Subsequent to mass vaccination programs against COVID-19, diverse side effects have been described, both at the injection site, such as pain, redness and swelling, and systemic effects such as fatigue, headache, muscle or joint pain. On rare occasions, a lymphadenopathic syndrome may develop, raising the clinical suspicion of a lymphoproliferative disorder. We present the case of a 30-year-old woman who developed self-limiting left axillary lymphadenopathy following COVID-19 vaccination. To date, only seven similar cases with a complete clinicopathological description have been published, and fourteen cases have been notified to the European adverse events databases (Eudravigilance) in relationship with vaccination against COVID-19.
It is important to be aware of this potential complication when a lymphadenopathic syndrome develops following vaccination, to avoid unnecessary treatment.
Tras la vacunación masiva frente a la COVID-19 se han comenzado a describir diversos efectos adversos incluyendo efectos locales en el lugar de la inyección, como dolor, enrojecimiento, hinchazón, etc., y efectos sistémicos como fatiga, dolor de cabeza, dolor muscular o articular. Más infrecuentemente se pueden desarrollar cuadros linfadenopáticos sospechosos clínicamente de proceso linfoproliferativo. Presentamos el caso de una mujer de 30 años que desarrolló linfadenopatía axilar izquierda tras la vacunación contra la COVID-19 con hallazgos histopatológicos de linfadenopatía necrotizante de tipo Kikuchi y resolución espontánea. Hasta el momento se han publicado 7 casos con descripción clinicopatológica completa en la literatura y notificado 14 casos en la Red Europea de Farmacovigilancia en relación con la vacunación.
Es importante tener en cuenta esta entidad en linfadenopatías sospechosas de procesos linfoproliferativos en este contexto, para evitar un tratamiento innecesario.
Since its emergence in 2019, COVID-19 virus has infected people worldwide, resulting in over six million deaths and putting a significant burden on public health services.1 More than a year after the start of this pandemic, numerous vaccines, from messenger RNA (mRNA)-based vaccines to inactivated virus vaccines, have been developed.
Reported side effects of the vaccine range from injection site reactions, such as pain, redness, swelling, etc., to systemic effects such as fatigue, headache, muscle or joint pain. 50–90% of vaccinated subjects reported some degree of adverse reaction,1 although the vast majority were mild.
One of the well-known adverse effects after vaccination is ipsilateral lymphadenopathy, occurring especially with mRNA-based vaccines, when it affects about 15% of people inoculated with this type of vaccine. Some of these cases may raise a suspicion of lymphoproliferative disorder and prompt a lymph node biopsy. In most instances, reactive follicular hyperplasia with paracortical expansion is found. Rarely, sarcoid-like reactions, hemophagocytosis or necrotizing lymphadenitis of the Kikuchi–Fujimoto disease (KFD) type has been described.2,3
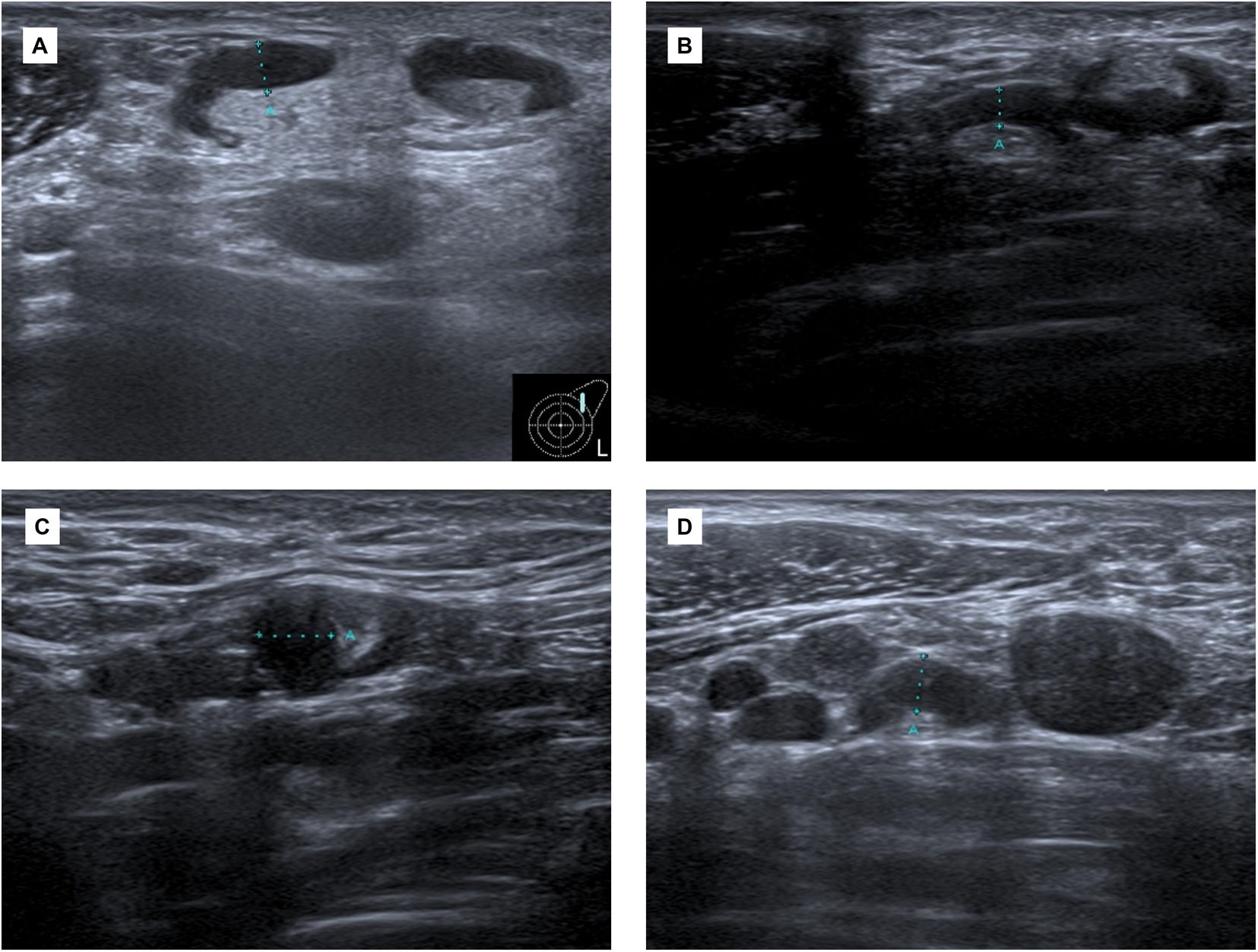
Case reportA 30-year-old woman with no significant past medical history presented with left axillary lymphadenopathy 12 days after the first dose of messenger RNA (mRNA)-based COVID-19 vaccine (Pfizer-BioNTech) in her left arm. She had no fever or other inflammatory symptoms. An ultrasound revealed lymphadenopathies in level I and II of Berg (UN3) reaching up to 5mm of cortical thickness, with reactive ultrasound features (Fig. 1).
(A) Initial ultrasound scan. Adenopathy at Berg's level I and II (UN3, cortical thickness >2.3mm) reaching up to 5mm cortical thickness, with reactive features. (B and C) Follow-up ultrasound scan 6 weeks after first dose. Adenopathy of inflammatory aspect in both axillary regions. The larger ones (UN3) are in level II of Berg in the left axilla and in level I of the right axilla. (D) Ultrasound scan (more than six weeks after the second dose). Increase in the number and size of the left adenopathy in the three axillary levels of Berg. A core needle biopsy was requested.
Laboratory studies performed 2 months after the first dose revealed subtle alterations with mild hypergammaglobulinemia (21.6%, range 11.1–18.8, ratio albumin/globulins 1.29, range 1.5–1.9) and slight elevation of the erythrocyte sedimentation rate (ESR) (26mm). Other parameters were normal including hemoglobin (Hb): 12.6g/dL, white blood cells (WBC): 6.7×103/μL (neutrophils: 4.3×103/μL, lymphocytes: 1.7×103/μL, monocytes: 0.5×103/μL), platelets: 297×103/μL, creatinine: 0.7mg/dl, C-reactive protein (CRP): <0.4mg/dl, and lactate dehydrogenase (LDH): 168Units/L. Liver function tests were normal: ALT 16U/L, AST 24U/L, and ferritin 36ng/ml. Further serological studies did not show ongoing infections for Toxoplasma gondii, Brucella, Coxiella or Bartonella henselae. The tuberculosis QuantiFERON test was also negative. Epstein Barr virus (EBV), Parvovirus B19, cytomegalovirus and herpes simplex virus IgM antibodies were also absent. Antinuclear antibodies (ANA) and rheumatoid factor test were absent. Serology testing for SARS-CoV-2 RNA by real-time reverse transcription polymerase chain reaction was negative.
Approximately one month after first dose, she was inoculated with the second dose of COVID-19 vaccine (Pfizer-BioNTech) in the right arm. The ultrasound performed 20 days later revealed lymph node enlargement with inflammatory features in both axillary regions. The larger ones with a morphology (UN3) were in level II of Berg in the left axilla and in level I of the right axilla (Fig. 1).
More than 6 weeks after the second dose, a new ultrasound scan revealed an increase in the number and size of the left adenopathies in the three axillary levels of Berg (Fig. 1). A core needle biopsy was requested.
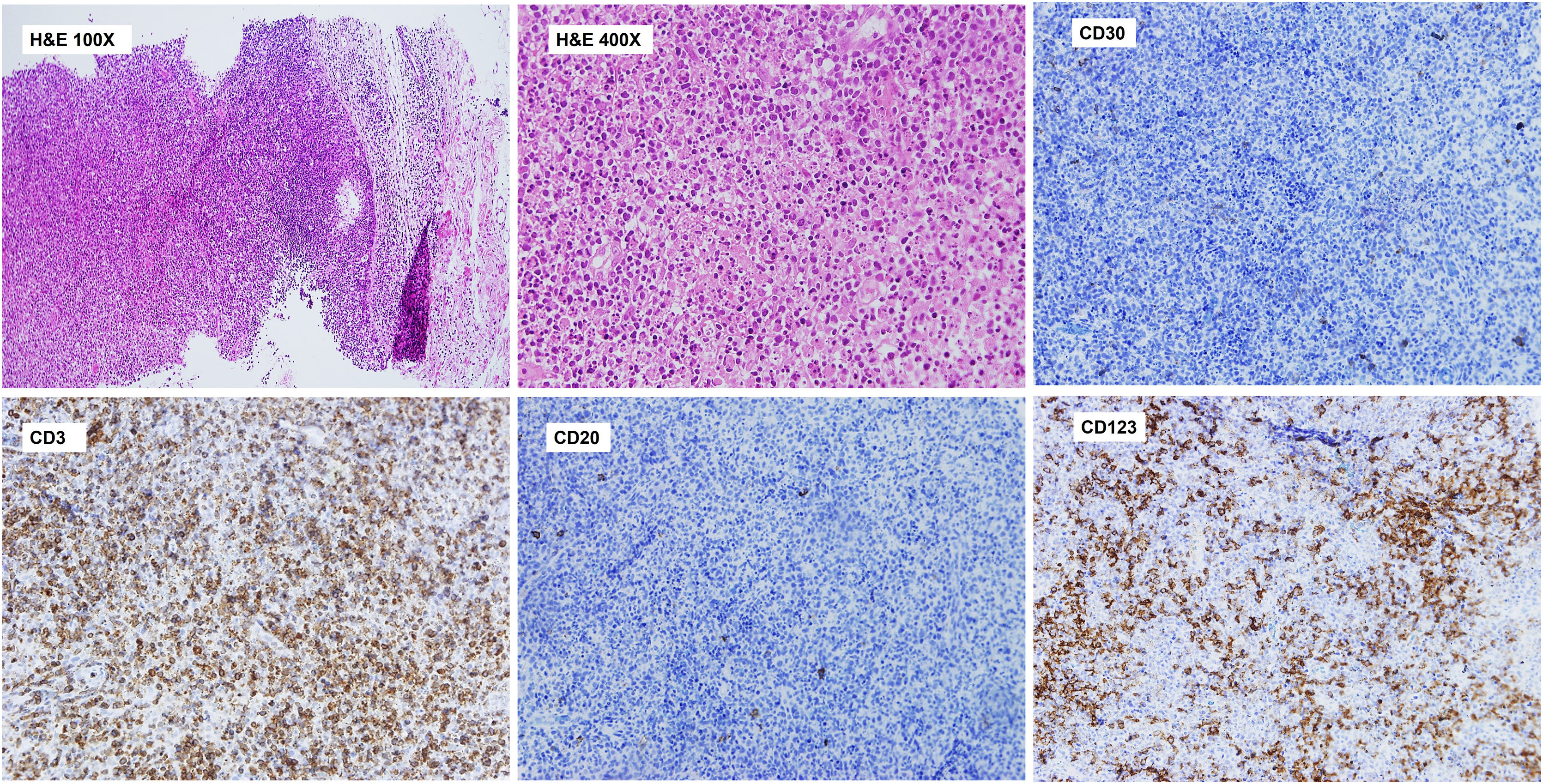
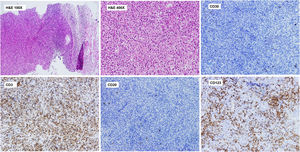
Histopathological study revealed a lymph node parenchyma with paracortical hyperplasia and multiple circumscribed foci of necrosis with abundant karyorrhexis and eosinophilic debris. Numerous histiocytes (some C-shaped) and mature plasmacytoid dendritic cells were seen in association with apoptotic cells. No neutrophils or hematoxylin bodies were observed (Fig. 2).
Axillary lymph node CNB. Histopathological study. Lymph node parenchyma (paracortical area) with multiple circumscribed foci of necrosis with abundant karyorrhexis and eosinophilic debris. Numerous histiocytes (some C-shaped) and plasmacytoid dendritic cells are seen. No neutrophils or hematoxylin bodies were observed. Immunohistochemistry of the lymph node shows a paracortical CD3+ T cell lymphoid reaction and perivascular nests of CD123+ mature plasmacytoid dendritic cells. Significant reduction of CD20+ B cells in the paracortical area. Scattered CD30+ blasts were identified and kappa and lambda showed polytypic populations (not shown).
Immunohistochemical analysis showed a predominance of CD3+ T cells with scattered CD20+ B cells in the paracortical area and patchy CD30 expression. Kappa and lambda showed polytypic plasma cell populations. Importantly, multiple perivascular groups of CD123+ plasmacytoid dendritic cells were identified (Fig. 2).
The lymph node biopsy was consistent with necrotizing lymphadenitis of the Kikuchi type.
The patient was not treated and a follow-up ultrasound six months after the biopsy showed regression of the nodes with a preserved morphology (UN1–UN2) in the left axillary levels. To date the patient is well and with no further symptoms.
DiscussionWe present a case showing a clear temporal association between the inoculation of a COVID-19 mRNA based vaccine and the development of local lymphadenopathies that increased in size after the second dose of the vaccine. Histopathological analysis demonstrated necrotizing lymphadenitis with prominent paracortical hyperplasia and plasmacytoid dendritic cell reaction. These changes are usually seen in so called Kikuchi–Fujimoto lymphadenitis. After follow-up the lymph node reaction regressed spontaneously.
Post-vaccination lymphadenitis has been described with multiple vaccines such as those against smallpox, influenza, varicella zoster, BCG, and pneumococcus. KFD type lymphadenopathy has been described, albeit infrequently, subsequent to the human papillomavirus, influenza vaccines and Japanese encephalitis virus vaccination.3
The association between COVID 19 vaccination and KFD lymphadenopathy as an adverse effect has been reported in Europe, with 14 cases reported at the European adverse events databases (Eudravigilance) and 1 additional case in the Spanish adverse events database FEDRA.4 Furthermore, some case reports have linked KFD type lymphadenopathy with active SARS CoV2 infection.5–10
Kikuchi–Fujimoto disease, also known as histiocytic necrotizing lymphadenitis, is a rare, benign, self-limiting inflammatory disease of unknown etiology, characterized mainly by cervical lymphadenopathy and/or fever. First described in 1972 by Kikuchi and Fujimoto et al., is more commonly observed in the Asian population, although it is present globally and usually affects young adult females. The pathogenesis is unknown, but an autoimmune/autoinflammatory basis has been suggested. The clinical presentation, course and laboratory and histologic findings suggest that immune responses by T cells, histiocytes and cytokines contribute to its pathogenesis.11,12 In our case there was an increase in the population of mature CD123+ pDCs (plasmacytoid dendritic cells), a major component of the type I IFN producing cells of the innate immune system.
Collectively these data suggest that COVID-19 related antigens, either mRNA or proteins, may elicit a post-viral immune dysregulation with increased IFN, producing plasmacytoid dendritic cells and necrotizing lymphadenitis of the Kikuchi–Fujimoto disease type in some cases.
To date, however, only 7 cases with a complete clinico-pathological description of Kikuchi–Fujimoto disease following SARS CoV2 vaccination have been reported,2,3,13–16 representing approximately 33% (7/21) of cases with lymphadenopathy associated with COVID-19 vaccine with available histopathological evaluation.2,3,13–16 We realize, though, that this figure could be an overestimation of the true prevalence of this association due to both a publication bias and the absence of histopathological analysis in most cases of clinically acute lymph node enlargements in which malignancy is not suspected on echography.
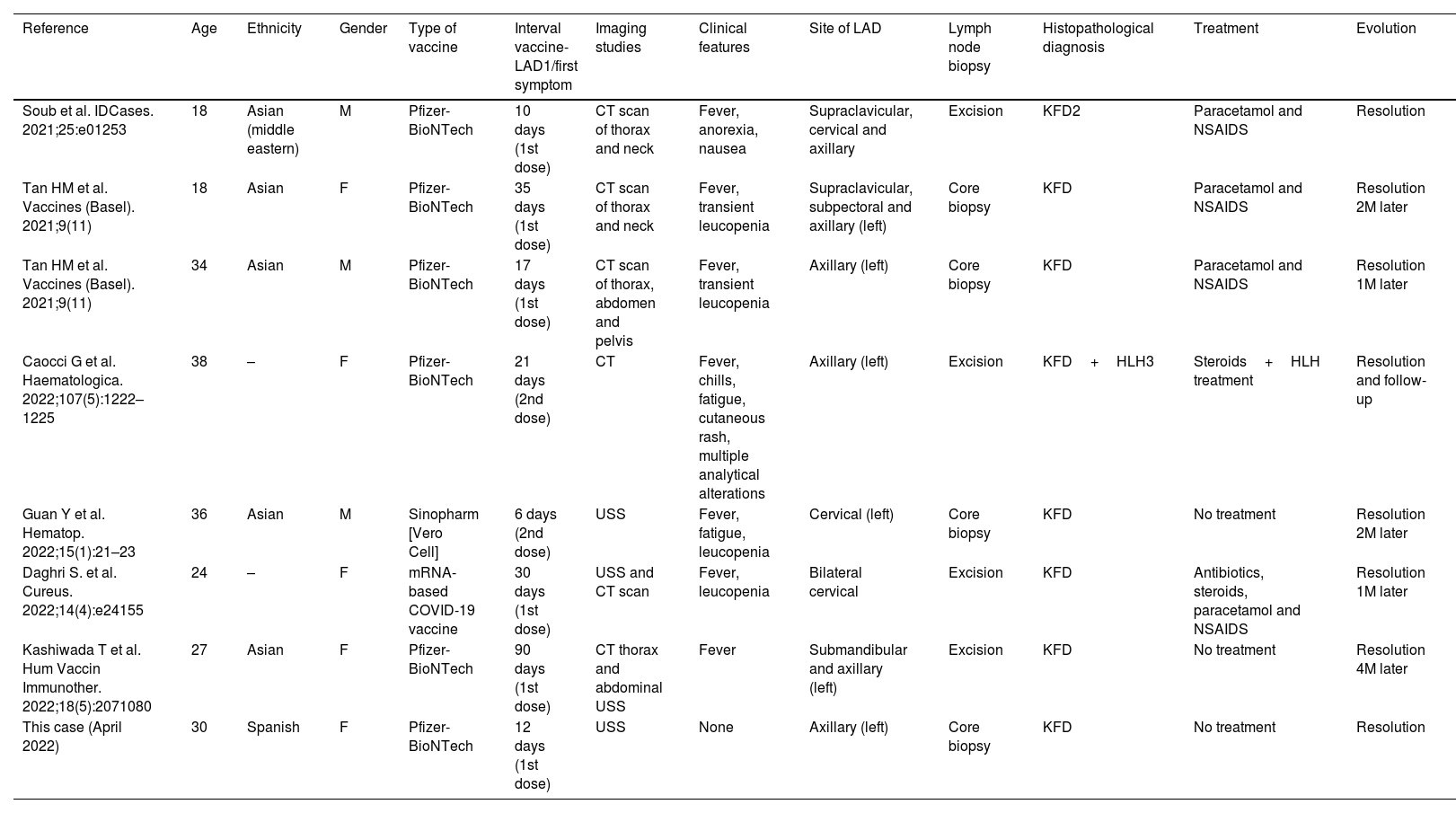
A summary of the clinical and pathological findings is shown in Table 1.
Details from the patients diagnosed as Kikuchi–Fujimoto disease following SARS CoV2 vaccination to date.
| Reference | Age | Ethnicity | Gender | Type of vaccine | Interval vaccine-LAD1/first symptom | Imaging studies | Clinical features | Site of LAD | Lymph node biopsy | Histopathological diagnosis | Treatment | Evolution |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Soub et al. IDCases. 2021;25:e01253 | 18 | Asian (middle eastern) | M | Pfizer-BioNTech | 10 days (1st dose) | CT scan of thorax and neck | Fever, anorexia, nausea | Supraclavicular, cervical and axillary | Excision | KFD2 | Paracetamol and NSAIDS | Resolution |
| Tan HM et al. Vaccines (Basel). 2021;9(11) | 18 | Asian | F | Pfizer-BioNTech | 35 days (1st dose) | CT scan of thorax and neck | Fever, transient leucopenia | Supraclavicular, subpectoral and axillary (left) | Core biopsy | KFD | Paracetamol and NSAIDS | Resolution 2M later |
| Tan HM et al. Vaccines (Basel). 2021;9(11) | 34 | Asian | M | Pfizer-BioNTech | 17 days (1st dose) | CT scan of thorax, abdomen and pelvis | Fever, transient leucopenia | Axillary (left) | Core biopsy | KFD | Paracetamol and NSAIDS | Resolution 1M later |
| Caocci G et al. Haematologica. 2022;107(5):1222–1225 | 38 | – | F | Pfizer-BioNTech | 21 days (2nd dose) | CT | Fever, chills, fatigue, cutaneous rash, multiple analytical alterations | Axillary (left) | Excision | KFD+HLH3 | Steroids+HLH treatment | Resolution and follow-up |
| Guan Y et al. Hematop. 2022;15(1):21–23 | 36 | Asian | M | Sinopharm [Vero Cell] | 6 days (2nd dose) | USS | Fever, fatigue, leucopenia | Cervical (left) | Core biopsy | KFD | No treatment | Resolution 2M later |
| Daghri S. et al. Cureus. 2022;14(4):e24155 | 24 | – | F | mRNA-based COVID-19 vaccine | 30 days (1st dose) | USS and CT scan | Fever, leucopenia | Bilateral cervical | Excision | KFD | Antibiotics, steroids, paracetamol and NSAIDS | Resolution 1M later |
| Kashiwada T et al. Hum Vaccin Immunother. 2022;18(5):2071080 | 27 | Asian | F | Pfizer-BioNTech | 90 days (1st dose) | CT thorax and abdominal USS | Fever | Submandibular and axillary (left) | Excision | KFD | No treatment | Resolution 4M later |
| This case (April 2022) | 30 | Spanish | F | Pfizer-BioNTech | 12 days (1st dose) | USS | None | Axillary (left) | Core biopsy | KFD | No treatment | Resolution |
LAD: lymphadenopathy; KFD: Kikuchi–Fujimoto disease; HLH: hemophagocytic lymphohistiocytosis; M: month.
In summary, this condition involves both sexes and has been reported mainly in Asiatic populations (5/7, 70%). The patients were all young adults (mean age 28 years old, range 18–38). The most common sites of involvement were the left axillary nodes (71% of the cases involved axillary lymph nodes) but additional involvement of other lymph node locations has been reported, including submandibular, cervical, supraclavicular and subpectoral nodes. Two cases showed only cervical lymph node involvement. Most patients also presented inflammatory symptoms such as fever. Occasionally, transient leukopenia, as well as symptoms such as fatigue, anorexia, chills, skin rash, hepatosplenomegaly and analytical alterations (elevation of acute phase reactants, alteration in the liver profile) were reported. It is notable that further analysis revealed no infectious processes in any of the patients. No active COVID-19 infection was detected in any case, either by serological or polymerase chain reaction tests.
The interval between the first symptom or lymphadenopathy detection and time of inoculation ranged from 10 to 90 days. In 5 of the 7 cases (71%), symptoms appeared after injection of the first dose of COVID-19 vaccine but in some patients the diagnosis was delayed.
All cases resolved spontaneously, within one to four months from the onset of symptoms and only two patients required immune-modulatory therapy with corticosteroids, due to persistent fever14 and associated hemophagocytic lymphohistiocytosis.13
We present the case of a 30-year-old Spanish woman who developed self-limiting lymphadenopathy following COVID-19 vaccination with histopathological features of necrotizing lymphadenitis of the Kikuchi–Fujimoto type. It is important to be aware of this potential complication when a lymphadenopathic syndrome develops following vaccination, to avoid unnecessary treatment.
Authors’ contributionsGMDJ performed research and wrote the paper. APDB and EGHR provided the radiological images, MGR provided epidemiological data, SMM designed research, performed research and wrote the paper.
Conflict of interestThe authors declare no potential conflict of interest in relationship with the work presented here.
The authors want to acknowledge the Valdecilla Anatomic Pathology technicians for their skillful handling and processing of tissue samples.