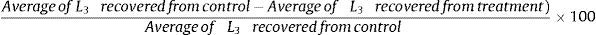Geohelminths are parasites that stand out for their prevalence and wide distribution, depending on the soil for their transmission.
AimsThe aim of this work was to evaluate the predatory capacity of the fungal isolate of the genus Duddingtonia (CG768) on third stage larvae (L3) of Ancylostoma spp. in beach sand under laboratory conditions.
MethodsIn the assay A five treatment groups and 1 control group were formed. The treatment groups contained 5000, 10,000, 15,000, 20,000 or 25,000 chlamydospores of the fungal isolate and 1000 Ancylostoma spp. L3 in pots containing 30g of sand. The control group (without fungus) contained only 1000 Ancylostoma spp. L3 and distilled water in pots with 30g of sand.
ResultsEvidence of predatory activity was observed at the end of 15 days, where we observed the following percentages of reduction of L3: Group 1 (4.5%); Group 2 (24.5%); Group 3 (59.2%); Group 4 (58.8%); Group 5 (63%). However, difference was noted (p<0.01) only at concentrations 15,000, 20,000 and 25,000 in relation to control group. In the assay B two groups were formed in Petri dishes of 9cm in diameter containing agar water 2% medium. In the treated group, each Petri dish contained 500 Ancylostoma spp. L3 and 5g of sand containing the isolate CG 768 at a concentration of 25,000 chlamydospores/g of sand, and the control group (without fungus) contained only 500 L3. At the end of 7 days the non-predation L3 of Petri dishes using the method of Baermann were recovered. Difference (p<0.01) between groups on reducing the average number of Ancylostoma spp. L3 (percent reduction of 84%) was observed.
ConclusionsThe results of this study confirm earlier work on the efficiency of the Duddingtonia genus in the control of Ancylostoma spp. infective larvae.
Los geohelmintos son parásitos que destacan por su prevalencia y amplia distribución, puesto que su transmisión depende del suelo.
ObjetivosEl objetivo del presente estudio fue evaluar la capacidad predatoria de aislamientos fúngicos del género Duddingtonia (CG768) sobre las larvas de estadio 3 (L3) de Ancylostoma spp. en arena de playa, en condiciones de laboratorio.
MétodosEn el ensayo A se formaron 5 grupos de tratamiento y un grupo de control. Los grupos de tratamiento contenían 5000, 10.000, 15.000, 20.000 o 25.000 clamidosporas del aislamiento fúngico y 1000 larvas L3 de Ancylostoma spp. en recipientes con 30g de arena. Los recipientes del grupo de control (sin clamidosporas) solo contenían 1000 larvas L3 de Ancylostoma spp. y agua destilada con 30g de arena.
ResultadosAl término de 15 días, fue evidente la actividad predatoria, con los porcentajes siguientes de reducción de larvas L3: grupo 1 (4.5%); grupo 2 (24.5%); grupo 3 (59.2%); grupo 4 (58.8%), y grupo 5 (63%). Sin embargo, en relación con el grupo control, solo se identificaron diferencias significativas (p<0.01) a las concentraciones de 15.000, 20.000 y 25.000. En el ensayo B, en placas de Petri de 9cm de diámetro, que contenían un medio de agar agua al 2%, se formaron 2 grupos. En el grupo tratado, cada placa de Petri contenía 500 larvas L3 de Ancylostoma spp. y 5g de arena con el aislamiento CG768 a una concentración de 25.000 clamidosporas/g de arena, y el grupo de control (sin hongo) solo contenía 500 larvas L3. Al cabo de 7 días, utilizando el método de Baermann, a partir de las placas de Petri se obtuvieron larvas L3 no sometidas a predación por el hongo. Entre los grupos se observó una diferencia significativa (p<0.01) en la reducción del número medio de larvas L3 de Ancylostoma spp. (porcentaje de reducción del 84%).
ConclusionesLos resultados del presente estudio confirman los datos de investigaciones previas sobre la eficiencia del género Duddingtonia en el control de las larvas infectantes de Ancylostoma spp.
Geohelminths are parasites that stand out for their prevalence and wide distribution, depending on the soil for their transmission. According to Silva et al.20 some factors are important to evaluate the context of the transmission of these parasites and, among them, the following stand out: (1) the presence of infected animals, in this case stray dogs, (2) the favoritism of the environmental conditions, (3) the presence of eggs and/or larvae in the environment and (4) faecal contamination of the soil.
In this context, the hookworm has a cosmopolitan distribution and is more prevalent in tropical and subtropical regions where the soil presents temperature and moisture conditions suitable for the development of pre-parasitic forms (egg and/or larvae).8,23 On the other hand, the literature has reported that larvae and eggs of these gastrointestinal parasites are commonly found in samples of sand.14,15 Moreover, this environment serves not only as a source of leisure but also presents risks to animal and human health.13,18 Also in relation to this fact, Guimarães et al.9 reported that in several cities of Brazil a considerable canine population circulates through the streets and public squares, where often their habits of defecation contaminate the soil with various types and potentially zoonotic parasitic forms. These authors observed the occurrence of Toxocara spp., and eggs or larvae of Ancylostoma spp. in 69.6% of soil samples collected in public squares. In this context these same authors showed that infected samples of sand from schools or kindergartens were positive only with larvae of Ancylostoma spp. In recent work Silva et al.21 demonstrated contamination of the sand on the beaches of southeastern of Pernambuco state by larvae of Ancylostoma spp.
Regarding the control of these parasites, the same is based on the use of antihelminthic drugs, however, there are problems with parasitic resistance already installed in production animals, and that in the future may represent a problem for the combat of parasitic nematodes of dogs and cats. High resistance of Ancylostoma caninum to anthelmintic-therapy with pyrantel was demonstrated by Kopp et al.10 Thus, much has been researched about the use of biological control in environmental decontamination of eggs and larvae of the genera Ancylostoma and Toxocara spp.7
Biological control by nematophagous fungi decreases the population of parasites in the environment since its action is concentrated in the fecal environment.2,11 Among the nematophagous fungi, we must highlight the fungus Duddingtonia flagrans, characterized by producing several conidia at the ends of conidiophores,25 but mainly by the production of numerous chlamydospores interspersed with vegetative hyphae.19 These spores are thick walled and may be ovoid and elliptical with the size of approximately 25–50mm in length by 10–15mm in width. The nematophagous activity is ensured by adhesive hyphae and dimensional adhesive networks.11 This species has been studied as a biocontrol organism in experiments in vitro and in vivo conditions.6,24 However, there are no reports regarding the biological control using nematophagous fungi on beach sand, representing a new approach.
The aim of this study was to evaluate the effect of different concentrations of chlamydospores of the fungus D. flagrans (isolate CG 768) on the destruction of Ancylostoma spp. L3 on beach sand under laboratory conditions.
MethodsOrganismThe predator nematophagous fungus Duddingtonia flagrans (CG768) obtained from samples of Brazilian soil and of animal feces was used in this experiment. The isolate was stored on integral rice grains inside 5mL BD Vacutainer® glass tubes (Becton Dickinson, Brazil) containing blue silica gel, based on the conservation technique described by Smith and Onions.22 The species was chosen for this study due to the success in the destruction of Ancylostoma spp. third stage larvae observed in previous studies and its great capacity for production of chlamydospores.
Production of chlamydosporesThe chlamydospore production was performed in 2.5L plastic bags with biofilter as described by Maciel et al.12 Corn meal was used as substrate. Bags containing 300g of substrate and 110ml of distilled water were closed and sterilized for 30min at 121°C. The sterilized substrate in each bag was inoculated with six mycelium discs of about 5mm diameter taken from the edges of culture D. flagrans after growth on plates containing agar–water medium 2% during 7 days at 25°C. The bags were then closed to allow growth of D. flagrans on the substrate for 15 days in the dark at 25°C. The substrate was agitated every 5 days until the end of the incubation period to ensure homogeneous mycelial growth. After the incubation period, six samples of 1g of colonized substrate were transferred from each bag to an Erlenmeyer flask containing 10mL of distilled water and 0.2% (v/v) dispersing polysorbate (Tween® 80) and shaken for 2min for dispersion of chlamydospores. Then two aliquots of 10μL were placed in a Neubauer chamber to estimate the number of chlamydospores per gram of substrate.
Ancylostoma spp. L3Ancylostoma spp. L3 was obtained from fresh feces of naturally infected stray dogs, by vermiculite coproculture kept for 10 days at 26°C in a BOD incubator. After this period, active larvae were harvested from culture using the Baermann funnel technique.4 The L3 suspension was homogenized and 3 aliquots of 10μL were placed on a microscope glass slide. Each aliquot was covered with a glass coverslip after adding 10μL of lugol's solution to kill L3. Then, they were counted using a light microscope (40× magnification) and the concentration of the suspension was adjusted for 1000 L3/10μL.
Collection and preparation of sand samplesThroughout the test, sand samples collected in the resort city of Guarapari, state of Espírito Santo were used and submitted regarding the particle size analysis and chemical analysis by the methodology of Ruiz16 (coarse sand: 76%; fine sand: 22%; silt 0%; clay: 2%; sodium: 342mg/dm3; phosphorus: 34.15mg/dm3; potassium: 20mg/dm3; calcium: 0.46cmol/dm3; magnesium: 0.32cmol/dm3; aluminum: 0cmol/dm3; pH: 8.2). The beaches of this resort are much visited by tourists throughout the year, and in this context we chose to perform this work. Samples were collected at a depth of 0–20cm and stored in plastic bags. The samples were autoclaved for 1h in order to eliminate possible pre-parasitic forms.
Experimental assayAssay AFive treatment groups and one control group were formed with 6 replicates for each group in accordance with the following description: Group 1 (5000 chlamydospores of the fungus D. flagrans per gram of sand and 1000 Ancylostoma spp., L3); Group 2 (10,000 chlamydospores of the fungus D. flagrans per gram of sand and 1000 Ancylostoma spp., L3); Group 3 (15,000 chlamydospores of the fungus D. flagrans per gram of sand and 1000 Ancylostoma spp. L3); Group 4 (20,000 chlamydospores of the fungus D. flagrans per gram of sand and 1000 Ancylostoma spp. L3); Group 5 (25,000 chlamydospores of the fungus D. flagrans per gram of sand and 1000 Ancylostoma spp. L3) and group 6 (control) containing only distilled water and 1000 L3.
The assay consisted of 30g of sand inside of transparent polypropylene pots (PP size). The sand in each pot was artificially infected with 1000 Ancylostoma spp. L3. Then the pots were closed with their own plastic lids and incubated for 15 days in the dark at 25°C. Every three days the pots were shaken to promote interaction between microorganisms. At the end of the incubation period the larvae were recovered from the sand by the method of Baermann and counting as previously described.
Assay BTwo groups were formed into Petri dishes of 9cm in diameter containing 2% water–agar medium, with 6 replicates for each group. In the treated group, each Petri dish contained 500 Ancylostoma spp. L3 and 5g of sand containing the isolated CG 768 at a concentration of 25,000 chlamydospores/g of sand, and the control group (without fungus) contained only 500 L3 in the plates with WA 2%. The plates were maintained for 7 days in a BOD incubator in the dark at 25°C. At the end of this period, the non-predated L3 from the content of Petri dishes by the method of Baermann were recovered.
Statistical analysisThe data obtained in the assays A and B were statistically interpreted by analysis of variance in levels of significance of 1% probability.3 The predation efficiency of L3 compared to control was evaluated by Tukey's test at 1% probability. Subsequently the percent reduction from the average of L3 was calculated according to the following formula:
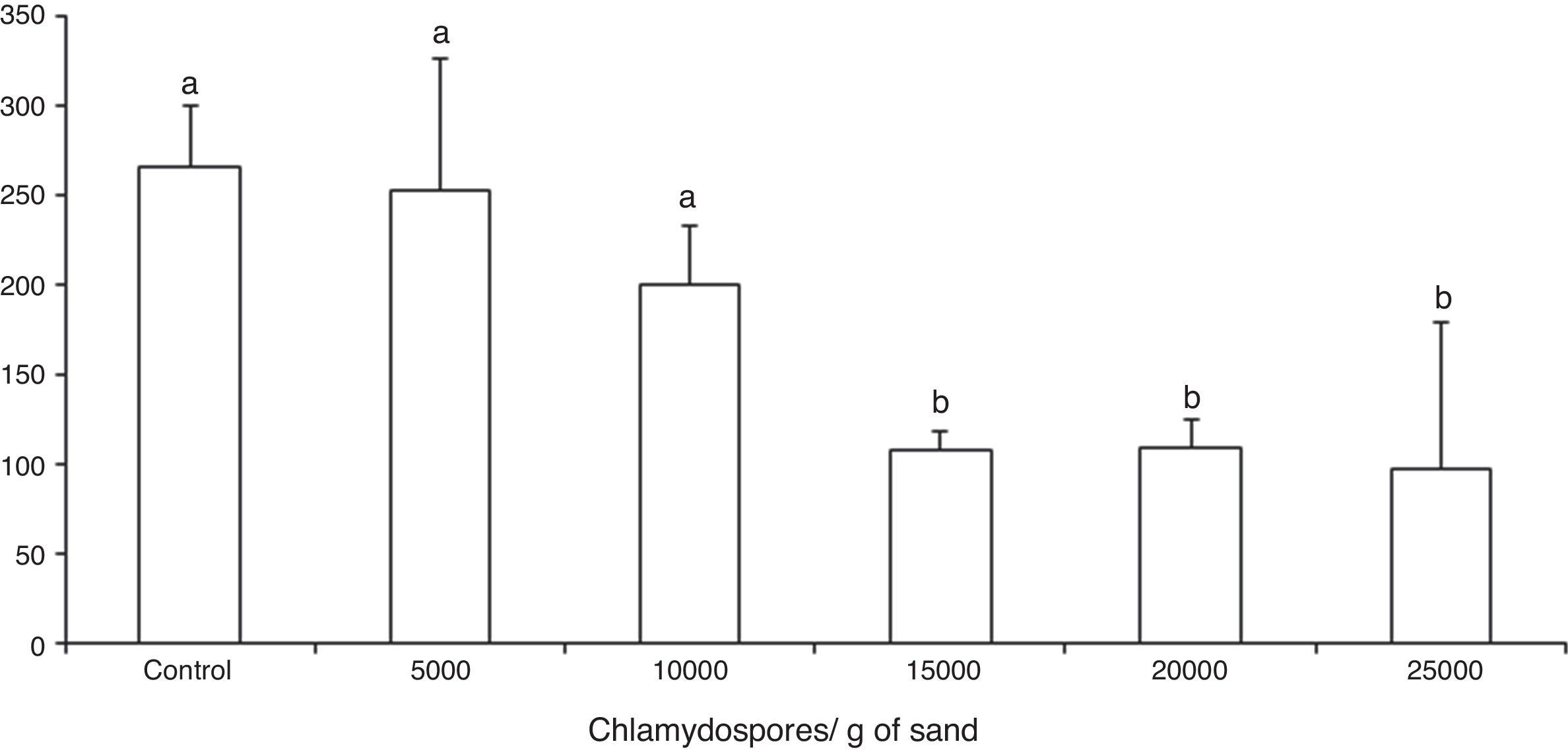
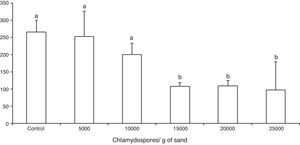
ResultsThe tested fungal isolate D. flagrans (CG768) was able to prey on the Ancylostoma spp. L3 in both in vitro experimental assays. In the assay A, the proof of predatory activity was observed at the end of the experiment (15 days), where we observed the following percentages of reduction of L3 for each concentration of chlamydospores used: Group 1 (4.5%); Group 2 (24.5%); Group 3 (59.2%); Group 4 (58.8%); Group 5 (63%). However, a statistical difference (p<0.01) was noted only at concentrations 15,000, 20,000 and 25,000 in relation to the control group (Fig. 1).
Average number of non-preyed infective larvae of Ancylostoma spp. recovered of sand by Baermann method on the fifteenth day of treatment after interaction with the fungus Duddingtonia flagrans (CG768) and control (without fungus). Bars represent the standard deviation. Asterisk denotes difference (p<0.01) of the treated groups compared to control group.
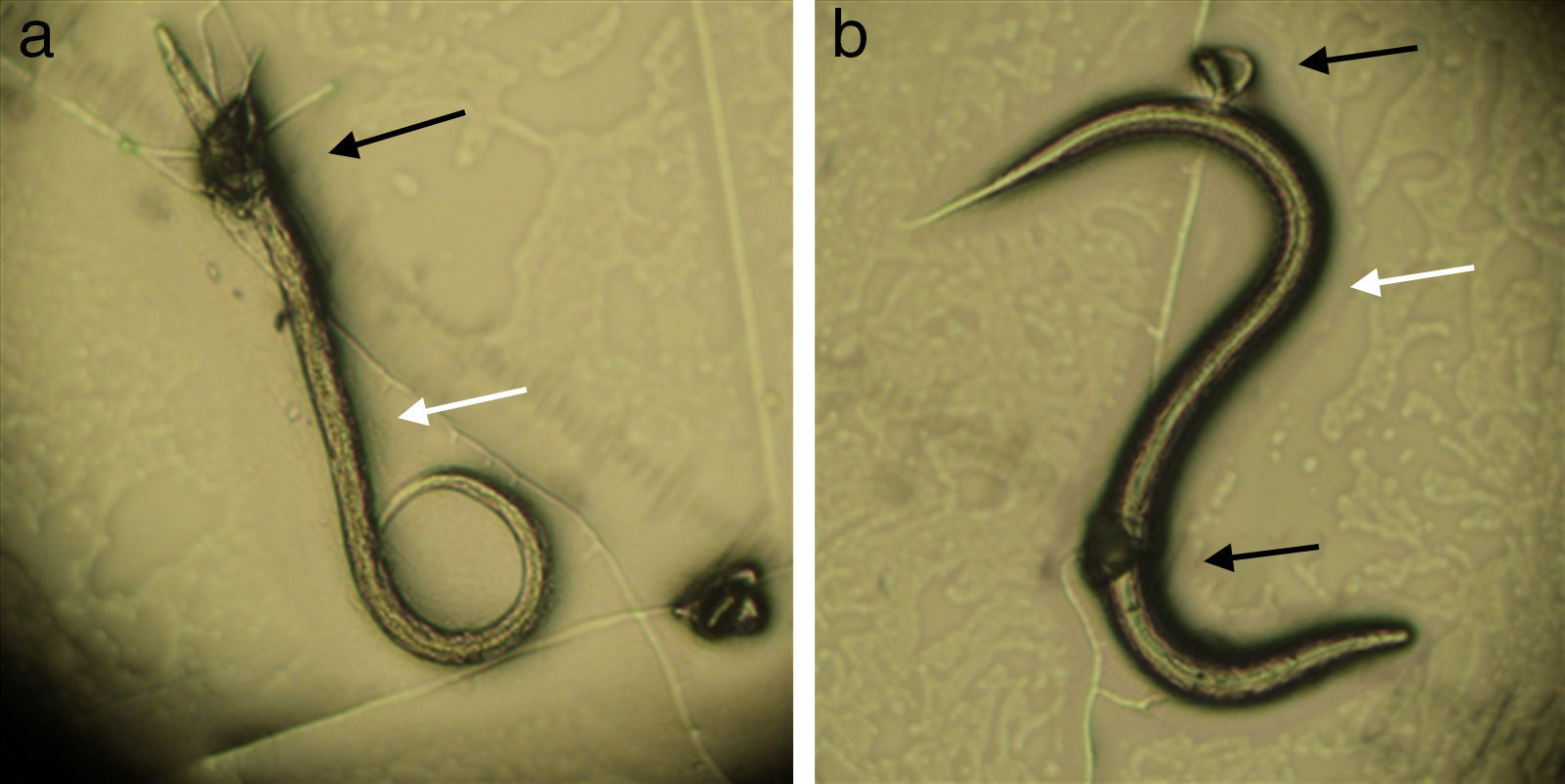
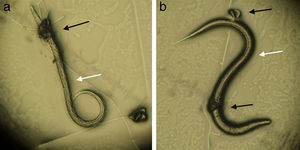
In assay B, a significant difference (p<0.01) was observed between the treated and control group in reducing the average number of Ancylostoma spp. L3 (reduction percentage of 84%). This fact proves that the fungus was able to establish itself in the sand from the substrate used, since, after 15 days mixed with sand, the inoculum was still viable and infective. The plates of the treated group were examined under the microscope during the first 24h, and formation of traps and predated larvae were observed (Fig. 2).
DiscussionSands of public areas represent a potential source of transmission to human and animal population because of being frequently infested by zoonotic parasite eggs in the faeces of dogs that are commonly infected5,17 and, in this context, Ancylostoma spp. Thus, the results of this work demonstrate that the nematophagous fungus D. flagrans reduced the number of L3Ancylostoma spp. in beach sand and therefore this work is justified in the context of the use of new approaches that can contribute to environmental decontamination.
The literature mentions some reports about the successful use of nematophagous fungi in the control of potential zoonotic geohelminths, especially in an experimental model with dogs.1,7 On the other hand, most studies are carried out under laboratorial conditions and even partially natural conditions, since the viability of tested nematophagous fungi after passage through the gastrointestinal tract is observed. Carvalho et al.7 demonstrated that the fungus D. flagrans (AC001) was able to withstand the passage through the gastrointestinal tract of dogs and was viable in predation on Ancylostoma spp L3 under laboratory conditions. In that work, using another approach, the authors observed that the administration of 0.5g/10kg of mycelial mass containing the fungus D. flagrans (isolate AC001) was effective in reducing egg counts per gram of faeces and in the recovery of A. caninum larvae in treated animals compared to the control group. In connection with this, in a recent work Araujo et al.1 demonstrated that one isolate of a menatophagous fungus was able to pass through the gastrointestinal tract of dogs and show its predation on Toxocara canis eggs in an in vitro assay.
Regarding the use of nematophagous fungi at ambient conditions, these must be inoculated in the environment (soil) together with a fungal growth substrate, in order to promote their growth and therefore promote their establishment. However, until now there are no studies about the biological control of hookworm in beach sand, serving as a constant source of contamination. In the constitution of the sand used in this work only minerals were shown, without evidence of organic matter that could help in the proliferation of the fungus. Therefore, the main nutrients for the fungus were the substrate (corn meal) and the nematode.
Moreover, there is a lack of studies on the use of different concentrations of chlamydospores. In recent work, Maciel et al.12 reported that in soil microcosms in the different concentrations of the fungus D. flagrans (5000, 10,000, 15,000, 20,000 and 25,000 chlamydospores per gram of soil) concentrations of 10,000–25,000 showed no difference (p>0.01) in its predatory activity on Ancylostoma spp L3. However, these results are not in agreement with the present work, where differences were observed (p<0.01) in all concentrations (5000, 10,000, 15,000, 20,000 and 25,000) of chlamydospores of this same fungal isolate and research group. However, these differences are interesting from the biological point of view and even when dealing with sand and not soil as described above. Furthermore, the authors of the present study suggest that possibly the environment of “soil” may be rich in organic matter and can directly influence the development of fungal isolates.
The results of this study confirm earlier work on the efficiency of the Duddingtonia genus in control of Ancylostoma spp. infective larvae. However, this is the first report of the use of this fungus in beach sand, suggesting that this fungal species may become part of an alternative and complement program to control this nematode on the environment, reducing the use of chemical control agents.
Ethical approvalNone.
FundingFAPEMIG, CAPES and CNPq.
Competing interestsNone declared.
The authors thank the Federal University of Viçosa (UFV) for its professionalism and competence in developing education, research and extension, without which this work would not have been accomplished.