Biofilm is known to contribute to the antifungal resistance of Candida yeasts. Aureobasidin A (AbA), a cyclic depsipeptide targeting fungal sphingolipid biosynthesis, has been shown to be effective against several Candida species.
AimsThe aim of this study was to investigate Candida biofilm growth morphology, its biomass, metabolic activity, and to determine the effects of AbA on the biofilm growth.
MethodsThe biofilm forming ability of several clinical isolates of different Candida species from our culture collection was determined using established methods (crystal violet and XTT assays). The determination of AbA planktonic and biofilm MICs was performed based on a micro-broth dilution method. The anti-biofilm effect of AbA on Candida albicans was examined using field emission scanning electron microscope (FESEM) analysis.
ResultsA total of 35 (29.7%) of 118 Candida isolates were regarded as biofilm producers in this study. Candida parapsilosis was the largest producer, followed by Candida tropicalis and C. albicans. Two morphological variants of biofilms were identified in our isolates, with 48.6% of the isolates showing mainly yeast and pseudohyphae-like structures, while the remaining ones were predominantly filamentous forms. The biofilm producers were divided into two populations (low and high), based on the ability in producing biomass and their metabolic activity. Candida isolates with filamentous growth, higher biomass and metabolic activity showed lower AbA MIC50 (at least fourfold), compared to those exhibiting yeast morphology, and lower biomass and metabolic activity. The observation of filament detachment and the almost complete removal of biofilm from AbA-treated C. albicans biofilm in FESEM analysis suggests an anti-biofilm effect of AbA.
ConclusionsThe variability in the growth characteristics of Candida biofilm cultures affects susceptibility to AbA, with higher susceptibility noted in biofilm cultures exhibiting filamentous form and high biomass/metabolic activity.
La biopelícula de las levaduras del género Candida contribuye a la resistencia de este género a los antifúngicos. La aureobasidina A (AbA), un depsipéptido cíclico cuya diana es la biosíntesis de los esfingolípidos en los hongos, ha mostrado tener cierta acción antifúngica frente a diferentes especies de Candida.
ObjetivosEl objetivo de este estudio fue investigar el desarrollo de las biopelículas de Candida, su biomasa y su actividad metabólica, y determinar el efecto de AbA en su desarrollo.
MétodosSe utilizaron los métodos de cristal violeta y XTT para determinar la formación de biopelícula en diferentes especies de Candida de nuestra colección. La determinación de los valores MIC de AbA sobre cultivos planctónicos o con biopelícula fue realizada mediante un método de microdilución en caldo. El efecto anti-biopelícula de AbA sobre Candida albicans fue analizado mediante el uso de un microscopio electrónico de barrido de emisión de campo (FESEM).
ResultadosTreinta y cinco aislamientos de Candida (29,7%) de un total de 118 produjeron biopelícula. Candida parapsilosis fue la especie más productora, seguida de Candida tropicalis y C. albicans. Se observaron dos variantes morfológicas en las biopelículas, con un 48,6% de los aislamientos con estructuras formadas fundamentalmente por levaduras y seudohifas, mientras que los restantes aislamientos mostraron mayoritariamente formas filamentosas. Los aislamientos productores de biopelícula fueron divididos en dos grupos en función de su capacidad de producir biomasa y de la actividad metabólica de la biopelícula. Aquellos aislamientos de Candida con estructuras filamentosas, mayor cantidad de biomasa y de actividad metabólica mostraron menores valores CMI50 (de hasta cuatro veces) respecto a aquellos aislamientos con morfología levaduriforme, y menor biomasa y actividad metabólica. El desprendimiento y casi completa eliminación de la biopelícula del aislamiento de C. albicans estudiado mediante FESEM y tratado con AbA sugiere el efecto antifúngico de esta molécula.
ConclusionesLas distintas particularidades de las biopelículas del género Candida condicionan la sensibilidad de los aislamientos a la AbA; la sensibilidad es mayor en aquellas biopelículas con estructura filamentosa, y mayor biomasa y actividad metabólica.
Candidiasis is a significant cause of morbidity and mortality among hospitalized immunocompromised patients. Among the Candida species, Candida albicans is the most frequently isolated yeast from clinical settings.1,15,17 However, in recent years, there has been an increase in the incidence of candidiasis caused by other species of the genus including Candida parapsilosis, Candida tropicalis and Candida glabrata.3,6,16Candida is known to produce biofilms on medical devices. Biofilms are defined as structured microbial communities that are attached to a surface and encased in a matrix of exopolymeric material. Yeast cells can be released from biofilms, migrate through the bloodstream and cause a systemic infection.15,19 Hence, mortality is greater in patients infected by biofilm-producing isolates than those infected by non-biofilm-forming isolates.26 Biofilm-associated infections are generally difficult to treat due to the decreased susceptibilities of the biofilm cultures to antimicrobial therapy.2,14,15,20 Removal of catheter has been recommended for management of biofilm-associated infections in catheterized patients with candidemia.5 Although candins and liposomal-amphotericin B have been reported to have good antibiofilm activity,2,19 the difficulty in completely recover from biofilm-associated infections has prompted studies to search for alternative therapies.18–19
Candida biofilms are different depending on the species, morphology and metabolic activity. It has been reported that up to 30% of C. albicans isolates exhibiting high metabolic activity in preformed biofilms showed the most marked antifungal effect (lower MICs), as seen in the case of micafungin.13 Aureobasidin A (AbA) is a cyclic depsipeptide antifungal produced by Aureobasidium pullulans with a strong fungicidal activity against a variety of fungi, including Candida.7,23,24 The antifungal inhibits inositol phosphorylceramide (IPC) synthase, a key enzyme absent in mammals responsible for sphingolipid biosynthesis in fungi.27 AbA has low toxicity and has shown effectiveness when given orally to mice with candidiasis.24A study in our laboratory reported in vitro susceptibility of several Candida species to AbA, and demonstrated that biofilms formed by 0, 1, 2, and 4h adherent populations were more susceptible to AbA treatment when compared with mature biofilms (represented by 24h yeast-adherent populations).25 However, studies are still lacking on the effects of AbA on the candidal biofilm morphology, biomass and metabolic activity. It has been reported that the antifungal activity of micafungin against C. albicans is dependent on biofilm metabolic activity; therefore isolates with lower metabolic activity were less susceptible to micafungin and isolates with higher biofilm metabolic activity were significantly more susceptible to micafungin.13
In this study, the biofilm morphology, biomass and metabolic activity of Malaysian isolates of several Candida species were studied and the effects of AbA on the biofilm growth were determined. The antibiofilm effect of AbA on C. albicans was also investigated using field emission scanning electron microscope (FESEM) analysis.
MethodsClinical isolates. A total of 118 clinical isolates from six Candida species were obtained from a culture collection at the Diagnostic Microbiology Laboratory, University Malaya Medical Centre (UMMC), Malaysia. Forty-eight isolates were obtained from blood cultures, and 70 isolates recovered from non-sterile sites (respiratory tract, vagina, urine, etc.) were included in this investigation. The isolates were identified as C. albicans (n=48, 40.7%), C. tropicalis (n=38, 32.2%), C. parapsilosis (n=16, 13.6%), C. glabrata (n=12, 10.2%), Candida nivariensis (n=3, 2.5%), and Candida kefyr (n=1, 0.8%) using routine mycological procedures (Table 1). C. albicans SC5314 strain was included as a control strain.
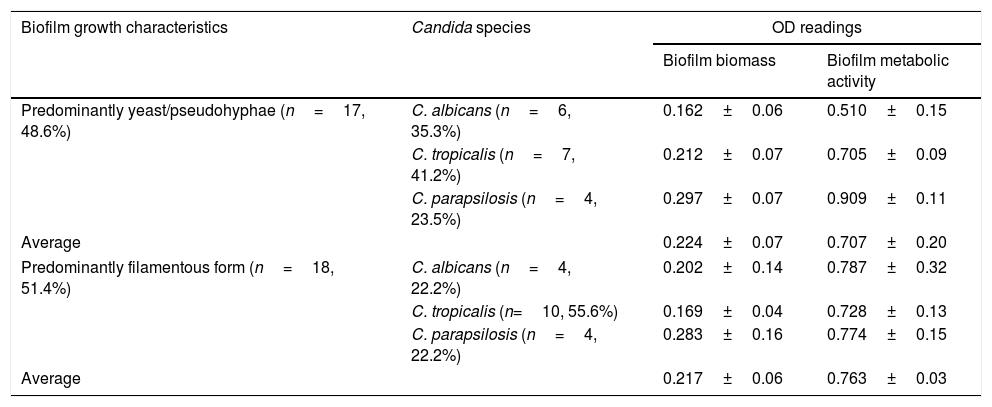
Growth morphology, biomass, and metabolic activity of the biofilm developed by different Candida isolates.
| Biofilm growth characteristics | Candida species | OD readings | |
|---|---|---|---|
| Biofilm biomass | Biofilm metabolic activity | ||
| Predominantly yeast/pseudohyphae (n=17, 48.6%) | C. albicans (n=6, 35.3%) | 0.162±0.06 | 0.510±0.15 |
| C. tropicalis (n=7, 41.2%) | 0.212±0.07 | 0.705±0.09 | |
| C. parapsilosis (n=4, 23.5%) | 0.297±0.07 | 0.909±0.11 | |
| Average | 0.224±0.07 | 0.707±0.20 | |
| Predominantly filamentous form (n=18, 51.4%) | C. albicans (n=4, 22.2%) | 0.202±0.14 | 0.787±0.32 |
| C. tropicalis (n=10, 55.6%) | 0.169±0.04 | 0.728±0.13 | |
| C. parapsilosis (n=4, 22.2%) | 0.283±0.16 | 0.774±0.15 | |
| Average | 0.217±0.06 | 0.763±0.03 | |
Determination of minimum inhibitory concentrations (MICs). For planktonic MIC determination, a microbroth dilution method was performed in accordance with the guidelines of the Clinical and Laboratory Standards Institute M27-A3 document.4 A stock solution of 10μg/ml of AbA (Clontech, Mountain View, CA, USA) dissolved in dimethyl sulfoxide (Fluka Chemika, USA) was used to prepare a dilution range from 8 to 0.0156μg/ml. The planktonic MIC is defined as the lowest concentration of AbA inhibiting the growth of the tested yeast after incubating for 48h.
Assessment of biofilm biomass and metabolic activity. The formation of Candida biofilm was performed using a modified protocol of Jin et al.9 Briefly, several yeast colonies were suspended in RPMI 1640 medium and adjusted to approximately 107cells/ml (OD520=0.38) using a Genesys spectrophotometer (Thermo Scientific, USA). The yeast suspension (100μl) was then inoculated into each designated well in a flat-bottomed polystyrene microtiter plate (Corning, USA) and incubated at 37°C for 1.5h with constant shaking (75rpm). After the attachment phase, unattached cells were removed and the wells were washed thrice with sterile phosphate buffered saline (PBS). Fresh RPMI 1640 medium was then added into each well and incubation continued for 24h.
For determining the biofilm biomass, crystal violet (CV) assay was performed as described by Reisner et al.22 Biofilm cultures (after 24h of growth) were stained with 0.1% (v/v) CV solution for 15–20min. After washing thrice with PBS, the CV stain was dissolved thoroughly in 96% (v/v) ethanol. The absorbance (A585nm) of the resultant solution was measured using an automated microplate reader (Epoch, BioTek, USA). Isolates giving absorbance readings of ≥0.1 at 585nm in the CV assay were regarded to have good adherence and biofilm forming ability.22 The experiments were repeated in quadruplicate. For determining the biofilm metabolic activity, XTT (2, 3-bis (2-methoxy-4-nitro-5-sulfophenyl)-5-[(phenylamino) carbonyl]-2H-tetrazolium hydroxide) reduction assay was used. Biofilm cultures were incubated with XTT-menadione-PBS solution in the dark for 1h at 37°C and the absorbance of the resultant solution (100μl) in each well was determined at 490nm. The experiment was performed in duplicate for each isolate. The absorbance readings of the negative control wells (containing no cells) were subtracted from the absorbance readings of the test wells.
Determination of biofilm MICs. For MIC determination, 24h-biofilm cultures in a microtiter plate were incubated with a series of AbA solutions ranging from 128 to 0.25μg/ml at 37°C for 48h. Antifungal-free wells and biofilm-free wells were included to serve as positive and negative controls, respectively. The biofilm MIC is defined as the lowest concentration of AbA which causes 50% reduction in the biofilm metabolic activity.
Field emission scanning electron microscope (FESEM) analysis. The preparation of the biofilm cultures of C. albicans SC5314 strain (biofilm MIC, 32μg/ml) for FESEM analysis was performed as described by Khan and Ahmad10 and Jayatilake et al.8 Biofilm cultures were developed on nucleopore membranes (0.2μm pore size, 13mm in diameter; Whatman, USA) which had been pre-sterilized by UV irradiation and placed in different flat-bottom wells of a 24-well plate (SPL Life Sciences, Korea). Briefly, yeast suspension (107cells/ml) was inoculated onto each membrane, and the cells were adhered on the surface for 1.5h. The cells were washed and incubated with fresh SD minimal medium at 37°C for 24h with shaking (75rpm). The biofilm cultures were then exposed to different concentrations of AbA (0, 8, 32μg/ml) for 2.5h. For FESEM analysis, the biofilm cultures were fixed overnight with 4% glutaraldehyde in cacodylate buffer, and treated with 2% (w/v) osmium tetroxide for 1h. The membranes were then subjected to dehydration in a series of ethanol solutions (10–100%), ethanol: acetone mixtures (3:1, 1:1 and 1:3), and acetone. After drying in CO2 (CPD 7501, Polaron, UK), each membrane was mounted onto an aluminum stub and coated with gold (Biorad E5100 Series 11, USA) for observation under a FEI Quanta 450 FEG (USA) field emission scanning electron microscope (FESEM). The diameters of the hyphae and yeast cells were determined using the measurement tools of FESEM.
Statistical analysis. All statistical analyses were performed using Statistical Package for the Social Sciences ver. 23.0 (SPSS Inc., Chicago, IL). Due to the small sample size, Mann Whitney test12 was used to compare the biofilm biomass and the metabolic activity among Candida species. A p-value lower than 0.05 was considered as statistically significant.
Results and discussionA total of 35 (29.7%) of 118 Candida isolates were regarded as biofilm producers based on the CV assay, of which the majority were isolates of C. parapsilosis (8/12 isolates, 66.7%), followed by C. tropicalis (17/38 isolates, 44.7%), and C. albicans (10/48 isolates, 20.8%). Interstrain and interspecies variability in biofilm morphology was observed microscopically. Amongst the 35 biofilm-producing isolates, 17 (48.6%) developed only yeast/blastospores or pseudohyphae, while the remaining 18 (51.4%) isolates exhibited a dense network of biofilms consisting mainly of filamentous cells (Table 1). Almost half (55.6%) of the biofilm producers showing filamentous growth were C. tropicalis. The results of CV assay correlated with microscopic observations, as C. glabrata, C. nivariensis, and C. kefyr isolates were poor biofilm producers, exhibiting no filamentation and producing low biofilm biomass. Amongst the biofilm-producing isolates, C. parapsilosis isolates showed the highest biofilm biomass, as assessed by CV assay; however, no significant difference was noted when compared to C. albicans and C. tropicalis (p>0.05). Nevertheless, the biofilm metabolic activity of C. parapsilosis was significantly higher compared to that of C. albicans (p=0.014) and C. tropicalis (p=0.012).
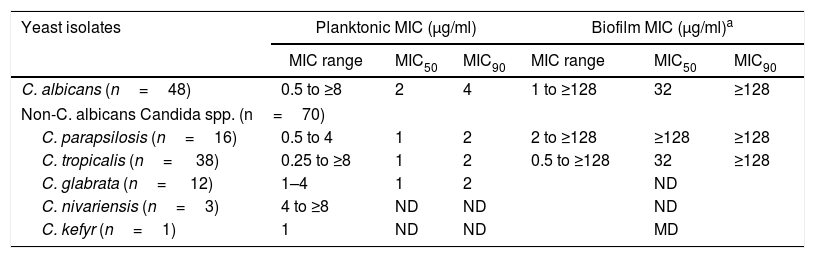
Table 2 shows the in vitro susceptibilities of the planktonic and biofilm cultures of Candida isolates to AbA. The planktonic MICs for 118 Candida isolates ranged from 0.25 to ≥8μg/ml, with the MIC50 and MIC90 being 1 and 4μg/ml, respectively. Eleven (22.4%) isolates of C. albicans out of 49, two out of 38 isolates of C. tropicalis, one out of 16 isolates of C. parapsilosis, and one out of 12 C. glabrata isolates had MICs of ≥4μg/ml. C. albicans isolates had apparently a twofold increase in MIC50 (2μg/ml) and MIC90 (4μg/ml) in the planktonic form when compared to the rest of the species. Three C. nivariensis isolates exhibited high planktonic MICs (ranging from 4 to ≥8μg/ml). Overall, the AbA planktonic and biofilm MICs of Candida yeasts in this study (Table 1) were higher than those reported by Tan and Tay in a previous study.25 This is probably due to the inclusion of a larger number of Candida isolates in this study.
In vitro susceptibilities to AbA of the planktonic and biofilm cultures of Candida isolates.
| Yeast isolates | Planktonic MIC (μg/ml) | Biofilm MIC (μg/ml)a | ||||
|---|---|---|---|---|---|---|
| MIC range | MIC50 | MIC90 | MIC range | MIC50 | MIC90 | |
| C. albicans (n=48) | 0.5 to ≥8 | 2 | 4 | 1 to ≥128 | 32 | ≥128 |
| Non-C. albicans Candida spp. (n=70) | ||||||
| C. parapsilosis (n=16) | 0.5 to 4 | 1 | 2 | 2 to ≥128 | ≥128 | ≥128 |
| C. tropicalis (n= 38) | 0.25 to ≥8 | 1 | 2 | 0.5 to ≥128 | 32 | ≥128 |
| C. glabrata (n= 12) | 1–4 | 1 | 2 | ND | ||
| C. nivariensis (n=3) | 4 to ≥8 | ND | ND | ND | ||
| C. kefyr (n=1) | 1 | ND | ND | MD | ||
The biofilm MICs were determined for 10 isolates of C. albicans, 17 isolates of C. tropicalis and 8 isolates of C. parapsilosis.
Planktonic MIC: The lowest concentration of AbA which inhibits the visible growth of the yeast after incubation at 37°C for 48h.
Biofilm MIC: The lowest concentration of AbA which causes 50% reduction in the metabolic activity.
MIC50 and MIC90 values were only calculated for Candida species with five or more isolates.
ND: not determined.
The biofilm MICs for 35 biofilm-producing Candida isolates ranged from 1 to ≥128μg/ml, with the MIC50 and MIC90 being 32 and ≥128μg/ml, respectively (Table 2). Overall, the AbA biofilm MICs for all Candida isolates in this study were at least twofold higher when compared with their respective planktonic MICs. C. parapsilosis had the highest biofilm MIC50. The reduced susceptibility to several antifungal drugs of Candida biofilm cultures in comparison to planktonic cultures has been related to several mechanisms of resistance such as reduced growth rate, expression of resistance genes, efflux pumps, and the existence of ‘persister’ cells.14,21
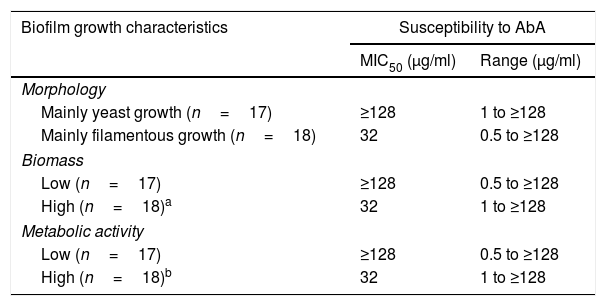
Table 3 shows the effects of AbA on biofilm growth morphology, biomass, and metabolic activity. Candida isolates exhibiting filamentous growth, high biomass and high metabolic activity showed at least fourfold lower MIC50 (32μg/ml) in comparison to those exhibiting yeast growth, lower biomass and metabolic activity (≥128μg/ml). In a recent study, Marcos-Zambrano et al.13 reported higher susceptibility of Candida isolates with high biofilm metabolic activity to micafungin. The authors explained that since a high degree of fungal wall biosynthesis was involved in biofilm formation, this may cause the yeasts to become more susceptible to the antifungal drug. A similar explanation may be applied to this study, as higher susceptibility to AbA was noted in biofilm cultures exhibiting high metabolic activity (Table 2). The high metabolic activity in Candida biofilm cultures is often correlated with filamentous growth of Candida biofilm (and thus higher biomass in comparison to yeast growth), thus it is not surprising a higher susceptibility to AbA in biofilm cultures exhibiting filamentous growth and high biomass (Table 3).
Effect of AbA on the biofilm growth morphology, biomass, and metabolic activity.
| Biofilm growth characteristics | Susceptibility to AbA | |
|---|---|---|
| MIC50 (μg/ml) | Range (μg/ml) | |
| Morphology | ||
| Mainly yeast growth (n=17) | ≥128 | 1 to ≥128 |
| Mainly filamentous growth (n=18) | 32 | 0.5 to ≥128 |
| Biomass | ||
| Low (n=17) | ≥128 | 0.5 to ≥128 |
| High (n=18)a | 32 | 1 to ≥128 |
| Metabolic activity | ||
| Low (n=17) | ≥128 | 0.5 to ≥128 |
| High (n=18)b | 32 | 1 to ≥128 |
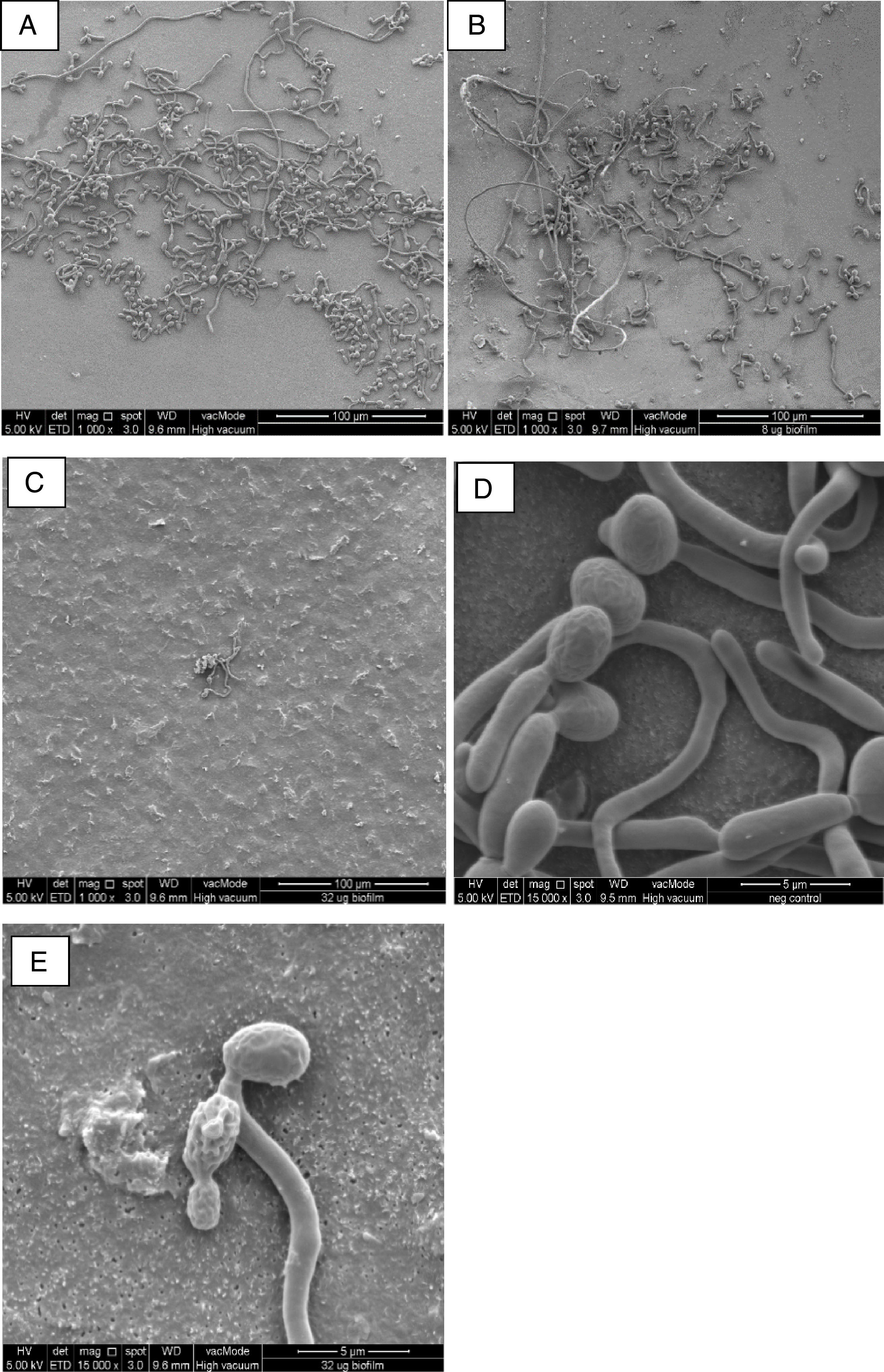
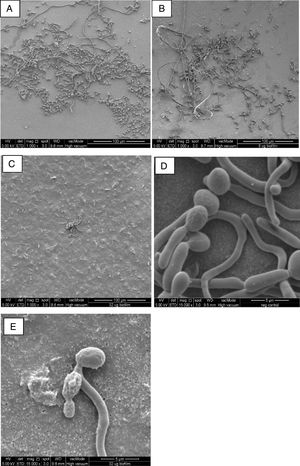
In comparison to the negative control (Fig. 1A), FESEM analysis revealed filament detachment in C. albicans SC5314 biofilm culture when treated with 8μg/ml AbA (0.25× of its biofilm MIC, Fig. 1B). Upon treatment with 32μg/ml AbA (1xMIC), almost the complete elimination of filamentous cells was shown as only scanty yeast cells were seen on the nucleopore membrane (Fig. 1C). Untreated yeast cells were mostly intact with well-defined and smooth surfaces (Fig. 1D), while rough and distorted cell surfaces were observed in the AbA-treated cells (Fig. 1E). Smaller diameter was also noted in the filaments of treated C. albicans biofilm cultures; however, the difference was not significant when compared to the untreated culture, (p>0.05). The precise mechanism for this phenomenon is not known but susceptibility to AbA has been related to lipid rafts which are important for Candida biofilm formation.11
FESEM images of C. albicans biofilm cultures upon exposure to AbA for 2.5h. (A) Untreated biofilm culture (1000×); (B) biofilm culture treated with 8μg/ml AbA (1000×); (C) biofilm culture treated with 32μg/ml AbA (1000×); (D) untreated biofilm culture (15,000×); (E) biofilm culture treated with 32μg/ml AbA (15,000×).
In conclusion, our results show that in comparison with planktonic cultures, AbA seems to be less active against Candida biofilms. The antifungal effects of AbA against Candida biofilm cultures should be further explored at the molecular level since little is known about its mechanism of action. The mechanism behind the higher susceptibility to AbA of Candida biofilm cultures exhibiting filamentous form and higher biomass/metabolic activity should also be explored.
Conflict of interestThe authors report no conflict of interest.
This study was supported by the research grants FP035-2014A, provided by Ministry of Higher Education (MOHE), PPP045-2013B and PG178-2015B, provided by University of Malaya. We would like to acknowledge Prof. Ng KP, Ms. Ng Shiang Lin, and Ms. Azadeh Lotfalikhani for providing the clinical isolates used in this study.