Microscopic assessment is essential in the study of mediastinal lymph nodes. Obtaining cytological samples through Endobronchial Ultrasound TransBronchial Needle Aspiration (EBUS-TBNA) has long been considered the gold standard procedure. The implementation of the World Health Organization (WHO) Reporting System for Lymph Node Cytopathology, along with the advancement of the CryoEBUS lymph node technique, has enhanced and refined diagnostic accuracy in this field.
Materials and methodDuring a two-year period, cases involving the parallel performance of EBUS-TBNA and CryoEBUS specimen collection were quantified. The implementation of the WHO Reporting System allowed for the comparison of diagnostic yield between cytological and CryoEBUS tissue samples.
ResultsA total of 178 EBUS-TBNA and CryoEBUS procedures were conducted, with a mean patient age of 63 years and a male predominance of 72.5%. Lymph node station 7 was the most sampled site, accounting for 38.76% of cases. Category V – malignant was the most common cytological diagnosis, representing 50% of cases, while 46.62% of CryoEBUS samples were malignant. The Pearson correlation coefficient between the two methods was calculated at 0.99.
ConclusionsThe combined implementation of the WHO Reporting System for Lymph Node Cytopathology and the simultaneous use of CryoEBUS resulted in enhanced performance and diagnostic accuracy, reducing non-diagnostic samples to less than 3%.
La evaluación microscópica es esencial en el estudio de los ganglios linfáticos mediastínicos. La obtención de muestras citológicas mediante la aspiración con aguja transbronquial guiada por ecografía endobronquial (EBUS-TBNA) ha sido considerada durante mucho tiempo el procedimiento de referencia. La aplicación del Sistema de Clasificación de la Organización Mundial de la Salud (OMS) para la Citopatología de Ganglios Linfáticos, junto con el avance de la técnica de CryoEBUS en ganglios linfáticos, ha mejorado y refinado la precisión diagnóstica en este campo.
Materiales y métodosDurante un período de 2 años, se cuantificaron los casos que incluyeron la realización conjunta de EBUS-TBNA y la obtención de muestras mediante CryoEBUS. La aplicación del Sistema de Clasificación de la OMS permitió comparar el rendimiento diagnóstico entre las muestras citológicas y las obtenidas mediante CryoEBUS.
ResultadosSe realizaron un total de 178 procedimientos de EBUS-TBNA y CryoEBUS, con una edad media de los pacientes de 63 años y un predominio masculino del 72,5%. La estación ganglionar 7 fue la más analizada, representando el 38,76% de los casos. El diagnóstico citológico más común correspondió a la categoría V – maligno, que constituyó el 50% de los casos, mientras que el 46,62% de las muestras obtenidas mediante CryoEBUS resultaron ser malignas. El coeficiente de correlación de Pearson entre ambos métodos se calculó en 0,99.
ConclusionesLa aplicación combinada del Sistema de Clasificación de la OMS para la Citopatología de Ganglios Linfáticos y el uso simultáneo de CryoEBUS resultó en una mejora del rendimiento y la precisión diagnóstica, reduciendo las muestras no diagnósticas a menos del 3%.
The mediastinal lymph node can harbour a wide range of primary and secondary diseases. Despite significant advancements in imaging techniques for the thoracic cavity, the definitive diagnosis of most mediastinal lymphadenopathies relies on microscopic evaluation of the lymph nodes. While surgical biopsy remains the gold standard specimen for lymph node studies, its invasive nature and potential complications make it a last resort in the diagnostic process, particularly for lymphadenopathies in hard-to-reach areas like the chest. The introduction of endobronchial ultrasound for lymph node examination, along with the capability to obtain samples via fine needle aspiration (EBUS-TBNA), represents a significant development.1 However, this poses a challenge for interventional pathologists, as they must work with small, cytological samples that now require not only a microscopic diagnosis but often a molecular one as well.2
In recent years, we have observed three significant milestones in the diagnosis of mediastinal lymph nodes. The first is the growing importance of molecular diagnostics and the identification of therapeutic targets. This shift has led to the need for optimizing samples obtained via EBUS, particularly in cases of non-small cell lung carcinoma metastasis or mediastinal lymphomas.3 The second milestone is the introduction of lymph node cytopathology reporting systems, such as the Sydney System in 20204 and more recently the World Health Organization (WHO) System.5 These systems aim to standardize and provide guidelines for lymph node cytological diagnosis. The third milestone involves the development and utilization of the CryoEBUS methodology for obtaining mediastinal lymph node samples. This technique allows for the collection of larger specimens with preserved architecture, resembling tissue fragments.6–8
In this paper, we present the findings from a tertiary hospital centre regarding the implementation of the WHO reporting system for the evaluation of mediastinal lymph nodes. In addition, we discuss the diagnostic efficacy of CryoEBUS and introduce a diagnostic algorithm for its recommended use, providing insights from the perspective of the interventional pathologist.
Materials and methodA retrospective study was conducted between January 2022 and May 2024, documenting all samples from patients who underwent bronchoscopy procedures at our institution. The study involved the parallel collection of lymph node cytological samples using both conventional EBUS-TBNA and CryoEBUS techniques.
Data on demographic characteristics, clinical suspicions, and the number of sampled lymph node stations were recorded. Four interventional pathologists independently reported microscopic diagnoses in two categories:
- 1.
Cytologic diagnosis based on the evaluation of cytologic smears stained with Diff Quik-Giemsa and haematoxylin–eosin (H&E), as well as cell blocks prepared using the self-clotting method. These blocks underwent formalin fixation, paraffin embedding, and H&E staining. The WHO Reporting System for Lymph Node Cytopathology was applied, categorizing diagnoses into one of the five established categories.5,9
- 2.
Histological diagnosis of CryoEBUS samples collected using the Ariza-Pallarés method, which involves an initial EBUS-TBNA followed by CryoEBUS if indicated.10 Standard tissue processing, including formalin fixation, paraffin embedding, and H&E staining of histological sections, was applied.2 Diagnoses were classified into five categories (insufficient, negative for malignancy, atypia, suspicious for malignancy, and malignant) for comparative statistical analysis similar to the WHO reporting system.
A descriptive analysis of the variables tested was conducted, including an assessment of diagnostic performance and a comparative study between EBUS-TBNA cytological samples and CryoEBUS specimens.
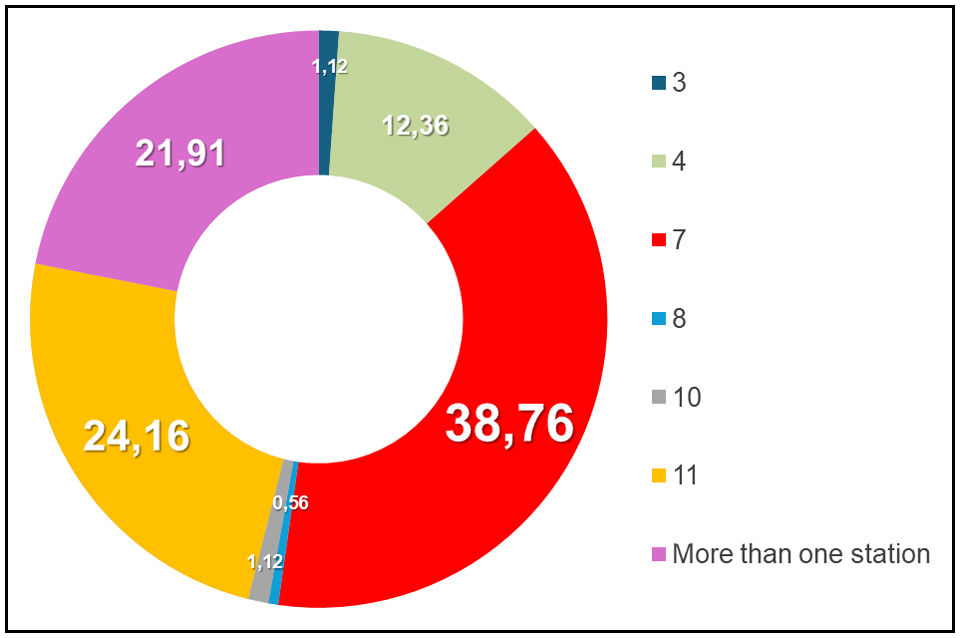
ResultsDuring the specified period, a total of 178 mediastinal lymph node sampling procedures were conducted, using EBUS-TBNA and CryoEBUS techniques. The average age of the patients was 63 years, with a range from 32 to 90 years. Gender distribution included 49 women (27.5%) and 129 men (72.5%). The primary indication for the sampling procedure was the diagnosis and staging of lung cancer in 106 patients (59.55%). Other indications included lymphoproliferative disorders (22 cases, 12.36%), sarcoidosis (18 cases, 10.11%), reactive lymphadenopathy (17 cases, 9.55%), suspected extrapulmonary metastasis (10 cases, 5.62%), as well as additional cases involving silicosis and tuberculosis. The most sampled mediastinal lymph node station was station 7 (69 cases), followed by station 11 (43 cases) (Fig. 1).
Mediastinal lymph node station sampled. Lymph nodes 7 and 11 were the most sampled, with a total of 112 cases. In 39 cases, EBUS and CryoEBUS were performed in more than one lymph node station. The non-diagnostic sample from both EBUS and CryoEBUS was obtained from lymph node 4R. Additionally the seven non-representative samples obtained by CryoEBUS corresponded to lymph nodes from the following stations: 3 (1 case), 7 (2 cases), 10 (1 case) and 11 (3 cases).
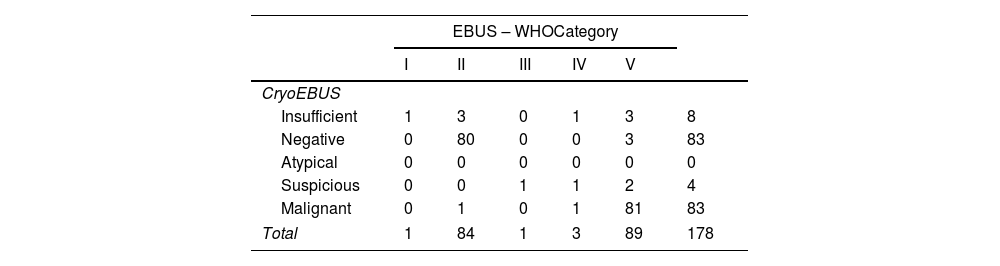
Table 1 displays the distribution of cytological diagnoses (samples obtained via EBUS-TBNA) and histological diagnoses (samples obtained via CryoEBUS). The Pearson correlation coefficient calculated between the two methods was 0.99.
Diagnostic distribution. EBUS-TBNA cytologic sample vs CryoEBUS tissue sample.
| EBUS – WHOCategory | ||||||
|---|---|---|---|---|---|---|
| I | II | III | IV | V | ||
| CryoEBUS | ||||||
| Insufficient | 1 | 3 | 0 | 1 | 3 | 8 |
| Negative | 0 | 80 | 0 | 0 | 3 | 83 |
| Atypical | 0 | 0 | 0 | 0 | 0 | 0 |
| Suspicious | 0 | 0 | 1 | 1 | 2 | 4 |
| Malignant | 0 | 1 | 0 | 1 | 81 | 83 |
| Total | 1 | 84 | 1 | 3 | 89 | 178 |
The percentage of insufficient cases was very low for both techniques, with only 1 case deemed insufficient for both methods, attributed to a subcentimeter lymph node in an unusual location. Among the 7 additional insufficient cases with CryoEBUS: 3 were negative, 3 were carcinoma metastases, and 1 was suspected of a lymphoproliferative disorder. Notably, in cases with a negative cytologic diagnosis, one was identified as lymphoma in the CryoEBUS sample, while 3 negative cases by CryoEBUS were positive for malignancy (2 carcinoma metastases and 1 lymphoma). The sole atypical case classified as suspicious for a lymphoproliferative disorder was based on the WHO reporting system. Among the three cases suspected of malignancy according to the WHO criteria, one was concordant (suspicious for lymphoproliferative disorder), one was non-diagnostic, and one was carcinoma metastasis in the CryoEBUS specimens.
As expected, reflecting patient selection bias, most cases yielded positive results for malignancy, at rates of 50% for EBUS-TBNA and 46.62% for CryoEBUS. Carcinoma metastases were the most prevalent entity, comprising 43% of cases.
In our hospital, similar to those in specialized health centres, a significant portion of cytological samples originates from the examination of mediastinal lymph nodes. This percentage notably increases, reaching up to 50% in specific areas such as the diagnosis and staging of lung cancer.1 Despite being a commonly sampled anatomical site, it was not until 2020 that the first initiative to standardize lymph node cytology was undertaken, leading to the publication of the Sydney System. Since its inception, this reporting system has been consistently implemented in our facility, yielding highly satisfactory outcomes.11,12
Following the principles of the Sydney System, albeit with slight variations we consider negligible, the WHO introduced its own cytologic reporting system in early 2024. Both systems consist of five similar diagnostic categories, making the comparison and transition process relatively straightforward. Since the integration of the new WHO reporting system, we have observed notable results, including a low incidence of non-diagnostic, atypical, and suspected malignant samples (1, 1, and 3 cases respectively) compared to existing literature.5,13,14 Furthermore, our findings indicate a comparable distribution between samples negative for malignancy (47.19%) and those identified as malignant (50%), aligning closely with results from other scholarly studies.5,15,16 This diagnostic distribution may be influenced by a patient selection bias, primarily from the pulmonology office, as well as the practice of the interventional pathologist conducting rapid on-site evaluation (ROSE) during sample collection procedures. This approach likely led to a higher yield of diagnostic samples, reducing the need for repeated sampling procedures.17 The requirement to assign a specific category to cytological diagnoses enhances accuracy by effectively categorizing and stratifying cases.
A comparison between the WHO System and the Sydney System is essential. While both classifications share striking similarities, the authors believe that the Sydney System excels in establishing diagnostic levels (level I: basic/mandatory and level II: recommended) and in distinguishing between primary nodal disease and secondary metastatic disease. This distinction facilitates communication between pathologists and non-pathologists. Conversely, it could be argued that the WHO System implicitly incorporates all the detailed information presented in the Sydney System. Rather than engaging in a debate over the preferred reporting system, the focus should be on selecting one and evaluating its performance in a practical setting.
While the correlation between EBUS-TBNA cytologic samples and CryoEBUS tissue samples is highly significant, with a Pearson coefficient approaching 1, it is crucial to examine certain findings and discrepancies within our series. The greater number of insufficiencies observed with CryoEBUS (8 vs 1) could be attributed to two primary factors: first, the challenge associated with sampling subcentimeter lymph nodes and second, the operator's proficiency in executing a novel technique for the first time. When examining the remaining cases with discrepancies between cytologic and CryoEBUS samples, although lacking statistical significance, it is important to note the apparent “enhanced diagnostic ease” provided by CryoEBUS samples for immunohistochemical and molecular techniques, including the assessment of therapeutic targets like PD-L1. This advantage arises from the architectural integrity and larger size of the sample, which particularly benefits the diagnosis of lymphomas and non-pulmonary-origin metastases.7,8,18 Furthermore, it is essential to address the false-negative cases observed in CryoEBUS samples and to explore potential reasons behind these discrepancies. The likely cause of these false-negative cases in CryoEBUS samples points to a sampling issue, highlighting the crucial role of the interventional pathologist in conducting rapid on-site molecular evaluation (ROME)19 alongside rapid on-site evaluation (ROSE). In the context of lung cancer, most studies evaluating the diagnostic performance of EBUS samples – whether or not ROSE is used – are predominantly from the pre-ROME era.20 These studies generally suggest minimal or non-existent discrepancies, underscoring the value of ROSE in ensuring optimal sample handling by the interventional pathologist.1,11,12 In the current landscape of personalized medicine, where samples are expected to provide more than just a microscopic diagnosis and undergo multiple molecular tests, it is imperative to question whether the diagnostic disparity between samples with ROSE/ROME and those without remains unchanged.3 Some publications suggest that a sample capable of providing a microscopic diagnosis but lacking relevant molecular determinations may be considered an insufficient or incomplete diagnosis.21
It is essential to recognize that a larger specimen obtained from CryoEBUS does not always ensure that the sample is representative. Therefore, it is advisable for pulmonologists to follow the guidance of the interventional pathologist during ROSE, identifying the most suitable area of the lymph node for CryoEBUS whenever feasible.
Two critical aspects concerning the procedure and sample management are worth noting. Our team has previously detailed the methodology utilized in CryoEBUS in published articles.7,10,18 By employing this systematic approach, we have identified a sample selection bias that may adversely affect the quality of the cytological sample or cell block. The CryoEBUS procedure for lymph nodes entails tunnelling through the bronchial wall, requiring at least 2–3 passes with the EBUS needle. Focusing solely on the passage for the cryoprobe may lead to suboptimal sampling, which could subsequently affect the EBUS-TBNA cytologic sample. Another important aspect to consider is the in-situ validation of the representativeness of the CryoEBUS sample, for which two proposed methods exist. The first method involves pre-validating the EBUS sample through ROSE to determine the most representative area within the lymph node for directing the cryoprobe. The second complementary approach involves using touch imprint cytology1,22 on the CryoEBUS sample. From the authors’ perspective, considering that one of the strengths of the CryoEBUS specimen lies in its tissue integrity, compromising it with prior manipulation – especially when ROSE has already been conducted on the EBUS specimen – may be counterproductive.
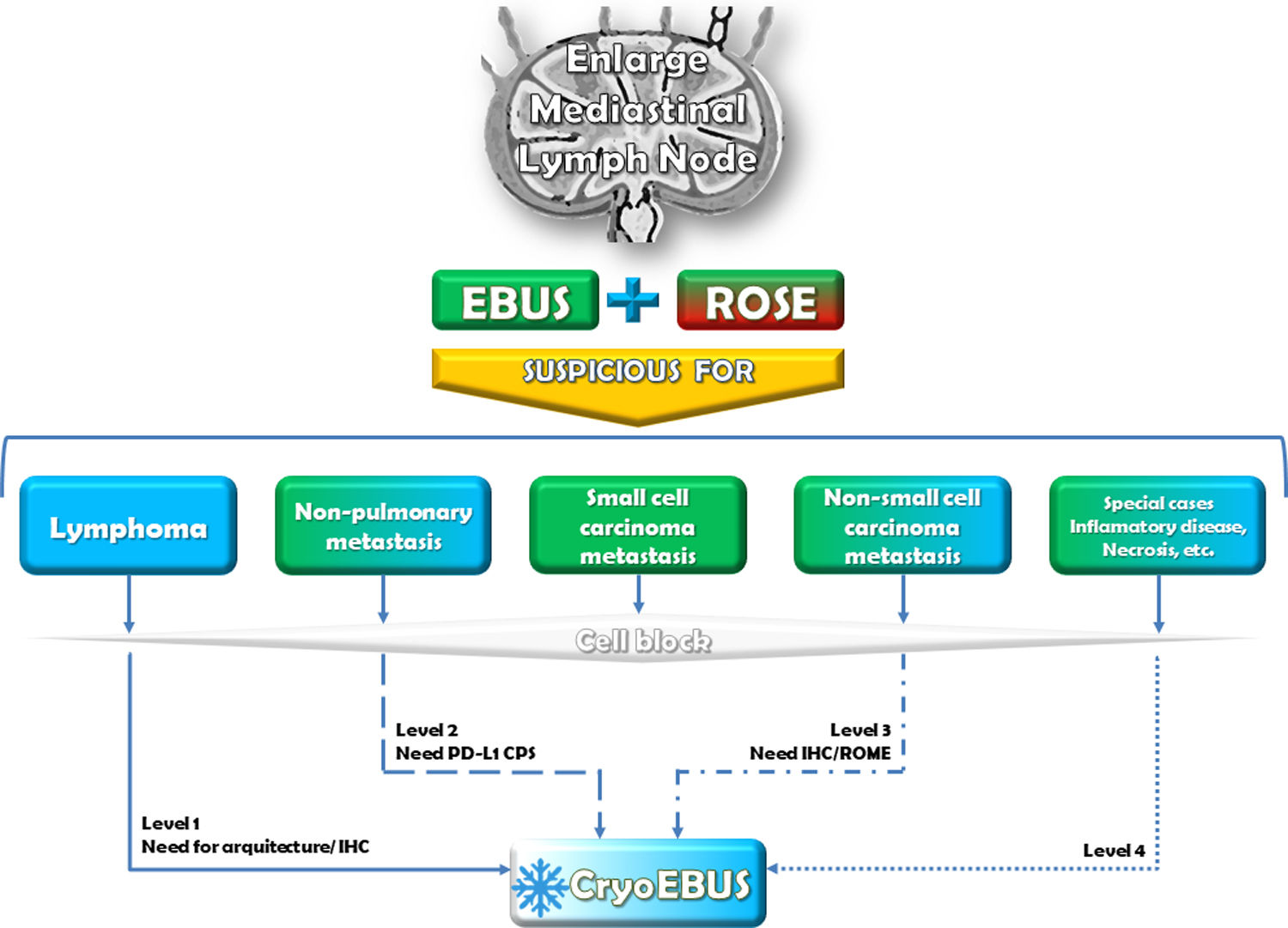
It is crucial to highlight that the procedures for obtaining mediastinal lymph node samples via EBUS-TBNA and CryoEBUS are complementary rather than mutually exclusive. They work in tandem to enhance diagnostic performance. The optimal scenario for mediastinal lymph node sampling involves an Interventional Pulmonology team trained in EBUS-TBNA and CryoEBUS techniques, along with an Interventional Pathology team proficient in guiding the sampling procedure. This collaboration ensures sample validity and appropriate handling to secure a comprehensive diagnosis (Fig. 2). While acknowledging that this ideal setup may not always be feasible due to resource availability and cost constraints, it remains essential for maximizing diagnostic outcomes. Given the established acceptance and diagnostic utility of lymph node cytologic sampling via EBUS-TBNA, it is advisable to consider the appropriate indications for when CryoEBUS should be utilised to optimize resources effectively. While recognising the expertise of the performing pulmonologist, we believe it is the pathologist who has the necessary experience to determine, based on clinical characteristics and ROSE information, whether EBUS-TBNA alone is sufficient or if combining both techniques with CryoEBUS is warranted. Drawing on our expertise in this field6,7,10,18 and our interpretation of relevant literature,8,23,24 we propose four levels of indication for CryoEBUS of the mediastinal lymph node (Box 1) as a guiding framework for pulmonologists and pathologists involved in mediastinal lymph node diagnosis. These levels of indication are not absolute; depending on the clinical context and sample collection scenario, the indication for CryoEBUS may vary, such as considering it the first option in granulomatous disorders (a priori level 4).
Diagnostic flowchart. Indication of EBUS-TBNA and CryoEBUS in the mediastinal lymph node. The study and sampling of mediastinal lymph nodes should ideally commence with EBUS-TBNA, incorporating the collection of cytologic smears and reserving a sample for a cell block. While ROSE during EBUS-TBNA plays a pivotal role in decision-making, the absence of an interventional pathologist may require the pulmonologist to rely on clinical suspicion for the subsequent steps. In such situations, the pulmonologist may decide on the next course of action based on the clinical context, which could involve additional EBUS-TBNA passes, transitioning to CryoEBUS, or concluding the procedure based on the available information and clinical assessment. The number of EBUS-TBNA and CryoEBUS passes to be performed depends on the specific scenario (without ROSE). Typically, this involves 1–3 passes for EBUS-TBNA and 2–3 for CryoEBUS. ROSE: rapid on-site evaluation; IHC: immunohistochemistry; PD-L1: programmed death ligand 1; CPS: combined positive score; ROME: rapid on-site molecular evaluation.
CryoEBUS diagnostic indication levels.
| Level 1 | The suspicion of a lymphoproliferative disorder serves as a priority indication for CryoEBUS, making its use essential whenever feasible. While the diagnosis of lymphomas remains a topic of debate, many authors advocate for diagnosing based on tissue sampling (incisional or excisional). CryoEBUS presents a valuable alternative before more invasive procedures, such as thoracotomy, are considered. In addition to examining lymph node architecture, CryoEBUS facilitates relevant immunohistochemical techniques and sample management, which should encompass evaluation through flow cytometry, cytogenetics, and/or molecular testing to provide a comprehensive diagnostic assessment. |
| Level 2 | The suspicion of metastases from non-pulmonary origins is considered a grade 2 indication for CryoEBUS. In addition to the common practice of immunohistochemical verification to determine the origin of the metastasis, assessments such as PD-L1 expression measured by combined positive score can be crucial. Although these determinations can be conducted on the EBUS cell block, the intact structure of CryoEBUS samples offers a superior approach for quantifying intratumoral inflammatory cells, specifically peritumoral cells displaying positivity. This preservation of the original position of these cells within the tissue allows for a more accurate assessment. |
| Level 3 | The need for molecular techniques in non-small cell carcinoma metastases is regarded as a relative indication for CryoEBUS. The expanding array of molecular tests in lung cancer highlights the need for larger samples or improved management to accommodate these evolving diagnostic requirements. In many instances, an EBUS-TBNA sample, featuring a sufficient number of cytologic smears and a representative cell block, suffices to meet diagnostic requirements. However, in certain situations, such as the growing inclusion in clinical trials, the CryoEBUS technique should be considered. This ensures a greater quantity of sample for potential molecular analyses and other diagnostic evaluations, thereby enhancing the capacity to meet these specific requirements. |
| Level 4 | Special cases falling under the category of miscellaneous are regarded as a marginal indication for CryoEBUS. In specific scenarios, such as when there is a suspicion of inflammatory or infectious diseases (e.g., sarcoidosis, tuberculosis), mediastinal lymph nodes displaying extensive necrosis, post-treatment staging, or instances of repeated insufficient sampling, CryoEBUS may be considered alongside EBUS-TBNA if available. The decision to use CryoEBUS in these cases may depend on factors such as non-representative ROSE results after multiple attempts, suggesting that CryoEBUS may yield a more comprehensive and diagnostic sample. |
In conclusion, the integration of the WHO reporting system alongside the implementation of CryoEBUS has achieved a diagnostic yield and accuracy of over 97% in the evaluation of mediastinal lymph nodes (Fig. 3).
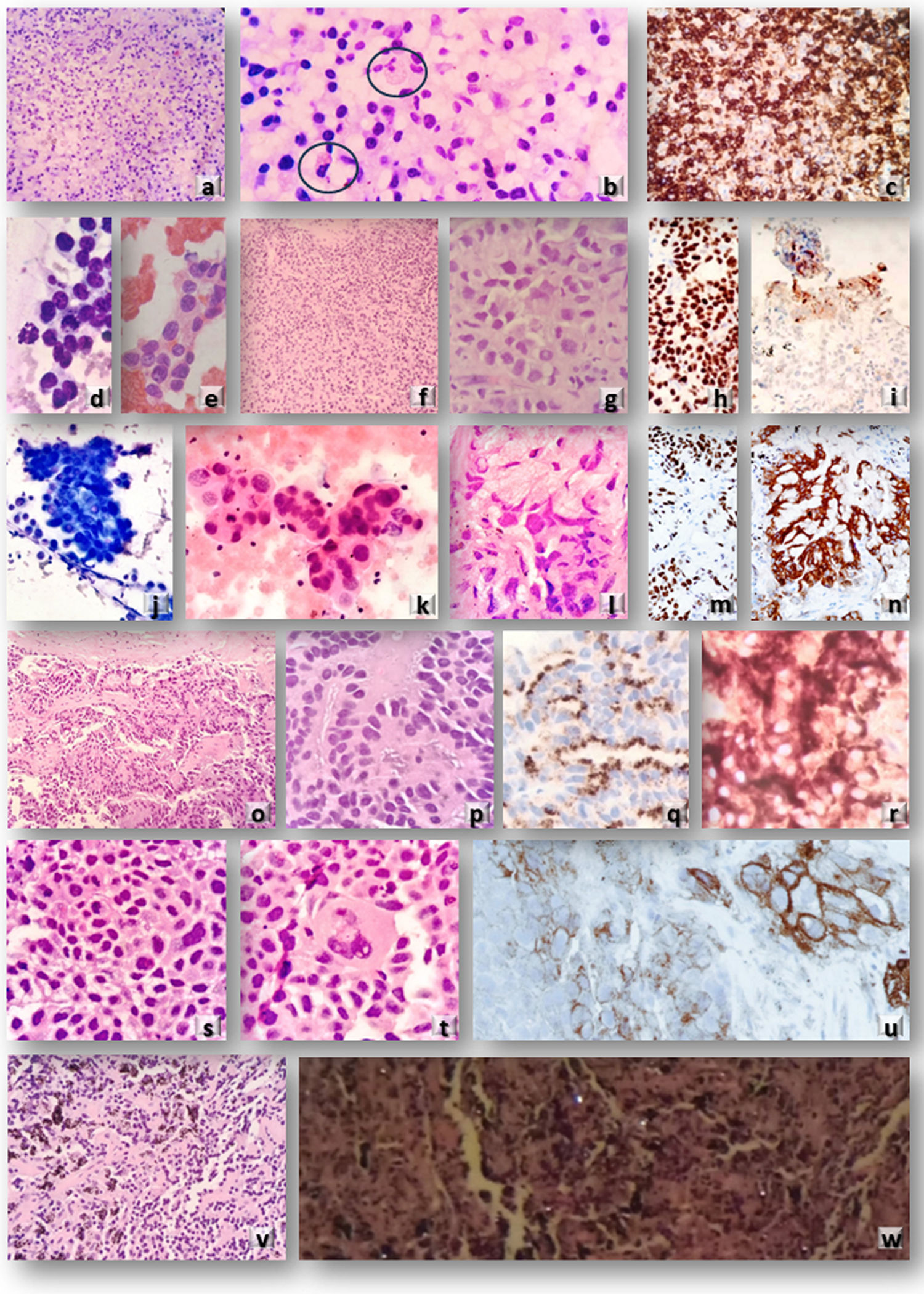
Microscopic evaluation of EBUS-TBNA cytologic and CryoEBUS tissue samples. Case 1: Clinical suspicion of lymphoproliferative disorder. (a and b) (H&E): CryoEBUS specimen from lymph node 7 revealed altered architecture with predominant plasma cells and occasional Russell bodies. Diffuse CD38 positivity confirmed the diagnosis of multiple myeloma (c). Case 2: Patient with a history of breast carcinoma and an enlarged mediastinal lymph node 4R. (d and e) Cytologic smears (Diff Quik© and H&E) demonstrated neoplastic epithelial cellularity. (f and g) (H&E): CryoEBUS confirmed neoplastic infiltration in solid nests. TRPS-1 positivity confirmed the breast origin of the metastasis (h). PD-L1 positivity in peripheral inflammatory cells was detectable due to sample architectural integrity (i). Case 3: Patient with a brain lesion, lung mass, and enlarged adenopathy. (j and k) Cytologic smears (Diff Quik© and H&E) from EBUS-TBNA indicated neoplastic epithelial cellularity consistent with adenocarcinoma metastasis (classified as WHO category 5). I (H&E): CryoEBUS showed neoplastic cellularity with a solid pattern. TTF1 positivity (m) and ALK positivity (n) confirmed using Idylla© orthogonal technique. Case 4: Patient with a history of prostatic carcinoma and an enlarged lymph node 10R. (o and p) (H&E): CryoEBUS revealed metastasis of adenocarcinoma, with prostatic origin confirmed through immunohistochemistry (q: p504s and r: PSA). Case 5: Patient with suspected recurrence of squamous cell carcinoma and 11L adenopathy with necrotic features. (s and t) (H&E): CryoEBUS demonstrated evidence of metastasis of squamous cell carcinoma, with PD-L1 positivity exceeding 50% (u). Case 6: Patient with occupational exposure to inorganic dust, lung parenchymal involvement, and mediastinal lymph node enlargement. (v) (H&E): CryoEBUS of 10L adenopathy exhibited abundant fibro-histiocytic cellularity along with anthracotic pigment deposits. Dark field microscopy revealed the presence of silica crystals (w).
Luis Manuel Fernández Fernández, MD. Conceptualization, data curation, formal analysis, resources, visualization, writing – original draft, and writing – review & editing.
María de la Paz González Gutiérrez, MD. Data curation, formal analysis, resources, visualization, writing – original draft, and writing – review & editing.
Miriam Rubiera, MD. Data curation, formal analysis, visualization, and review & editing.
Mario Luis Berrios Hernández, MD. Data curation, formal analysis, resources, visualization, writing – original draft, and writing – review & editing.
Clara González Rodríguez, MD. Visualization and review & editing.
Miguel Ariza Prota, MD. Formal analysis, resources, visualization, writing – original draft, and writing – review & editing.
José Fernando Pérez Fontán. Review & editing.
Karen Villar Zarra, MD. Visualization and review & editing.
Jesús Nieves-Alonso, MD. Formal analysis, visualization, and review & editing.
Javier Gómez-Román, PhD, MD. Formal analysis, visualization, and review & editing.
María Dolores Lozano, PhD, MD. Review & editing.
Miguel Ángel Pérez-Machado, PhD, MD. Review & editing.
Enrique Colado, MD. Review & editing.
Héctor-Enrique Torres Rivas, MD. Conceptualization, data curation, formal analysis, resources, visualization, writing – original draft, and writing – review & editing.
Ethical approvalThis study is exempt from the approval of the ethics committee because it is an observational study; therefore, the diagnosis or treatment of the patients was not affected in any way. Statistical analysis was performed using anonymised patient data.
FundingThere are no funding sources.
To cytotechnician staff (Pathology Department – HUCA).
To nursing staff (Pathology Department – HUCA).