Oral squamous cell carcinoma (OSCC) is the most prevalent head and neck cancer. Few studies have analyzed the expression of proteins related to inflammation (COX-2) and tumor progression according to the histological grade of OSCC.
ObjectiveAnalyze the immunohistochemical expression of COX-2, Ki-67 (cell proliferation), Bcl-2/Bax (apoptosis), VEGF, and CD105 (angiogenesis) according to histological grades of OSCC.
Material and methodsThe immunohistochemical expression of COX-2, Ki-67, Bcl-2, Bax, VEGF, and CD105 of 58 cases of OSCC was analyzed. 13 cases of oral mucosa (OM) were analyzed as controls.
ResultsCOX-2, VEGF, CD105, and Ki-67 were higher in OSCC than in OM, particularly in poorly differentiated OSCC (p<0.05). Bax expression was lower in poorly differentiated OSCC (p<0.001). The Bcl-2/Bax ratio was higher in OSCC compared to MO (p<0.05).
ConclusionThere are immunohistochemical differences according to histological grades of OSCC, which could influence clinical behavior.
El carcinoma oral de células escamosas (COCE) es el cáncer de cabeza y cuello más prevalente. Escasos estudios analizan la expresión de proteínas relacionadas a inflamación (COX-2) y progresión tumoral según el grado histológico de COCE.
ObjetivoAnalizar la expresión inmunohistoquímica de COX-2, Ki-67 (proliferación celular), Bcl-2/Bax (apoptosis), VEGF y CD105 (angiogénesis) según grados histológicos de COCE.
Material y métodosSe analizó la expresión inmunohistoquímica de COX-2, Ki-67, Bcl-2, Bax, VEGF y CD105 de 58 casos de COCE. Trece casos de mucosa oral (MO) fueron analizados como control.
ResultadosLas expresiones de COX-2, VEGF, CD105 y Ki-67 fueron mayores en el COCE comparadas con la MO, particularmente en el COCE pobremente diferenciado (p < 0,05). La expresión de Bax fue menor en el COCE pobremente diferenciado (p < 0,001). La razón Bcl-2/Bax fue mayor en COCE comparado con MO (p < 0,05).
ConclusiónExisten diferencias inmunohistoquímicas según grados histológicos de COCE, lo que podría determinar una evolución clínica diferenciada.
Oral cancer is the most prevalent head and neck cancer (HNC).1 About 90% of HNC cases are classified as oral squamous cell carcinoma (OSCC) and represent one of the ten most common cancers worldwide.2 OSCC arises from oral cavity epithelium and its histological features show cellular atypia and squamous differentiation.3 The World Health Organization (WHO) identifies three histologic grades of OSCC based on Broder's grades: Well-Differentiated (WD-OSCC), Moderately Differentiated (MD-OSCC) and Poorly Differentiated (PD-OSCC).4 Although this classification does not correlate with the prognosis of OSCC, the analysis of the histological differentiation contributes to the pathological diagnosis and might reflect tumor aggressivity.5
Several markers are associated with cell proliferation rate, cell survival capacity, angiogenesis and immunosuppression in the tumor microenvironment (TME) of OSCC.6 Interestingly, extensive research has demonstrated a relationship between a persisted expression of the inducible enzyme cyclooxygenase-2 (COX-2) and the development and progression of OSCC.7
COX-2, responsible for the synthesis of prostanoids, is a critical promoter of the inflammatory profile of the TME,8 inducing tumor angiogenesis, cell proliferation, immunosuppression and metastasis.9 COX-2 overexpression raises B-cell lymphoma-2 (Bcl-2) expression in OSCC epithelial cells, which causes marked anti-apoptotic signaling.9 In addition, COX-2 expression promotes new lymphatic vessels and metastasis to lymph nodes, predominantly explained through vascular endothelial growth factor (VEGF) stimulation.10 However, most studies did not examine the correlation between histologic grades of OSCC and biological characteristics of the TME of OSCC, such as inflammatory profile, angiogenesis, apoptosis or cell proliferation.9
We hypothesized that the immunohistochemical expression of COX-2 may increase in less differentiated histologic grades of OSCC and be correlated to markers of cell proliferation (Ki-67), cell survival and apoptosis (Bax and Bcl-2), and tumor vascularity (VEGF and CD105).
Material and methodsPatient samplesAll cases and demographic data between 1994 and 2016 were obtained from the Pathology Department, University of Chile. 71 cases were collected, a sample size similar to previous studies.11–13 Hematoxylin & Eosin (H&E)-stained slides were studied by two pathologists and re-evaluated and diagnosed according to the 2017 WHO classification for OSCC. Previously, a Cohen's Kappa (κ) coefficient was performed between two calibrated pathologists (κ-value=0.64±0.14). Cases were distributed into categories: WD-OSCC (n=20), MD-OSCC (n=24), and PD-OSCC (n=14). Cases from OM were collected from patients undergoing oral surgery for impacted mandibular third molars and benign diseases and disorders such as Sjögren's syndrome and cleft lip (n=13 cases). Finally, we examined clinical and pathological characteristics, such as evolution, clinical aspect, tumor size record according to TNM classification,14 symptomatology, and smoking habits.
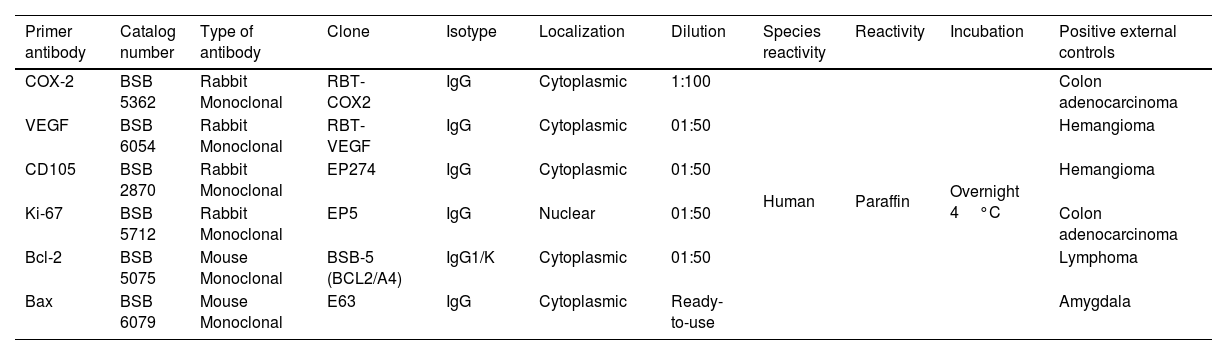
Immunostaining method4-μm-Thick sections from paraffin-embedded blocks in a Reichert-Jung® Biocut Microtome (Mod.1130/Biocut) were placed on positively charged slides (CellPath Ltd., UK). Slides were deparaffinized in xylene for 20minutes (min) and hydrated in graded ethanol for 10min each and later placed in distilled water for 10min. Deparaffinized slides were then put in phosphate-buffered saline (PBS) (pH=7.4±0.2) for 10min. The slides were immersed in 10mM citrate buffer solution (pH 6.0) for antigen retrieval and were transferred to Oster® Food Steamer until boiling point. After 20min they were left to cool to 21°C. Internal peroxidase activity was blocked using 3% hydrogen peroxide and mouse/rabbit immune-detector peroxidase blocker solution (BSB 0003, Bio SB, CA, USA) at room temperature for 20min each. Details of the primary antibodies used are summarized in Table 1. The slides were incubated with mouse/rabbit immuno-detector biotin-link and mouse/rabbit immuno-detector HRP label (BSB 0003) for 20min at room temperature. PBS was used as a wash buffer for 30min when required between each stage. Positive external controls are summarized in Table 1. Negative external controls were samples of external positive controls incubated with PBS (PBS-9990, BION, USA) instead of the primary antibodies. The peroxidase reaction was performed with chromogen 3,3′-diaminobenzidine reagent. All antibodies were from Bio SB, CA, USA. Finally, cases were counterstained with H&E solution for one minute, dehydrated and coverslipped.
Details of primary antibodies employed.
| Primer antibody | Catalog number | Type of antibody | Clone | Isotype | Localization | Dilution | Species reactivity | Reactivity | Incubation | Positive external controls |
|---|---|---|---|---|---|---|---|---|---|---|
| COX-2 | BSB 5362 | Rabbit Monoclonal | RBT-COX2 | IgG | Cytoplasmic | 1:100 | Human | Paraffin | Overnight 4°C | Colon adenocarcinoma |
| VEGF | BSB 6054 | Rabbit Monoclonal | RBT-VEGF | IgG | Cytoplasmic | 01:50 | Hemangioma | |||
| CD105 | BSB 2870 | Rabbit Monoclonal | EP274 | IgG | Cytoplasmic | 01:50 | Hemangioma | |||
| Ki-67 | BSB 5712 | Rabbit Monoclonal | EP5 | IgG | Nuclear | 01:50 | Colon adenocarcinoma | |||
| Bcl-2 | BSB 5075 | Mouse Monoclonal | BSB-5 (BCL2/A4) | IgG1/K | Cytoplasmic | 01:50 | Lymphoma | |||
| Bax | BSB 6079 | Mouse Monoclonal | E63 | IgG | Cytoplasmic | Ready-to-use | Amygdala |
Immunostaining of the tumor cells and keratinocytes from OM was assessed quantitatively by estimating the percentage of cells stained. Five micrographs were taken from each case containing normal cells from the OM and neoplastic epithelial cells from OSCC. Each microphotography was equivalent to a high-power field (HPF) with a magnification of 400×. The photomicrographs were captured with a digital camera in a light microscope (Olympus BX41, Japan) with the software program Micrometrics SE Premium 4.4. All images were stored in JPGE format. Cells were counted by two independent observers using the ImageJ free software package. Ki-67 immunoexpression was determined for the nuclear staining. COX-2, VEGF, Bcl-2 and Bax immunomarcation were assessed for the cytoplasmic staining. Positively stained cells were counted in five randomly selected fields at a magnification of ×400. The total number of positive cells/100 per case was calculated. Immunohistochemical expression of the microvascular density (MVD) was assessed through the expression of CD105/endoglin (MVD-CD105). MDV was quantified by counting intratumor microvessels positive for CD105 by five HPF to a total area equivalent to a magnification of 200× (0.7386mm2).
Subsequently, we performed the Bland–Altman test (a bias of 3.02±6.71 with a 95% Confidence Interval of −10.12 to 16.16) for the inter-observer agreement of protein immunoexpression analysis.
Statistical analysisStatistical analysis was performed using R Statistical Software (version 4.1.1; R Foundation for Statistical Computing, Vienna, Austria). We conducted a heteroskedasticity examination through Levene's Test using the “car” R package. Subsequently, we considered comparisons with Gaussian distributions using the Shapiro–Wilk test. We utilized one-way ANOVA with Tukey's multiple comparison test or Kruskal–Wallis with Dunn's multiple comparisons (with Holm's correction), depending on the data distribution. Spearman correlation analysis was performed to inspect the possible correlation between the variables. Statistical significance was defined as p<0.05. All graphics were performed with “ggplot2” and “ggcorrplot” R packages.
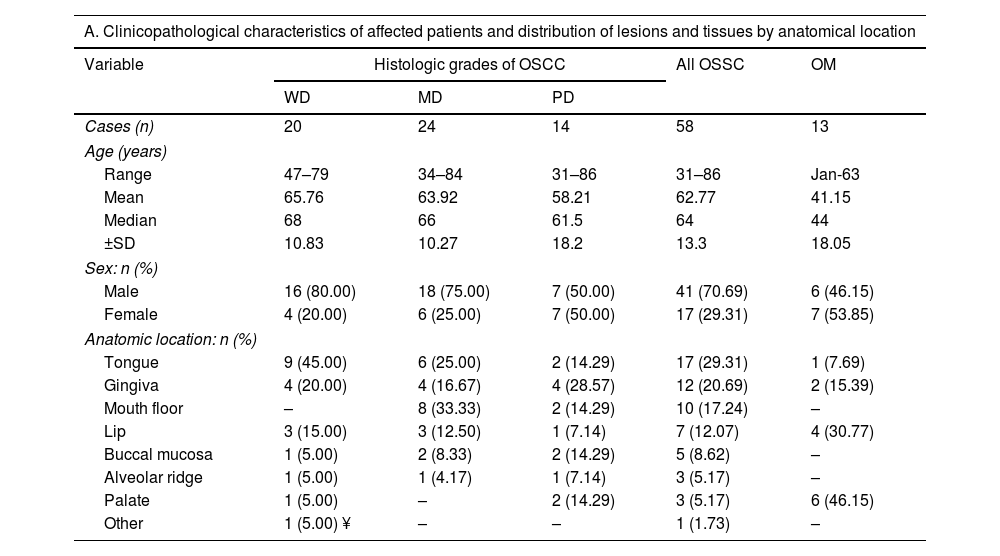
ResultsClinical featuresThe demographic data of the patients and the clinical and pathological characteristics of the samples are summarized in Table 2. For OSCC, 41 cases were male (70.69%), and 17 were female (29.31%). The age range was 31–86 years, with a mean of 62.77 years and a median of 64 years. We did not observe a statistical difference between ages according to histologic grades of OSCC. The tongue was the most common site (17 cases), followed by gingiva (11 cases) and mouth floor (10 cases). Moreover, most OSCC cases were classified as T1 or T2 category (75.86%) according to the TNM AJCC 2017 classification and were principally recognized as an ulcer (55.17%), followed by tumor lesions (36.21%). The evolution of cases was symmetrically distributed between less than three months (37.93%) and more than six months (31.03%). Finally, OSCC cases were not associated with symptomatology or history of smoking. For OM, seven cases were male (50.00%), and seven were female (50.00%). The age range of OM samples was 1 – 63 years, with a mean of 41.15 years and a median of 44 years.
Demographic and clinicopathological characteristics of samples analyzed.
| A. Clinicopathological characteristics of affected patients and distribution of lesions and tissues by anatomical location | |||||
|---|---|---|---|---|---|
| Variable | Histologic grades of OSCC | All OSSC | OM | ||
| WD | MD | PD | |||
| Cases (n) | 20 | 24 | 14 | 58 | 13 |
| Age (years) | |||||
| Range | 47–79 | 34–84 | 31–86 | 31–86 | Jan-63 |
| Mean | 65.76 | 63.92 | 58.21 | 62.77 | 41.15 |
| Median | 68 | 66 | 61.5 | 64 | 44 |
| ±SD | 10.83 | 10.27 | 18.2 | 13.3 | 18.05 |
| Sex: n (%) | |||||
| Male | 16 (80.00) | 18 (75.00) | 7 (50.00) | 41 (70.69) | 6 (46.15) |
| Female | 4 (20.00) | 6 (25.00) | 7 (50.00) | 17 (29.31) | 7 (53.85) |
| Anatomic location: n (%) | |||||
| Tongue | 9 (45.00) | 6 (25.00) | 2 (14.29) | 17 (29.31) | 1 (7.69) |
| Gingiva | 4 (20.00) | 4 (16.67) | 4 (28.57) | 12 (20.69) | 2 (15.39) |
| Mouth floor | – | 8 (33.33) | 2 (14.29) | 10 (17.24) | – |
| Lip | 3 (15.00) | 3 (12.50) | 1 (7.14) | 7 (12.07) | 4 (30.77) |
| Buccal mucosa | 1 (5.00) | 2 (8.33) | 2 (14.29) | 5 (8.62) | – |
| Alveolar ridge | 1 (5.00) | 1 (4.17) | 1 (7.14) | 3 (5.17) | – |
| Palate | 1 (5.00) | – | 2 (14.29) | 3 (5.17) | 6 (46.15) |
| Other | 1 (5.00) ¥ | – | – | 1 (1.73) | – |
| B. Clinicopathological characteristics of the samples and habits of the patients in this study | |
|---|---|
| T score according to the 2017 AJCC classification: n (%) | |
| T1 | 24 (41.38) |
| T2 | 20 (34.48) |
| T3 | 2 (3.45) |
| T4a | 8 (13.79) |
| T4b | 3 (5.17) |
| NI | 1 (1.72) |
| Clinical evolution: n (%) | |
| <3 months | 22 (37.93) |
| 3–6 months | 11 (18.97) |
| >6 months | 18 (31.03) |
| Symptomatology: n (%) | |
| Yes | 26 (44.83) |
| No | 29 (50.00) |
| NI | 3 (5.17) |
| Clinical presentation: n (%) | |
| Erythroplakia | 2 (3.45) |
| Leukoerythroplakia | 1 (1.72) |
| Tumor | 21 (36.21) |
| Ulcer | 32 (55.17) |
| NI | 2 (3.45) |
| Smoke history: n (%) | |
| Yes | 23 (39.66) |
| No | 18 (31.03) |
| NI | 17 (29.31) |
Abbreviations: OSCC, oral squamous cell carcinoma; WD, well differentiated; MD, moderately differentiated; PD, poorly differentiated; ¥, retromolar trigone; NI: not informed.
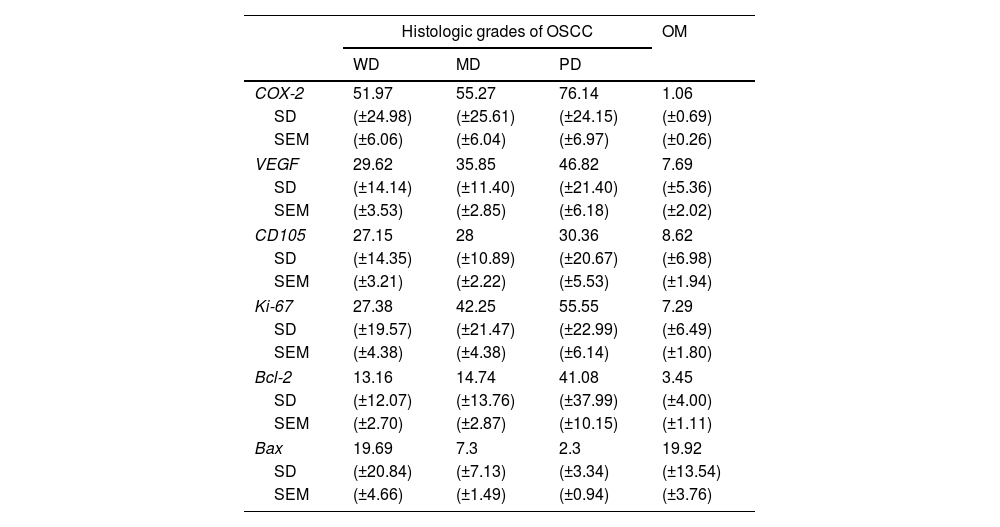
The mean COX-2, Ki-67, Bcl-2, Bax, VEGF and MDV (CD105) expression (±SD) for WD-OSCC, MD-OSCC, PD-OSCC and OM are summarized in Table 3.
Count of positive cells immunostaining (%) for OSCC and OM (Mean±SD and ±SEM).
| Histologic grades of OSCC | OM | |||
|---|---|---|---|---|
| WD | MD | PD | ||
| COX-2 | 51.97 | 55.27 | 76.14 | 1.06 |
| SD | (±24.98) | (±25.61) | (±24.15) | (±0.69) |
| SEM | (±6.06) | (±6.04) | (±6.97) | (±0.26) |
| VEGF | 29.62 | 35.85 | 46.82 | 7.69 |
| SD | (±14.14) | (±11.40) | (±21.40) | (±5.36) |
| SEM | (±3.53) | (±2.85) | (±6.18) | (±2.02) |
| CD105 | 27.15 | 28 | 30.36 | 8.62 |
| SD | (±14.35) | (±10.89) | (±20.67) | (±6.98) |
| SEM | (±3.21) | (±2.22) | (±5.53) | (±1.94) |
| Ki-67 | 27.38 | 42.25 | 55.55 | 7.29 |
| SD | (±19.57) | (±21.47) | (±22.99) | (±6.49) |
| SEM | (±4.38) | (±4.38) | (±6.14) | (±1.80) |
| Bcl-2 | 13.16 | 14.74 | 41.08 | 3.45 |
| SD | (±12.07) | (±13.76) | (±37.99) | (±4.00) |
| SEM | (±2.70) | (±2.87) | (±10.15) | (±1.11) |
| Bax | 19.69 | 7.3 | 2.3 | 19.92 |
| SD | (±20.84) | (±7.13) | (±3.34) | (±13.54) |
| SEM | (±4.66) | (±1.49) | (±0.94) | (±3.76) |
Abbreviations: OSCC, oral squamous cell carcinoma; WD, well differentiated; MD, moderately differentiated; PD, poorly differentiated; OM, oral mucosa; SD, standard deviation; SEM, standard error of the mean.
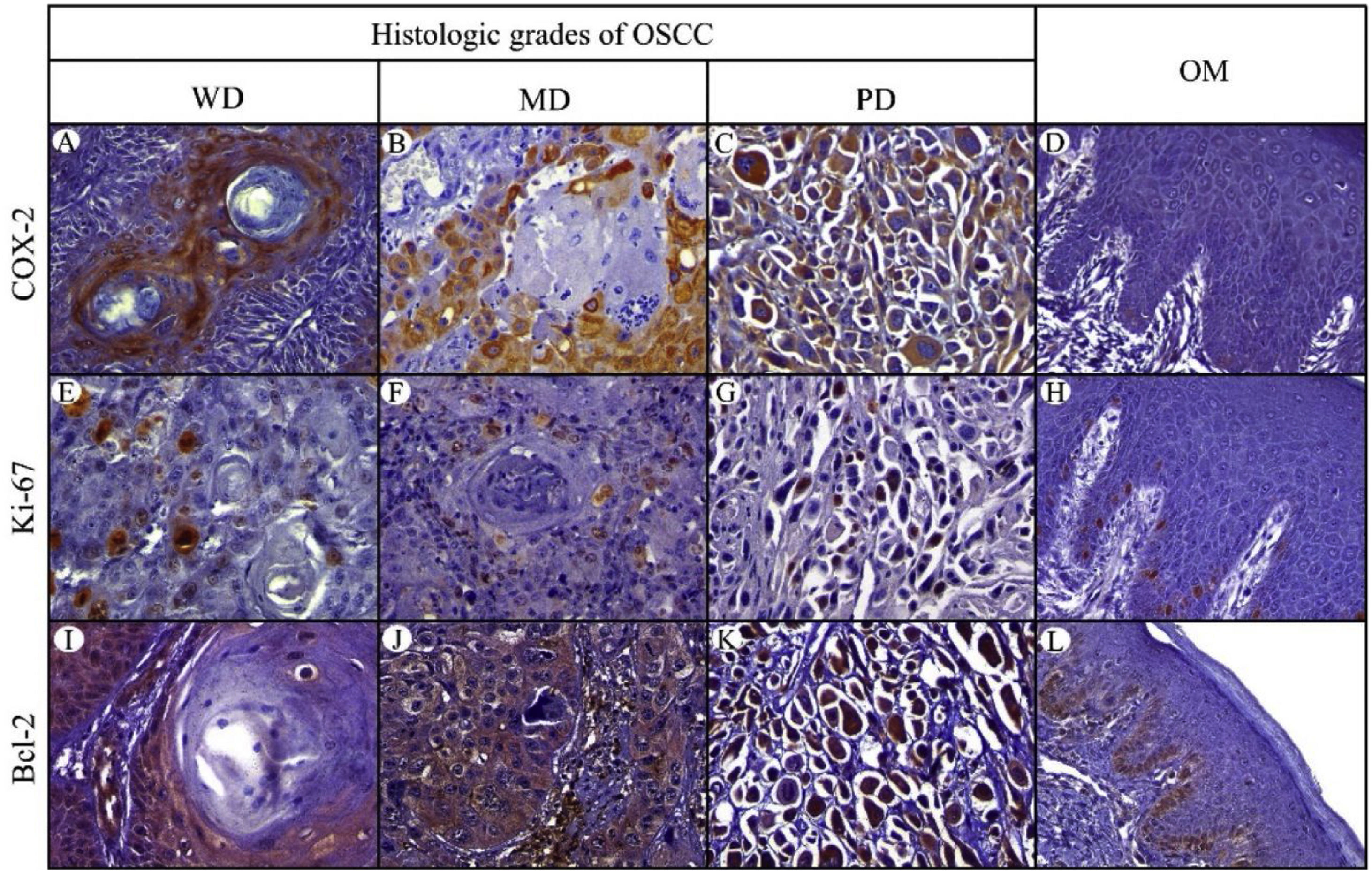
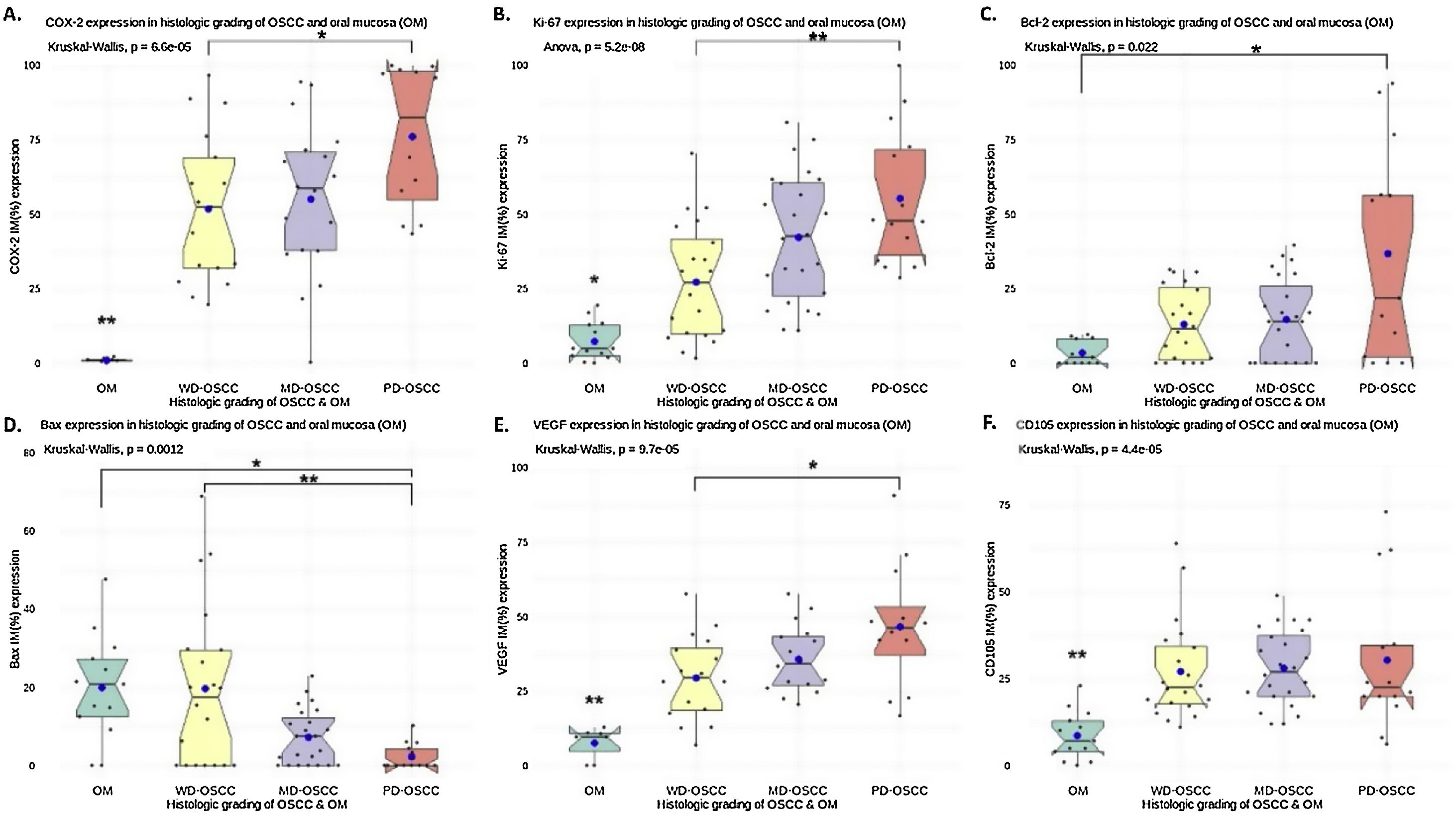
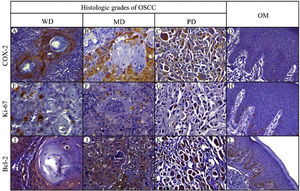
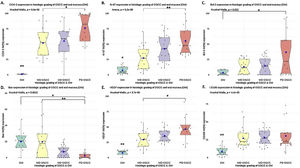
Positive yellowish-brown granular COX-2 immunostaining was cytoplasmatic and expressed mainly in tumor epithelial cells peripheral to keratin pearls in WD-OSCC, whereas in MD-OSCC it was preferentially observed in peripheral tumor epithelial cells located in epithelial nests (cords) and areas of intratumoral necrosis (Fig. 1, A, B). In contrast, no specific immunolocalization was observed in the tumor-parenchyma of PD-OSCC (Fig. 1, C). All OSCC cases presented a higher expression of COX-2 than OM (WD-OSCC, p=0.004; MD-OSCC, p=0.0015; PD-OSCC, p<0.0001). Moreover, there was a statistical difference between WD-OSCC and PD-OSCC (p=0.0418) (Fig. 3, A).
Immunohistochemical protein expression of COX-2, Ki-67 and Bcl-2. COX-2 protein expression in: A, Well-differentiated (WD) OSC; B, Moderately differentiated (MD) OSCC; C, Poorly differentiated (PD) OSCC; and D, Oral mucosa (OM). Ki-67 protein expression in: E, WD OSCC; F, MD OSCC; G, PD OSCC; and H, OM. Bcl-2 protein expression in: I, WD OSCC; J, MD OSCC; K, PD OSCC; and L, OM. (400×).
Positive Ki-67 nuclear immunostaining was observed in OSCC and OM (Fig. 1, E–H). In OM, Ki-67 was mainly detected in the basal compartment (basal and parabasal stratum) of the stratified squamous epithelium as yellowish-brown nuclear staining (Fig. 1, H). All OSCC cases presented a higher expression of Ki-67 than in OM (WD-OSCC, p=0.0250; MD-OSCC, p<0.0001; PD-OSCC, p=0.0004). Moreover, there was a statistical difference between WD-OSCC and PD-OSCC (p=0.0005) (Fig. 3, B).
Bcl-2 expressionPositive Bcl-2 was detected in the cytoplasm of epithelial cells from OSCC with yellowish staining (Fig. 1, I–K). In WD-OSCC and MD-OSCC, Bcl-2 immunolocalization was detected mainly in neoplastic cells surrounding keratin pearls. In OM, expression of Bcl-2 was infrequent and found exclusively in the basal cell layer (Fig. 1, L). In addition, PD-OSCC cases exhibited a higher expression of Bcl-2 than in OM (p=0.007) (Fig. 3, C).
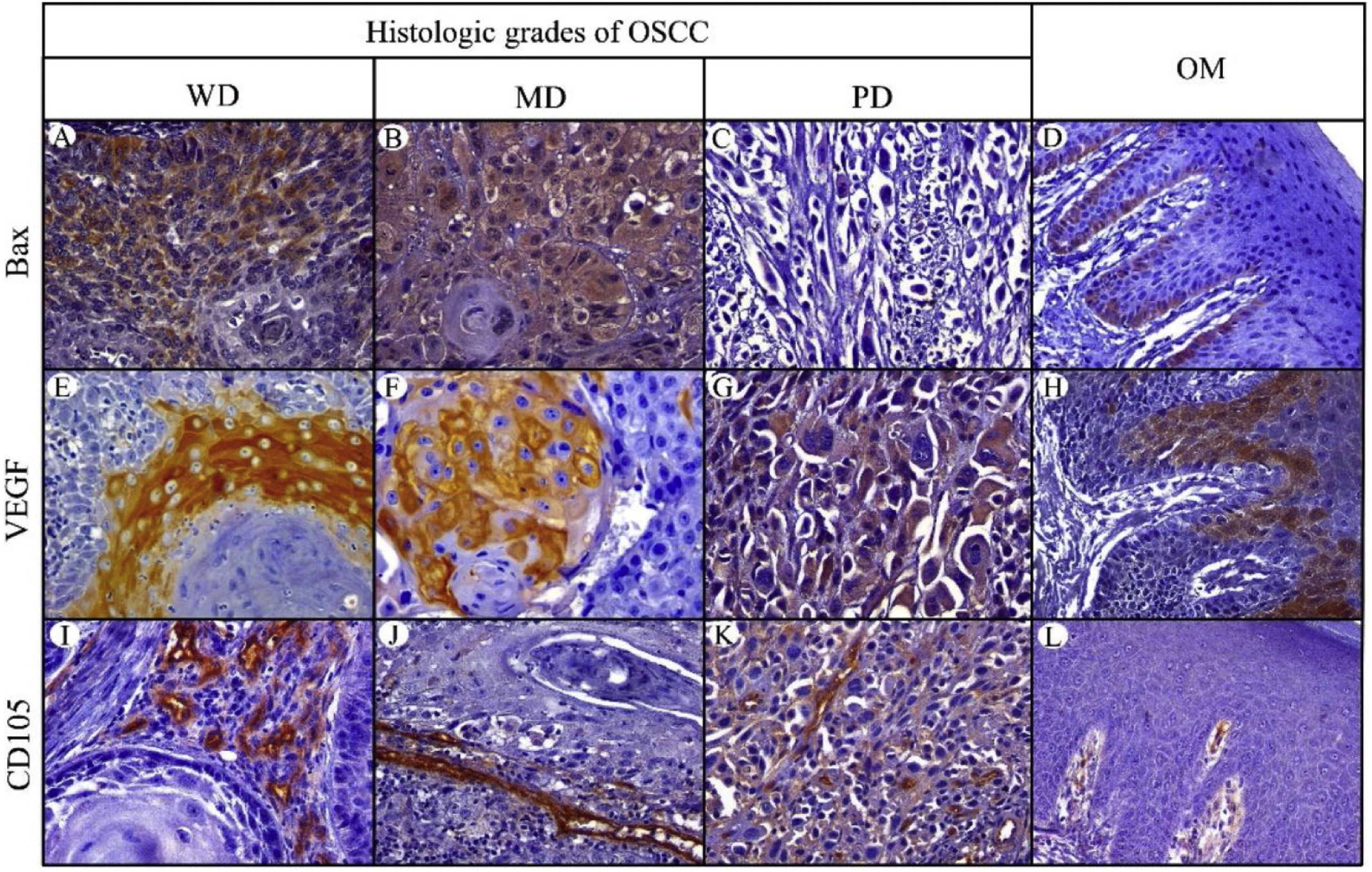
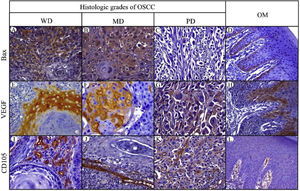
Bax expressionBax was mainly localized in the cytoplasm of tumor cells with yellowish staining (Fig. 2, A–D). In OM, expression of Bax was found only in the basal cell layer (Fig. 2, D). OM and WD-OSCC displayed a higher expression of Bcl-2 than PD-OSCC (p=0.0066 and p=0.0009, respectively) (Fig. 3, D).
Immunohistochemical protein expression of Bax, VEGF and CD105. Bax protein expression in: A, Well-differentiated (WD) OSCC; B, Moderately differentiated (MD) OSCC; C, Poorly differentiated (PD) OSCC; and D, Oral mucosa (OM). VEGF protein expression in: E, WD OSCC; F, MD OSCC; G, PD OSCC and H, OM. CD105 protein expression in: I, WD OSCC; J, MD OSCC; K, PD OSCC; and L, OM. (400×).
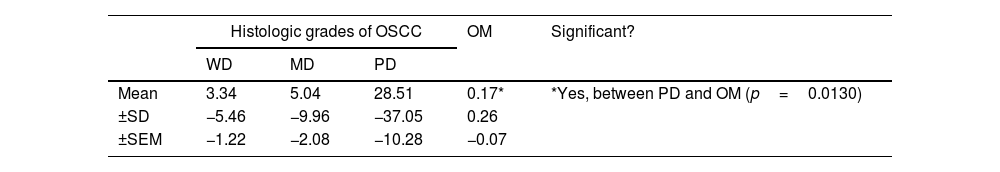
The ratio of Bcl-2/Bax>1 was found in eight cases in WD-OSCC (40%), fifteen cases in MD-OSCC (65.22%), nine cases in PD-OSCC (69.23%) and no cases in OM. The difference between OSCC and OM was significant (p=0.0272) and higher in PD-OSCC (p=0.0130). However, no difference was found between OSCC histological grading (Table 4).
Mean (±SD, ±SEM) Bcl-2/Bax ratio in histologic grades of OSCC and OM (*p<0.05).
| Histologic grades of OSCC | OM | Significant? | |||
|---|---|---|---|---|---|
| WD | MD | PD | |||
| Mean | 3.34 | 5.04 | 28.51 | 0.17* | *Yes, between PD and OM (p=0.0130) |
| ±SD | −5.46 | −9.96 | −37.05 | 0.26 | |
| ±SEM | −1.22 | −2.08 | −10.28 | −0.07 | |
Abbreviations: OSCC, oral squamous cell carcinoma; WD, well differentiated; MD, moderately differentiated; PD, poorly differentiated; OM, oral mucosa; SD, standard deviation; SEM, standard error of mean.
Positive brown VEGF immunostaining was cytoplasmatic and also on the cell surface, predominantly in the epithelial cells of the periphery tumor islands in WD-OSCC and MD-OSCC (Fig. 2, E, F). In contrast, no specific immunolocalization was detected in PD-OSCC (Fig. 2, G). In OM, immunostaining was observed mainly in the suprabasal strata of the stratified epithelium (Fig. 2, H). All OSCC cases presented a higher expression of VEGF than OM (WD-OSCC, p=0.0095; MD-OSCC, p=0.0006; PD-OSCC, p<0.0001). Moreover, there was a statistical difference between WD-OSCC and PD-OSCC (p=0.0167) (Fig. 3, E).
CD105 expressionThe number of vessels positive for endothelial cells with cytoplasmic staining for CD105 was counted. Immunoreaction was detected in the cytoplasm of endothelial cells of intratumor microvessels in OSCC (Fig. 2, I–K). The immunostaining was present in blood vessels with different diameters and morphology (oval, round, or irregular). In OM, immunostaining of endothelial cells was observed in capillaries of the lamina propria (Fig. 2, L). All OSCC cases presented a higher expression of CD105 than OM (WD-OSCC, p=0.0003; MD-OSCC, p<0.0001; PD-OSCC, p=0.0003) (Fig. 3, F).
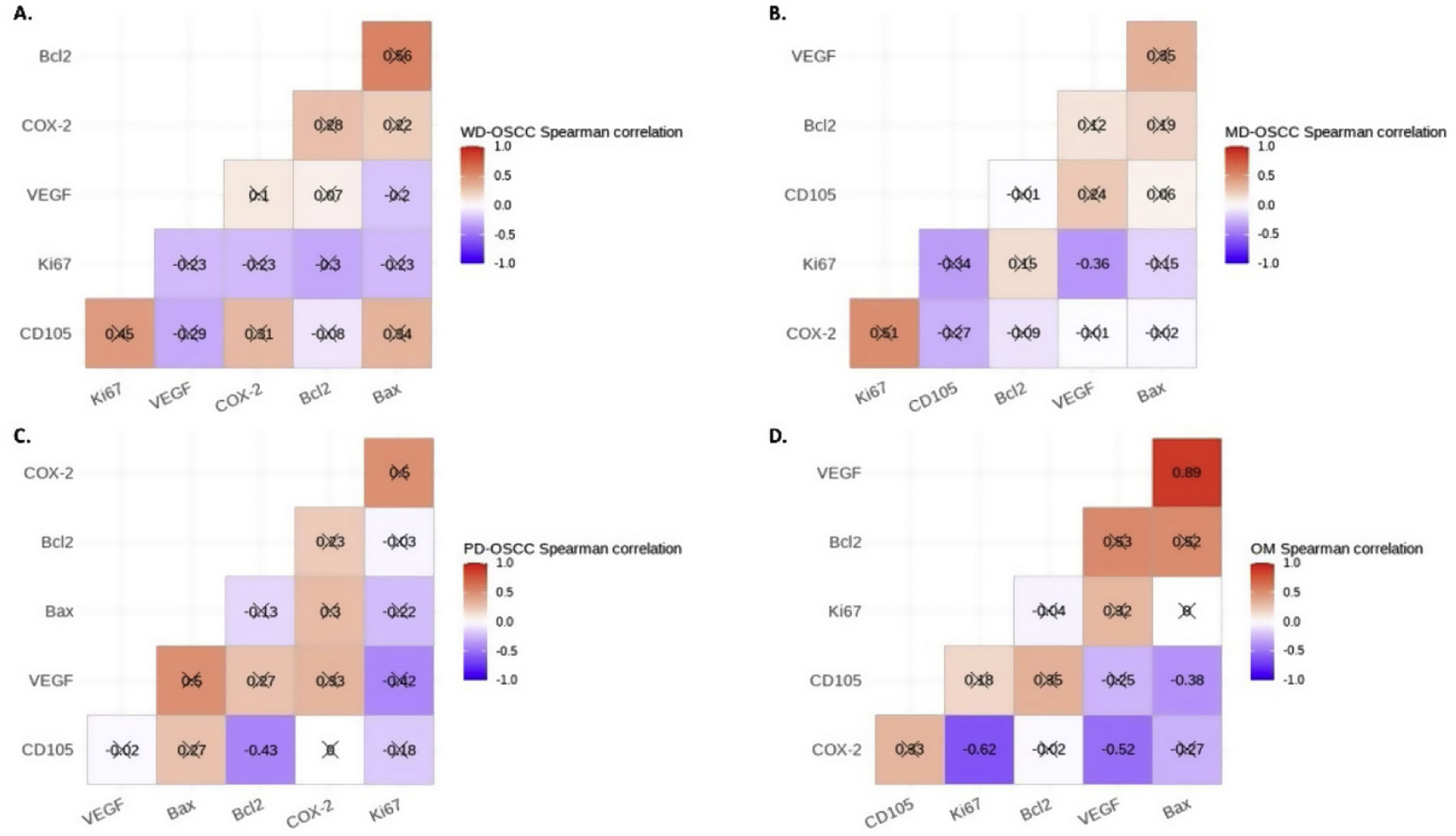
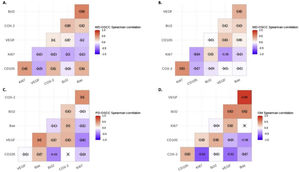
Correlation analysisSpearman's correlation test showed a statistical negative correlation between Ki-67 and VEGF in MD-OSCC (rho=−0.36, p=0.0366) (Fig. 4, B) and CD105 and Bcl-2 in PD-OSCC (rho=−0.43, p=0.0440) (Fig. 4, C). Interestingly, in OM we found a strong positive correlation between VEGF and Bax (rho=0.89, p=0.0044) and negative correlations of COX-2 with Ki-67 (rho=−0.62, p=0.0411) and VEGF (rho=−0.52, p=0.0408) (Fig. 4, D).
Spearman's correlation test between COX-2, Ki-67, Bcl-2, Bax, VEGF and CD105 in A, Well-differentiated (WD) OSCC; B, Moderately differentiated (MD) OSCC; C, Poorly differentiated (PD) OSCC; D, oral mucosa (OM). Crossed out rho-value indicates that the correlation was not significant.
COX-2 overexpression is associated with alterations in the architecture and cellularity that contribute to tumor development in the intrinsic cell population of the oral epithelium. Likewise, COX-2 protein expression is associated with inflammation which favors cell proliferation and survival, angiogenesis, cell invasion and metastasis.8 Hence, the study of proteins related to tumor development could be crucial for new therapeutic strategies which could be employed according to the histological grading and morphological presentations of OSCC.2 In our study, elevated levels of COX-2 protein were detected in OSCC, particularly in PD-OSCC. Similar findings were described by Thomas et al.,11 who recognized a higher percentage of COX-2 expression in PD-OSCC compared to WD-OSCC and MD-OSCC. In contrast, some studies suggested that early stages of carcinogenesis exhibited increased levels of COX-2 expression.15,16 Nevertheless, these differences may be related to different methodologies used to quantify the immunomarcation or the intensity of the immunostaining for COX-2 expression.
During OSCC carcinogenesis, the activation of transcription factors related to tumor progression enhances the expression of genes associated with cell survival and proliferation, angiogenesis, metastasis and pro-inflammatory pathways.17 Moreover, persistent inflammation may induce DNA damage at the initial stages of carcinogenesis. COX-2 protein is induced upon activation by mitogens and inflammatory mediators.17 Likewise, this inflammatory environment induces the expression of COX-2 and its principal metabolite prostaglandin E2 (PGE2), which promotes cell proliferation and tumor development.8,17 Furthermore, COX-2/PGE2 activation promotes various pro-inflammatory signaling mediators, such as interleukin-6 (IL-6), nuclear factor-kappa B (NF-κB), tumor necrosis factor-alpha (TNF-α), reactive oxygen species (ROS), and signal transducer and activator of transcription 3 (STAT3). Hence, pro-inflammatory signallings markedly increase and perpetuate COX-2 overexpression in OSCC.7
A high level of COX-2 protein plays an essential role in tumor development and progression by increasing cell proliferation.8 Ki-67 is a nuclear protein valuable as a proliferation marker in normal and tumor tissue. Several workers found that the expression of Ki-67 in OSCC is greater than in OM, and that its expression varies in histologic grades of OSCC.12
Consistent with our results, Pereira et al. observed a higher Ki-67 expression in PD-OSCC than in other OSCC histologic grades.12 Interestingly, although a correlation between the expression of Ki-67 and COX-2 has not been elucidated, the inhibition of COX-2 diminished cell proliferation in preclinical and clinical models of HNC.18 Moreover, we identified a robust negative correlation between Ki-67 and COX-2 in the OM, which agrees with previous reports.19 This suggests that COX-2 may regulate the expression of these death-regulatory proteins and thus inhibit apoptosis and contribute to carcinogenesis.
COX-2 stimulates anti-apoptotic Bcl-2 protein and reduces pro-apoptotic Bax protein expression.7 Hence, in the inflammatory context of TME, the overexpression of COX-2/PGE2 promotes Bcl-2 expression and reduces susceptibility to apoptosis of OSCC cells.7,8 In addition, the aberrant expression of Bcl-2 and Bax contributes to the molecular carcinogenesis of OSCC20 and might be correlated with OSCC prognosis.21
Previous reports showed different mean protein expressions of Bcl-221 and Bax21,22 in OSCC. We found that Bcl-2 protein was notably overexpressed in PD-OSCC, while the expression of Bax protein was suppressed in PD-OSCC. Similarly, Zhang et al.21 found that the expression of Bax was higher in WD-OSCC than in PD-OSCC. These results supported the role of the down-regulation of Bax protein in the development of a more aggressive OSCC phenotype.20 In OM, Bcl-2 expression was observed exclusively in the basal cell layer, agreeing with Zhang et al.21 In other studies, Bax expression was detected mainly in the suprabasal layers.23 Furthermore, Bax protein was maintained in epithelial dysplasia and WD-OSCC.24 Consequently, the reduction of Bax protein would be critical for the development and progression of oral carcinomas,25 particularly when COX-2/PGE2 is overexpressed.
In general, cases of OSCC expressing elevated levels of Bax and reduced levels of Bcl-2 were associated with a better prognosis.26 We found that the Bcl-2/Bax ratio in OSCC was significantly higher than in OM. When we analyzed Bcl-2/Bax according to the histologic degree of OSCC, we found a significantly higher Bcl-2/Bax ratio in PD-OSCC compared with OM. Interestingly, our results were similar to Bhutami et al.,13 who described that Bcl-2 expression increases in less differentiated OSCC. Therefore, more studies are required to understand the expression of Bcl-2 and Bax in the early stages of OSCC carcinogenesis. Our observation of a decreased Bcl-2/Bax ratio in oral carcinomas might indicate that cell death by apoptosis is favored.27 Furthermore, the lack of long-term studies precludes the potential utility of the Bcl-2/Bax ratio as a prognostic marker in OSCC.
Angiogenesis is the appearance of new microvessels from the preexisting vasculature.28 The angiogenic activity represents a pivotal event in OSCC carcinogenesis.29 A substantial increase in vascularity occurs during the transition of normal OM to different grades of dysplasia.29 Moreover, a significant increase in vascularity occurs during normal OM carcinogenesis to OSCC.28 Angiogenesis can be determined by immunohistochemical assessment of endothelial cell markers such as endoglin (CD105) expressed in activated endothelial cells and VEGF, a signaling protein that stimulates the division and migration of endothelial cells.29 VEGF protein expression is a potent inducer of angiogenesis and facilitates tumor progression.28
Various reports have analyzed the expression of the VEGF protein in OM and OSCC. In agreement with Astekar et al.,28 our study reported a higher VEGF expression in OSCC than OM. In addition, we observed a statistical difference between WD-OSCC and PD-OSCC. A probable explanation could be the necessary nutrition for the initial establishment and tumor growth. Nevertheless, the differentiating tumor presents several modulating factors.28 CD105 is strongly expressed in tumor endothelial cells and is considered a marker of neovascular microvessels.30 MVD assessment using CD105 as a marker has been proposed to predict tumor progression and prognosis.31 In our study, the expression of CD105 was significantly increased in all OSCC samples compared to OM. Astekar et al.28 showed higher MVD scores in OSCC compared with OM. Likewise, they compared this expression between histologic grades of OSCC, indicating a decrease of the mean MVD from WD-OSCC to MD-OSCC to PD-OSCC. Furthermore, MVD could indicate OSCC progression.28 These findings suggest that angiogenesis increases with disease progression and may be a critical mitogen responsible for neovascularization. Hence, the overexpression of COX-2 would be one of the crucial mechanisms that favor angiogenesis in OSCC, particularly by promoting VEGF expression.9 COX-2 may stimulate neovascularization in early stages of OSCC development, but also in late stages, in conjunction with other proangiogenic factors.30 Therefore, elevated levels of COX-2 protein could induce neoangiogenesis.
To conclude, we emphasize the potential benefits of employing selective COX-2 inhibitors as an adjuvant treatment in OSCC cases that express elevated levels of COX-2 or in resistant-OSCC cases.9 Indeed, Santoro et al.32 showed that COX-2 inhibitors would be helpful in the advanced stages of OSCC. Nevertheless, more studies are necessary to increase knowledge of the potential utility of selective COX-2 inhibitors as an adjuvant for OSCC treatment. We noted interesting differences in the expression of proteins related to multiple biological events when we compared the degrees of histological differentiation of OSCC. Although we did not identify a correlation between COX-2 and these proteins, we realized the importance of the histological and immunohistochemical examination of OSCC.
Furthermore, we recognized that less differentiated stages of OSCC could be related to a higher rate of cell proliferation and a decrease in pro-apoptotic events, which promote tumor progression. However, some limitations should be noted: We did not include the immunohistochemical comparison of potentially malignant events, such as oral dysplasia, or follow-up data and other clinical variables (regional lymph node involvement, metastasis, etc.) which are important factors in predicting prognosis and survival.
ConclusionsWe showed that the inflammation inducing enzyme COX-2, as well Ki-67, Bcl-2, and VEGF proteins, are increasingly expressed in less-differentiated histologic grades of OSCC, especially in poorly differentiated OSCC.
Authors’ contributionsEnrico Escobar: Conceptualization; Data curation; Formal analysis; Investigation; Methodology; Supervision; Writing-original draft; Writing-review & editing. Fernán Gómez-Valenzuela: Data curation; Formal analysis; Investigation; Supervision; Writing-original draft; Writing-review & editing. Cristian Peñafiel: Data curation; Methodology; Resources. Alondra Hormazábal Hevia: Data curation; Methodology; Writing-review & editing. Constanza Herrera Fuentes: Data curation; Methodology; Writing-review & editing. Diana Mori Aliaga: Data curation; Methodology; Writing-review & editing.
EthicsThis study was approved by the Ethics Committee (N°: 2017/10) and the Institutional Biosecurity Committee (FDO N° 101) of the Faculty of Dentistry, University of Chile, Santiago, Chile.
FundingEnrico Escobar: Project FIOUCH 17-017, Faculty of Dentistry, University of Chile, Grant/Award Number: FIOUCH 17-017; Fernán Gómez-Valenzuela: CONICYT-PFCHA/Doctorado Nacional 2019, Grant/Award Number: Folio 21190421.
Conflict of interestsThe authors declare that there is no conflict of interests regarding the publication of this paper.
The authors would like to thank the Faculty of Dentistry, University of Chile, Santiago, Chile, for financially supporting this project (supported by FIOUCH 17-017). FGV acknowledges partial support from CONICYT-PFCHA/Doctorado Nacional 2019-Folio 21190421 and the Faculty of Medicine, Pontificia Universidad Católica de Chile, Santiago, Chile (Doctoral Program in Medical Sciences). Thanks to Mr. Juan Fernández de los Ríos, from the Language and Translation services, Faculty of Dentistry, Universidad de Chile, for kindly proofreading and checking the spelling and grammar of this article.