD in COPD patients can exceed that of “classic” at-risk IFD patients, and its evolution is nearly always unfavorable.10 The incidence of IA in hematological patients is between 4% and 22%, depending on the publication.28 In this study we actually observed a high incidence among the COPD patients analyzed (specifically, 34.9% of IFD and 20% of IA). On the other hand, the mortality rate in our COPD patients stood at 32.6%. Compared to the mortality data relating to hematological patients (20–50%),16 this study shows a higher mortality rate. Mortality associated with candidemia ranges from 35% to 49%, depending on the study.3,27 Mortality among our patients with candidemia was 72%; in ICU, mortality in patients with candidemia was 76.9%. This high mortality rate compared to that described in the literature may be explained by the fact that data collection in the present study did not guarantee that the patient's death was caused by IFD. Given the critical condition of the patients, mortality may have been influenced by comorbidities and the highly variable risk factors presented by these patients (SOFA score>12 in 23% of our patients in ICU). However, according to other publications, mortality associated with IC exceeds 40%, with the highest rates in critically ill patients.29,30
In several studies where (1-3)-β-d-glucan was evaluated in the diagnosis of IFD,20,21 the NPV exceeded 90%. The data presented here are similar. This supports the work conducted by Karageorgopoulos et al.,13 in which the (1-3)-β-d-glucan determination was helpful in ruling out IFD in critically ill patients. The sensitivity figures of Moragues et al.19 concerning CAGTA marker in critically ill patients with IC were similar to those obtained in the present study (Se=70–89% vs. Se=85.7% in this study). In terms of specificity, our data shows lower values (Sp=91–100% vs. Sp=72.2% in this study). It is well known that patients with invasive infections caused by species other than C. albicans usually have lower CAGTA titers.25 The high percentage of non-C. albicans Candida species in these patients (66.6%) could explain why the specificity results were lower.
In the meta-analysis conducted by Pfeiffer et al.24 – with a predominance of oncohematologic patients – both sensitivity and specificity in galactomannan detection in serum to diagnose IA were similar to those calculated in this study (Se=61% and Sp=93% vs. Se=62.5% and Sp=88.5% in this study). NPV results were particularly good for both studies (>91%). Therefore, it is suggested that a negative galactomannan could be sufficient to exclude the diagnosis of IA. When considering an index ≥0.5 for galactomannan, several prospective studies in adult oncohematologic patients have confirmed low levels of false positives (3–10%).2,18,19 A galactomannan false positive rate of 2.4% was observed among our patients, which is lower than that described in the literature for oncohematologic patients. The extreme care of the clinicians to avoid cross-contamination could explain the low false positive rate found. Furthermore, the false positives for (1–3)-β-d-glucan and CAGTA were 4.5% and 3.1%, respectively. These are very acceptable rates and well below the usual published values (up to 25% (1-3)-β-d-glucan false positive in the first three days after the surgery in critically ill patients according to Pemán et al.23).
The IFD spectrum is currently undergoing changes that raise questions about how to treat and prevent IFD. At present, it is essential to pay attention to new populations at risk for IFD. The definitive diagnosis is made using histopathology and identifying the causative agent. However, in recent years, culture-independent techniques have permitted earlier diagnosis. On the other hand, while autopsy is considered a gold-standard arbiter of the presence of IFD, one must consider whether, in the last days of life of a critically ill patient, the collapse of normal physiology and normal anatomical integrity (the loss of gut mucosal permeability barrier integrity due to anoxia, uremia, dysbiosis or inflammation) may lead to the inappropriate presence of microorganisms as a mortality-associated process. The results of this study suggest that culture-independent techniques are particularly useful in COPD patients, especially for ruling out IFD. Besides, it is necessary to conduct a thorough selection of COPD-patients at risk for IFD. In these patients at risk, the finding of sequentially positive galactomannan and / or (1-3)-β-d-glucan tests are indicative of IFD when associated with a strong clinical suspicion. Anyway, the limitations of this study were the following: (i) Lack of a reliable gold standard that may lead to misdiagnose or underdiagnose IFD; (ii) the inclusion of cases of probable IFD along with proven IFD when calculating sensitivity, specificity and predictive values of biomarkers that are used to define precisely the concept of probable IFD; (iii) the difficulty in discriminating between colonization and fungal disease; (iv) insufficient galactomannan and (1-3)-β-d-glucan positive cases to draw conclusions about the usefulness of these techniques in such samples.
Conflict of interestsThe authors declare no conflict of interests.
The authors would like to thank Malcolm Finkelman, Ph.D. for the revision of the manuscript.