Posaconazole is used for the prophylaxis of invasive fungal disease (IFD). Previous studies have shown it to be cost-effective compared to fluconazole/itraconazole. However, posaconazole has never been economically evaluated in developing countries.
AimsThe aim of the present study was to perform a cost-effectiveness analysis of posaconazole compared to fluconazole in public (SUS) and private hospitals (PHS) in Brazil.
MethodsA cost-effectiveness simulation was conducted on the basis of a pivotal study on the use of posaconazole in acute myeloid leukemia (AML) patients, adjusting the costs to Brazilian data.
ResultsA pharmacoeconomic analysis was performed on a hypothetical sample of 100 patients in each drug group. The total cost of posaconazole use alone was USD$ 220,656.31, whereas that for fluconazole was USD$ 83,875.00. Our results showed that patients with IFD remain hospitalized for an additional 12 days, at an average cost of USD$ 850.85 per patient per day. The total money spent by PHS for 100 patients for 100 days was USD$ 342,318.00 for the posaconazole group and USD$ 302,039.00 for the fluconazole group. An analysis of sensitivity (10%) revealed no intergroup difference.
ConclusionsIn Brazil posaconazole is cost-effective, and should be considered for the prophylaxis of patients with AMD/myelodysplasia (AML/MDS) undergoing chemotherapy.
El posaconazol se utiliza para la profilaxis de la enfermedad fúngica invasora (EFI). Algunos estudios han demostrado su rentabilidad en comparación con el fluconazol o el itraconazol. Sin embargo, el posaconazol nunca se había evaluado económicamente en el contexto de los países en vías de desarrollo.
ObjetivosEl objetivo de este estudio fue realizar un análisis de rentabilidad del posaconazol en comparación con el fluconazol en hospitales públicos (SUS) y hospitales privados (PHS) de Brasil.
MétodosSe realizó una simulación de rentabilidad basada en un estudio fundamental para el uso de posaconazol en pacientes con leucemia mieloide aguda (LMA) que adaptaba los costes a los datos brasileños.
ResultadosSe realizó un análisis farmacoeconómico de 100 pacientes con cada grupo tratado. El gasto total de 100 días para los antifúngicos evaluados fue 220.656,31 $ para el posaconazol y de 83.875,00 $ para el fluconazol. Los pacientes con EFI permanecen en el hospital una media de 12 días más a un coste medio de 850,85 $ por día y paciente. El gasto total en PHS de 100 pacientes fue 342.318,00 $ para el grupo del posaconazol y 302.039,00 $ para el del fluconazol. No hubo diferencias entre los grupos al realizar un análisis de sensibilidad al 10%.
ConclusionesEn Brasil, el posaconazol es rentable y debe tenerse en cuenta al elegir la profilaxis ideal para pacientes con LMA tratados con quimioterapia.
Invasive fungal disease (IFD) is a complication observed mainly in patients hospitalized for long periods in intensive care units after invasive procedures and in specific populations, such as severe neutropenia patients undergoing chemotherapy for leukemia.6 The costs associated with antifungal therapy for IFD depend greatly on the choice of drugs for each situation, as well as the total treatment duration. Besides the cost of the drug, costs related to an increased length of stay (LOS) in hospitals because of IFD must also be considered. Typically, the LOS increases by 12–20 days on average in patients with acute myeloid leukemia (AML).9,11 In a previous meta-analysis, antifungal prophylaxis was shown to decrease both the number of IFD episodes and mortality.10
In 2007, Cornely et al.3 demonstrated the superiority of posaconazole when compared to fluconazole/itraconazole in patients with AML. Some authors have previously reported the cost-effectiveness of posaconazole in Europe and the United States.5,12 However, such an evaluation has never been performed in the context of developing countries. In many developing countries, there are different sectors of healthcare, broadly divided into private and public systems. In Brazil, costs calculated for the Unified Health System (SUS – Sistema Único de Saúde) differ from those calculated for private health services (PHS). For these reasons, it is important to have knowledge of the cost-effectiveness of different drugs used for IFD prophylaxis in Brazil.
The aim of this study was to determine the cost-effectiveness of posaconazole compared to that of fluconazole/itraconazole in public (SUS) and private (PHS) Brazilian hospitals.
MethodsStudy typeThe present study was a cost-effectiveness simulation study based on a pivotal study assessing posaconazole's use in AML/myelodysplasia (MDS) patients receiving myeloablative chemotherapy, resulting in neutropenia.3
Pharmacoeconomic modelThe analytical decision model used to evaluate and compare the cost-effectiveness of posaconazole with that of fluconazole in the prevention of IFD was previously described by Grau et al.4 In this study, the model was stretched only over 100 days horizon.
The decision tree starts with a decision node that describes the prophylaxis selection for invasive fungal infection (IFI), either posaconazole or comparator agents. After prophylaxis initiation, patients can develop an IFI according to the probabilities described in Cornely's clinical trial. In addition, the model also takes into consideration the probability of survival or death once the IFI develops. The model not only accounts for patients who do not develop an IFI, but also for those who survive the IFI, as well as the probability of death from other causes not related to the IFI during the initial 100 days of prophylaxis.
The dose and duration of different prophylaxes were based on the study by Cornely et al.3
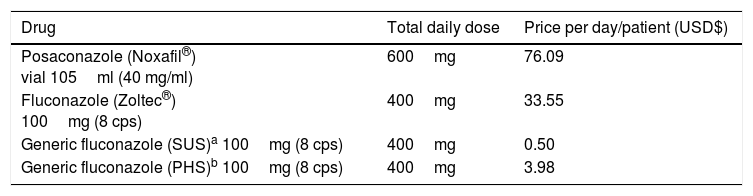
Cost of prophylaxisThe cost of prophylaxis involving posaconazole was based on the drug's market price in Brazil, as stated in the DOU (Brazilian Official Gazette) #175. To determine the cost of prophylaxis with fluconazole, three different prices were considered: (a) the cheapest value of the generic drug acquired from a public hospital in Curitiba (Hospital Evangélico de Curitiba); (b) the cost of generic/similar drug paid for by a major PHS, which was eight times more expensive; (c) the cost of the original drug (Zoltec®, Pfizer) paid for by the same PHS, which was 18 times more expensive.
Cost of invasive fungal diseaseDirect costs of IFDs were obtained from a database analysis of one of the largest private healthcare providers in Brazil (regarding the total amount of money spent by the PHS per IFD episode). For this purpose, a survey aimed at collecting all direct costs was performed during the admission of all AML patients with possible, probable, or proven IFD caused by invasive aspergillosis, fusariosis, and/or mucormycosis between 2011 and 2014 in the largest private hospital in the area of Curitiba, PR, Brazil. The costs of voriconazole (IV and/or PO) and/or amphotericin B lipid formulations were considered to estimate the direct costs of only the antifungal drugs for each IFD treatment. Data from all 38 patients were used to estimate direct costs from the PHS's viewpoint.
The total daily cost for hospitalization was analyzed (including that of non-antifungal drugs, laboratory tests, images, and medical procedures) to determine the cost of one day of hospitalization in such a population, without considering the costs of the antifungal agents, which were separately analyzed. After obtaining an average daily cost, it was multiplied by an average additional length of stay (LOS) for AML/MDS patients receiving myeloablative chemotherapy, which results in neutropenia and IFD. According to the literature, IFD patients reported an additional 12 days of LOS, on average.9 Indirect costs, patient-assigned costs, and costs accrued from outside the hospital were not considered.
Estimated costsOn the basis of the study by Cornely et al., a sample size of 100 patients was estimated to calculate IFD incidence and mortality in each study arm and for the final analysis.3
Presentation of valuesCalculated values are presented as means, medians, maximum and minimum values, and percentiles (10th, 25th, 75th, and 90th). Monetary values are presented in United States dollars (USD$), and each value was up-to-date as of the first day of 2016 based on the Non-revised Consumer Price Index (IPCA). Discounts were not calculated because of the short time period.
Sensitivity analysisWe performed a deterministic univariate sensitivity analysis with the objective of assessing the robustness of the model, as well as the consistency of the assumptions used in the model. The modified single parameters were the most relevant in the model and those with the greatest uncertainty. The cost of prophylaxis and treatment of the IFIs with a value of 75% or 125% of the value was used for base case estimations, as recommended by Brazilian authorities.1 Other parameters, such as the efficacy of prophylaxis and the risk of death from IFIs, have been investigated earlier.4,13
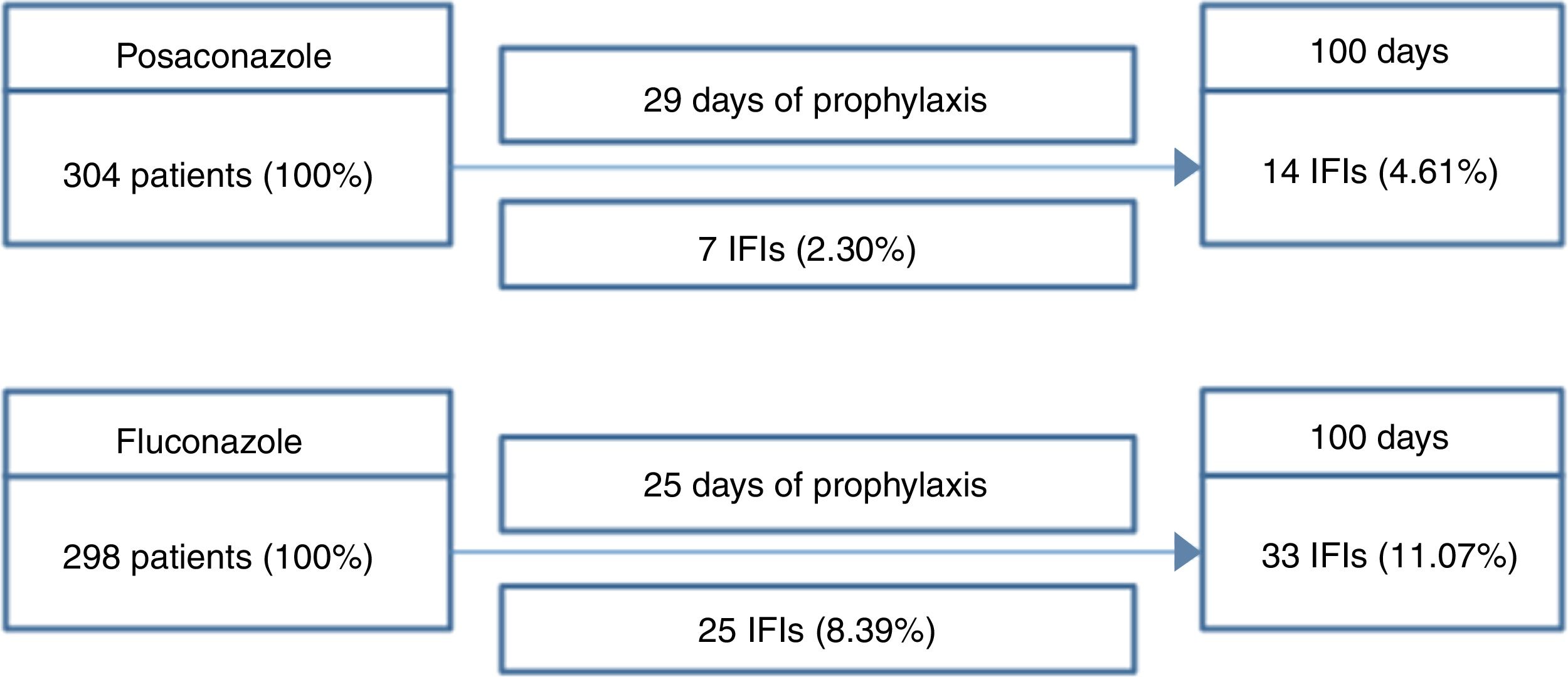
ResultsThe basic model described in Fig. 1 considered results from a major study in the area to calculate the direct cost of antifungal prophylaxis and treatment. At the end of the 100-day period, the final cost of prophylaxis was obtained using the average prophylaxis time, which was 29 days for posaconazole and 25 days for fluconazole. Thus, the direct cost of patient prophylaxis alone for the 100-day period was USD$ 2206.87 for posaconazole, USD$ 12.50 for generic fluconazole (SUS), USD$ 838.75 for Zoltec®, and USD$ 99.68 for PHS fluconazole (Table 1).
Scheme used to calculate the number of IFIs in patients with AML/MDS.
IFI: invasive fungal infection. Over the 100-day period, patients received, on average, 29 days of posaconazole prophylaxis or 25 days of fluconazole prophylaxis. Thus, the direct cost of prophylaxis for each patient over the 100-day period was USD$ 2206.87 for posaconazole, USD$ 12.50 for generic fluconazole (SUS), USD$ 838.75 for Zoltec®, and USD$ 99.68 for PHS fluconazole.
Cost of prophylaxis for hematological-oncological drugs according to the product.
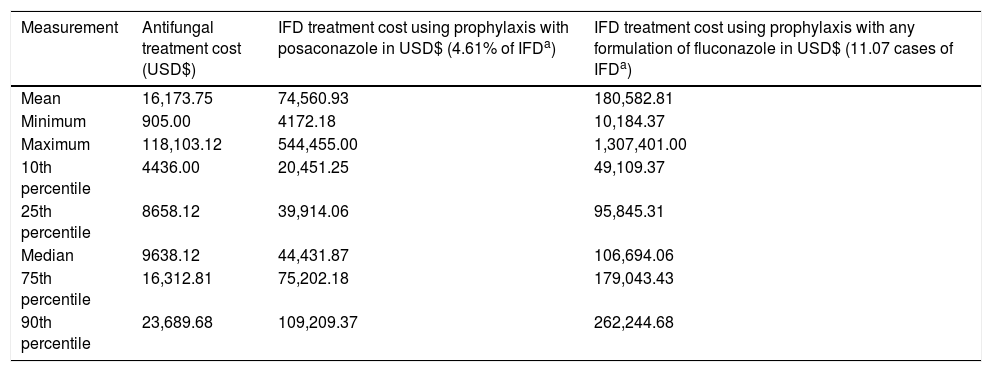
To the best of our knowledge, the burden of fungal disease treatment has not yet been described in Brazilian literature. The values presented are based on data collected from 38 patients with AML undergoing chemotherapy who experienced IFD (35 cases of invasive aspergillosis, three cases of fusariosis) and were receiving the right treatment as directed by the Infectious Diseases Society of America (2016) for a minimum of 5 days and who had a diagnosis of invasive aspergillosis.8 In this institution, the biomarker galactomannan, used in the diagnosis of invasive aspergillosis (IA), was not available. Therefore, most cases used in this economic model were classified as “possible IA.” This fact would be relevant for clinical analysis, but it does not interfere with the economic analysis presented, as all patients were fully treated. Costs of antifungal agents were excluded when establishing the costs of the average daily hospital stay for patients to avoid double charges. An average hospital day costs USD$ 850.85 in PHS. Direct costs of treatment were calculated using the average time of use of antifungals (voriconazole, amphotericin B lipid formulations) during the treatment of each IFD event of the same 38 patients. PHS spent USD$ 16,173.75 on average, only for antifungals, for each IFD episode. The average number of days to treat an episode of IFD and the 25th and 75th percentile values were 28.3 days, 9.5 days, and 37 days, respectively. Importantly, all these values are up-to-date for 2016, and refer only to direct costs accrued during hospitalization (Table 2).
IFD treatment cost for AML/MDS patients according to different prophylaxes in a 100-patient model.
| Measurement | Antifungal treatment cost (USD$) | IFD treatment cost using prophylaxis with posaconazole in USD$ (4.61% of IFDa) | IFD treatment cost using prophylaxis with any formulation of fluconazole in USD$ (11.07 cases of IFDa) |
|---|---|---|---|
| Mean | 16,173.75 | 74,560.93 | 180,582.81 |
| Minimum | 905.00 | 4172.18 | 10,184.37 |
| Maximum | 118,103.12 | 544,455.00 | 1,307,401.00 |
| 10th percentile | 4436.00 | 20,451.25 | 49,109.37 |
| 25th percentile | 8658.12 | 39,914.06 | 95,845.31 |
| Median | 9638.12 | 44,431.87 | 106,694.06 |
| 75th percentile | 16,312.81 | 75,202.18 | 179,043.43 |
| 90th percentile | 23,689.68 | 109,209.37 | 262,244.68 |
For pharmacoeconomic analysis, a budget impact projection for 100 hypothetical patients in each prophylaxis study was made. Prophylaxis costs for posaconazole alone was USD$ 220,656.31 and USD$ 83,875.00 for fluconazole (Zoltec®). Prophylaxis using generic fluconazole was USD$ 9968.75 in the PHS and USD$ 1250.00 in the SUS. Additional USD$ 10,210.31 must be added for each IFD episode, referring to additional LOS (12 extra hospital days, at an average cost of USD$ 850.85 per day with a daily hospital charge, medical fees, other drugs, and treatments) (Table 3). Finally, direct costs of antifungals were added according to the incidence in each group of prophylaxis.
IFD treatment and prophylaxis cost in AML/MDS patients according to different prophylaxes in a 100-patient model.
| Measurement | IFD treatment cost+posaconazole prophylaxis cost in R$ (4.61% of IFDa)b | IFD treatment cost+fluconazole (generic SUS) prophylaxis cost in R$ (11.07 cases of IFSa) | IFD treatment cost+fluconazole (generic PHS) prophylaxis cost in R$ (11.07 cases of IFDa) |
|---|---|---|---|
| Mean | 342,318.00 | 293,321.00 | 302,039.00 |
| Minimum | 271,928.00 | 124,296.00 | 133,014.00 |
| Maximum | 812,212.00 | 1,421,679.00 | 1,430,398.00 |
| 10th percentile | 288,208.00 | 163,387.00 | 172,105.00 |
| 25th percentile | 307,670.00 | 210,123.00 | 218,842.00 |
| Median | 312,188.00 | 220,971.00 | 229,690.00 |
| 75th percentile | 342,958.00 | 294,860.00 | 303,579.00 |
| 90th percentile | 376,966.00 | 376,522.00 | 385,241.00 |
The prophylactic cost of posaconazole was USD$ 220,656.31 and the prophylactic cost of fluconazole (Zoltec®) was USD$ 83,875.00 for 100 patients. Prophylaxis with generic fluconazole for 100 patients totaled USD$ 9968.75 in PHS and totaled USD$ 1250.00 in SUS. The additional USD$ 10,210.31 referred to 12 days of hospital costs, with an average of USD$ 850.85 per day.
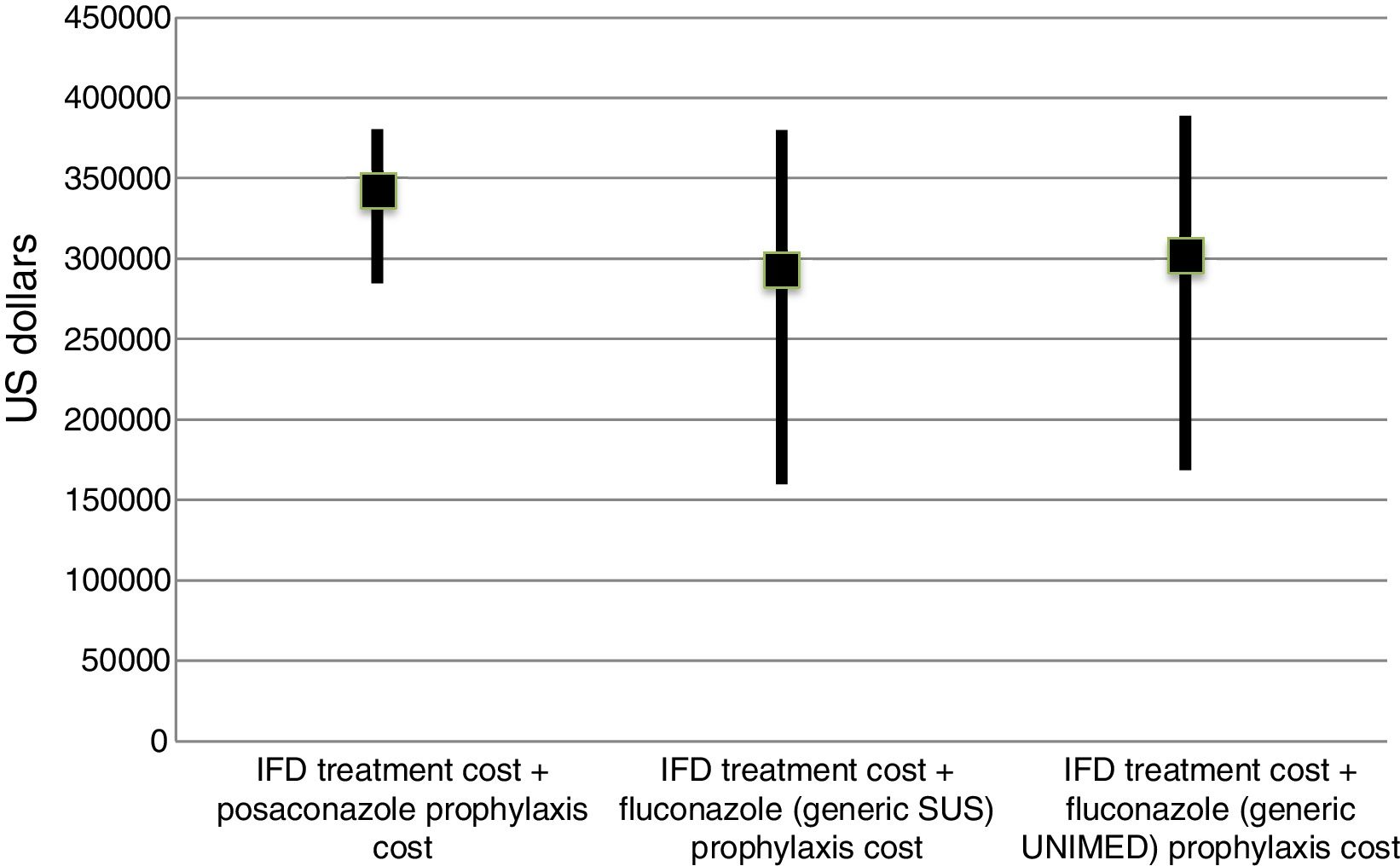
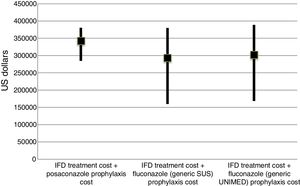
The incremental cost-effectiveness ratio (ICER) was not calculated because of the lack of difference in the final cost of each group, considering a 90% interval (Fig. 2). However, the ICER could be considered zero with less episodes of IFD (6 cases in 100 prophylaxes) and up to six additional lives saved.
IFD treatment and prophylaxis costs in AML/MDS patients according to different prophylaxes in a 100-patient model. Data are presented as medians and values of the 25th and 75th percentiles.
IFD=invasive fungal disease. The prophylaxis cost of posaconazole was USD$ 220,656.31 and the prophylaxis cost of fluconazole (Zoltec®) was USD$ 83,875.00 for 100 patients. The prophylaxis cost with generic fluconazole for 100 patients was USD$ 9968.75 in PHS and USD$ 1250.00 in SUS. The additional USD$ 10,210.31 referred to 12 days of hospital costs, with an average of USD$ 850.85 per day.
The results of the present study indicate that posaconazole may be considered more cost-effective than fluconazole because of the reduction in the number of IFD episodes and the number of lives saved with no additional costs. In addition, other studies have also indicated that posaconazole is more cost-effective than other azole antifungal agents.2,13 We believe that posaconazole could be even more cost-effective than that in the tested population; however, in Brazil, the initial costs of novel drugs are very high. In Brazilian hospitals antifungal agents accounted for 38.6% of the total hospital expenditure for AML/MDS patients admitted with an IFD.
In future studies, attention should be focused on calculating the total number of days patients will require prophylactic antifungal therapy: in case of overuse of therapy direct costs associated with prophylactic posaconazole will rise. For example, if posaconazole prophylaxis is provided for 60 days, the cost of this product alone will reach USD$ 456,594.64. In such a scenario of product misuse, total cost of IFD treatment, prophylaxis, and additional LOS will be approximately USD$ 578,224.82 in the posaconazole group versus USD$ 492,695.74 in the Zoltec® group (still not significant). On the other hand, if prophylaxis with posaconazole is provided for 70 days, the total cost of IFD treatment, prophylaxis, and additional LOS will increase from USD$ 625,000 and become significantly higher than that for the Zoltec® group. An ICER must be presented in such cases; however, it will most probably prove to be cost-effective.
IFD incidence may vary between different hospitals. In this study, we used the incidence presented by Cornely et al. in their pivotal study of posaconazole.3 In the Brazilian literature, there are evidences that the incidence of IFD is higher in a similar population of patients who received prophylactic fluconazole than in those who received prophylactic posaconazole. According to Nucci et al.,7 the incidence of IFD was 18.3% without high-efficiency particulate air (HEPA) filter protection. If we consider such a high incidence in the fluconazole group, the total cost of IFD treatment, prophylaxis, and additional LOS costs reaches USD$ 566,420.72. It would be a scenario where posaconazole could be considered not only lifesaving but also a cost-saving product.
Importantly, the availability of HEPA filters in Brazilian care centers that treat IFD patients remains rare. Therefore, the incidence of IFD may vary widely among different hospitals, which extensively favor the use of posaconazole. It is essential to recommend HEPA filters as a preventive measure for all patients with prolonged neutropenia. Considering the additional LOS used in our model, it is important to keep in mind some ideas. For this study, we used the PHS perspective and values, and the total number of additional days was calculated from the literature (12 days), which is shorter than that expected for a typical Brazilian scenario; however, there may be differences according to regional populations.
The limitations of our study include the following situations: indirect costs were not calculated, and the direct costs of any IFD outside the hospital were not calculated as well. It is difficult to preview prescribers’ behavior in treating IFD in patients under prophylaxis with posaconazole. If there is an increase in the use of amphotericin lipid formulation, the direct hospital cost of IFD may increase significantly. For example, if we treat patients for 28 days (the average number of days for treatment in hospital), the direct cost of these antifungals may vary from USD$ 45,000 to USD$ 100,000 per IFD episode.
In conclusion, although there are major differences in direct costs between several prophylaxis strategies, there were no statistically significant differences between them when calculating the final cost. However, mortality and the number of IFD episodes in the posaconazole group following treatment were lower than those in the fluconazole group. This should be considered before selecting the ideal prophylaxis for AML/MDS patients undergoing chemotherapy.
Conflict of interestsAll authors received grants from MSD.