To standardize acquisition protocols for 18F-Choline PET/CT to prevent from urine interference, to determine the best time point for the whole-body study, and to assess whether “dual point” acquisition allows for differentiating malignant vs. benign lesions.
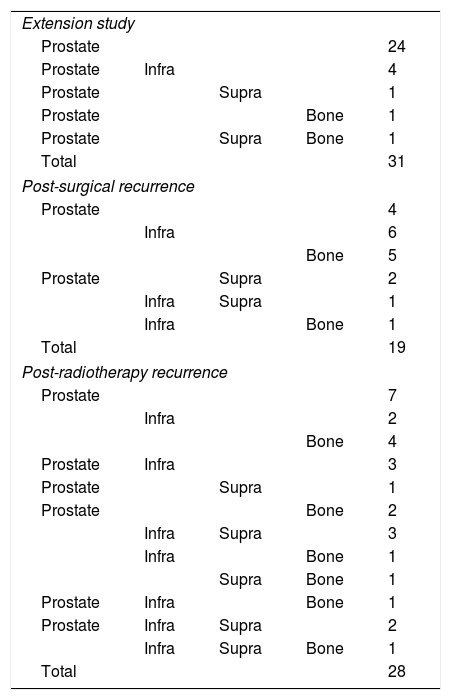
MethodsOne hundred consecutive patients with prostate cancer were prospectively studied. Immediately after 18F-Choline injection, a pelvis study was acquired, and a whole-body was subsequently obtained 1 and 2h p.i. Mean SUVmax was obtained in regions and for every sequential imaging. Mean analysis (χ2) and SUV percentage change (2/1h; 1h/0min) were obtained. Metabolic pattern dynamics were assessed: accumulative vs. clearance. Patient follow-up after therapy and directed classification whenever ethically possible were performed.
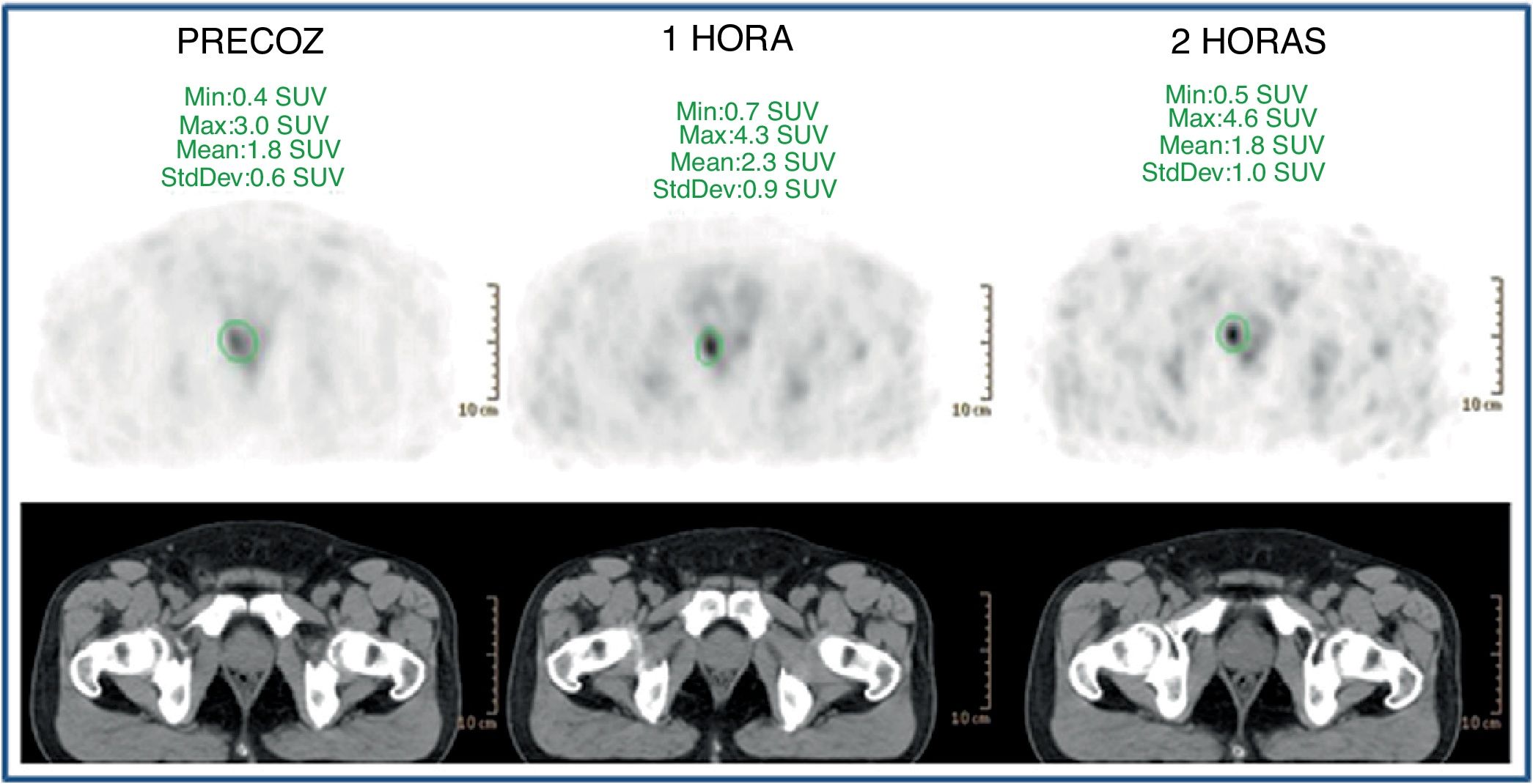
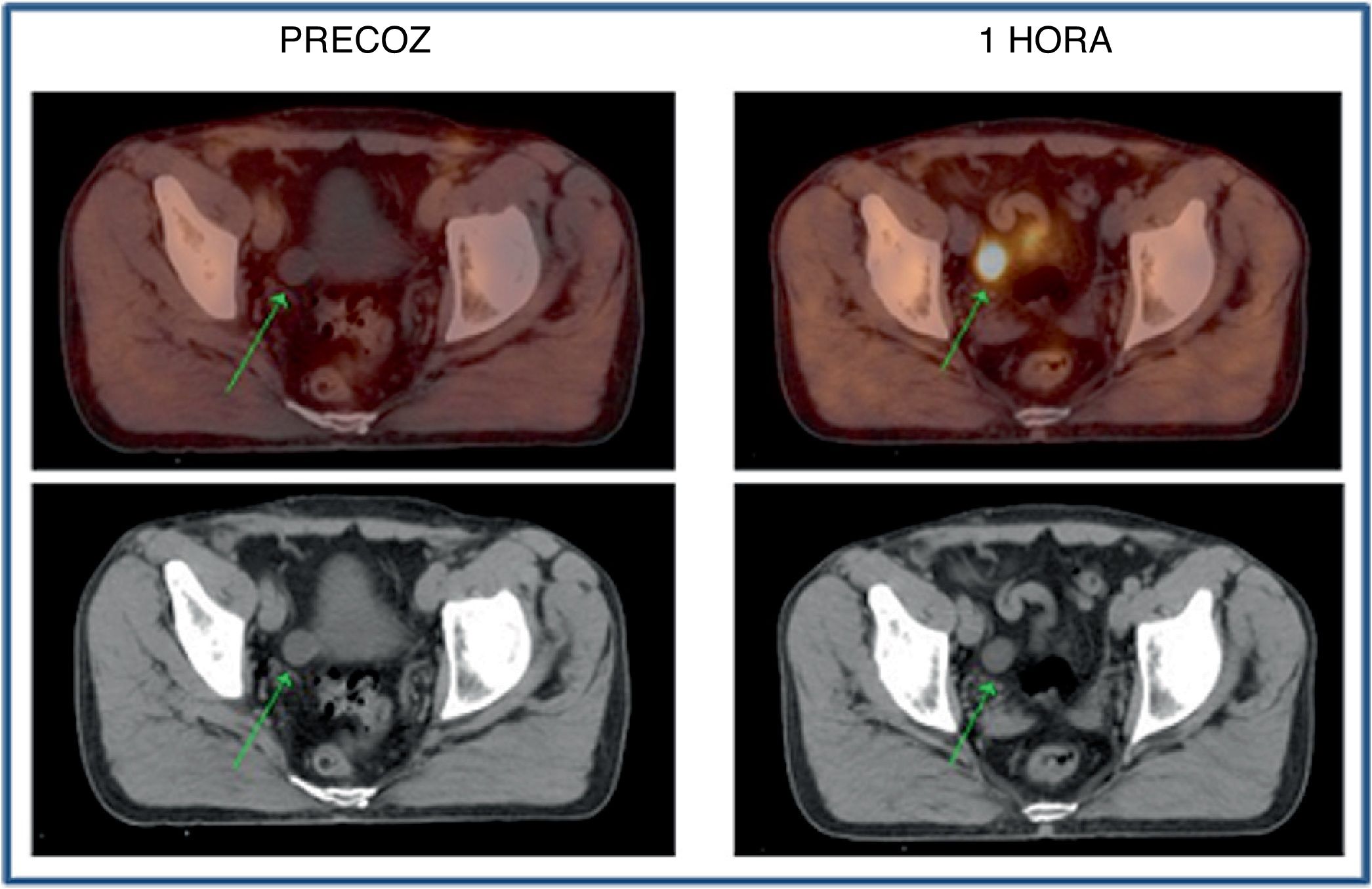
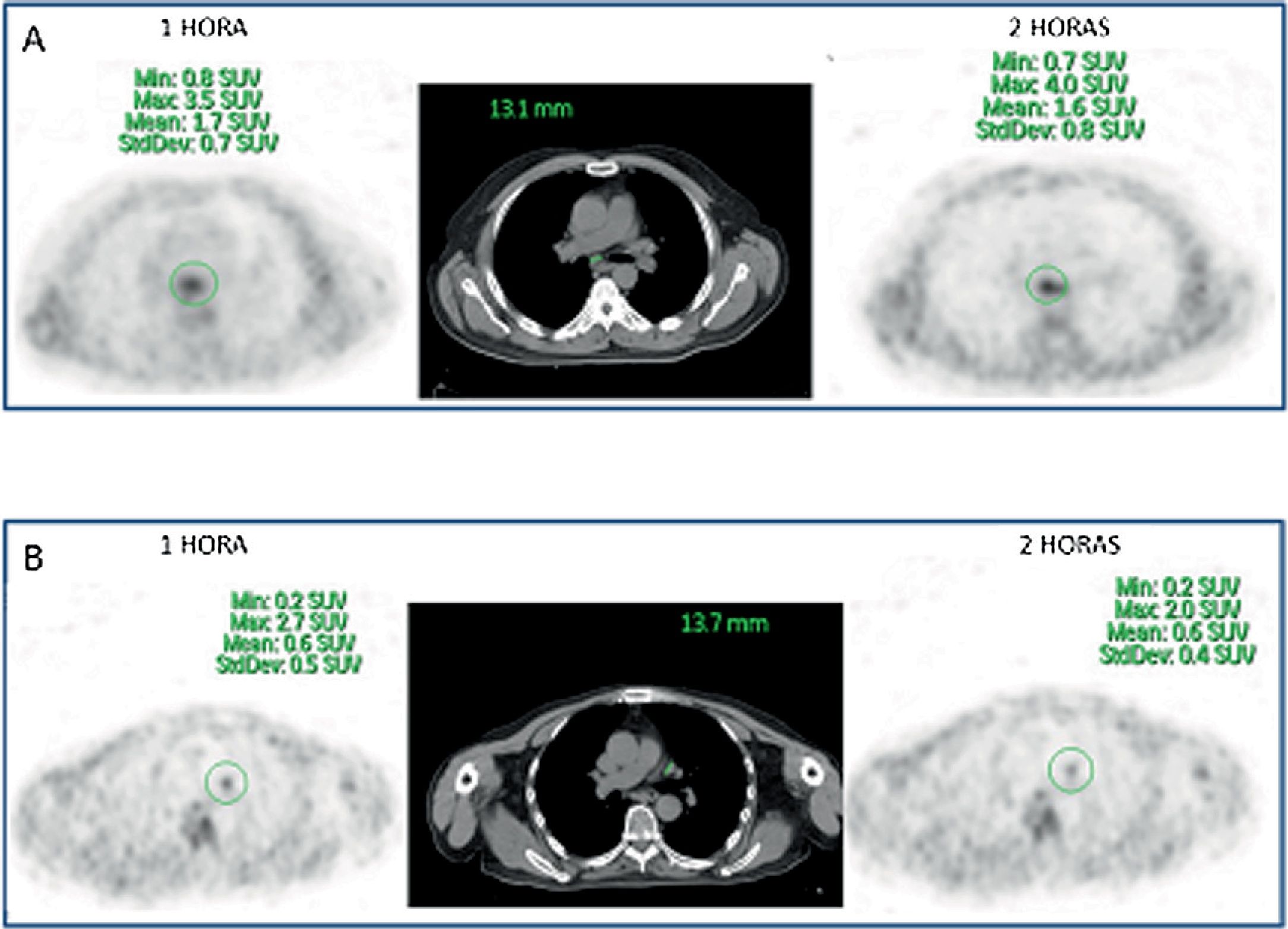
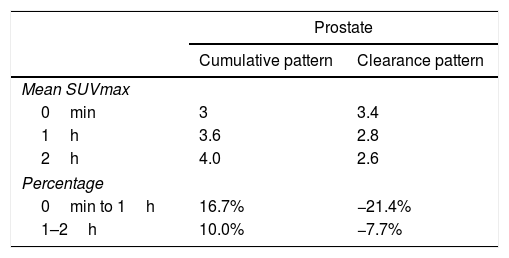
ResultsFifty-three prostate foci, without disturbing urinary activity was ever found on early images. Accumulative pattern in 42, with percentage increase was: 0min/1h: +16.7% (χ2 0.94); 1/2h: +10.0% (χ2 0.83). Clearance pattern in 11, with percentage decrease: 0min/1h: −21.4% (χ2 0.91): −7.7% (χ2 0.85), corresponding in 7 to initial staging and in 4 post-radiotherapy biochemical recurrence.
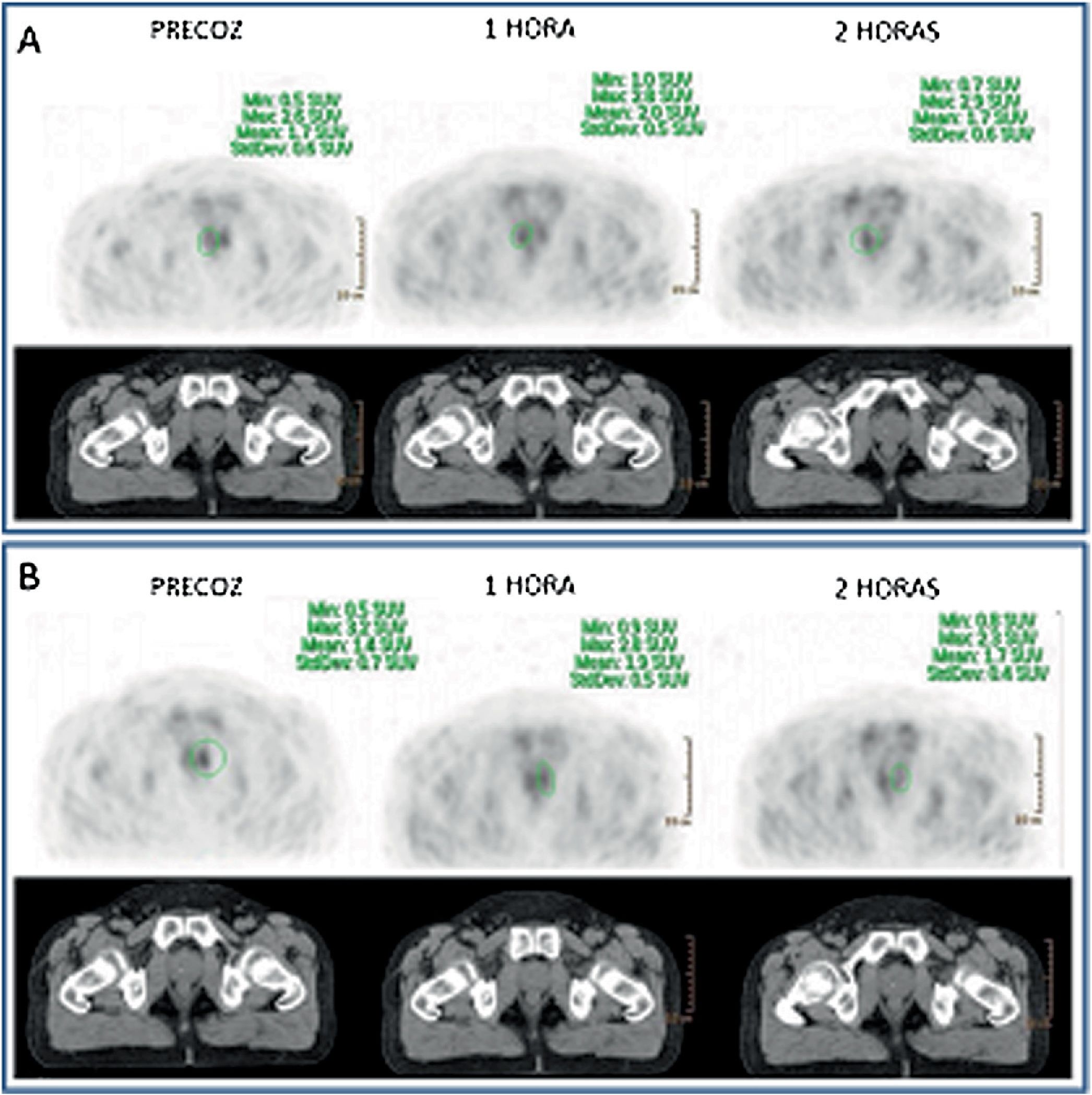
Every infradiaphragmatic uptake (n: 24) showed accumulative pattern, with percentage increase of +9.1% (χ2 0.97), all of them depicted on early imaging.
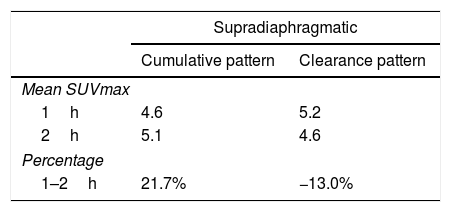
As for 12 supradiaphragmatic uptake, 8 of them showed clearance pattern with percentage decrease: −13.0% (χ2 0.95). Accumulative pattern showed in 4 of them with percentage increase +13.0% (χ2 0.96), thus being assessed as invasive/malignant.
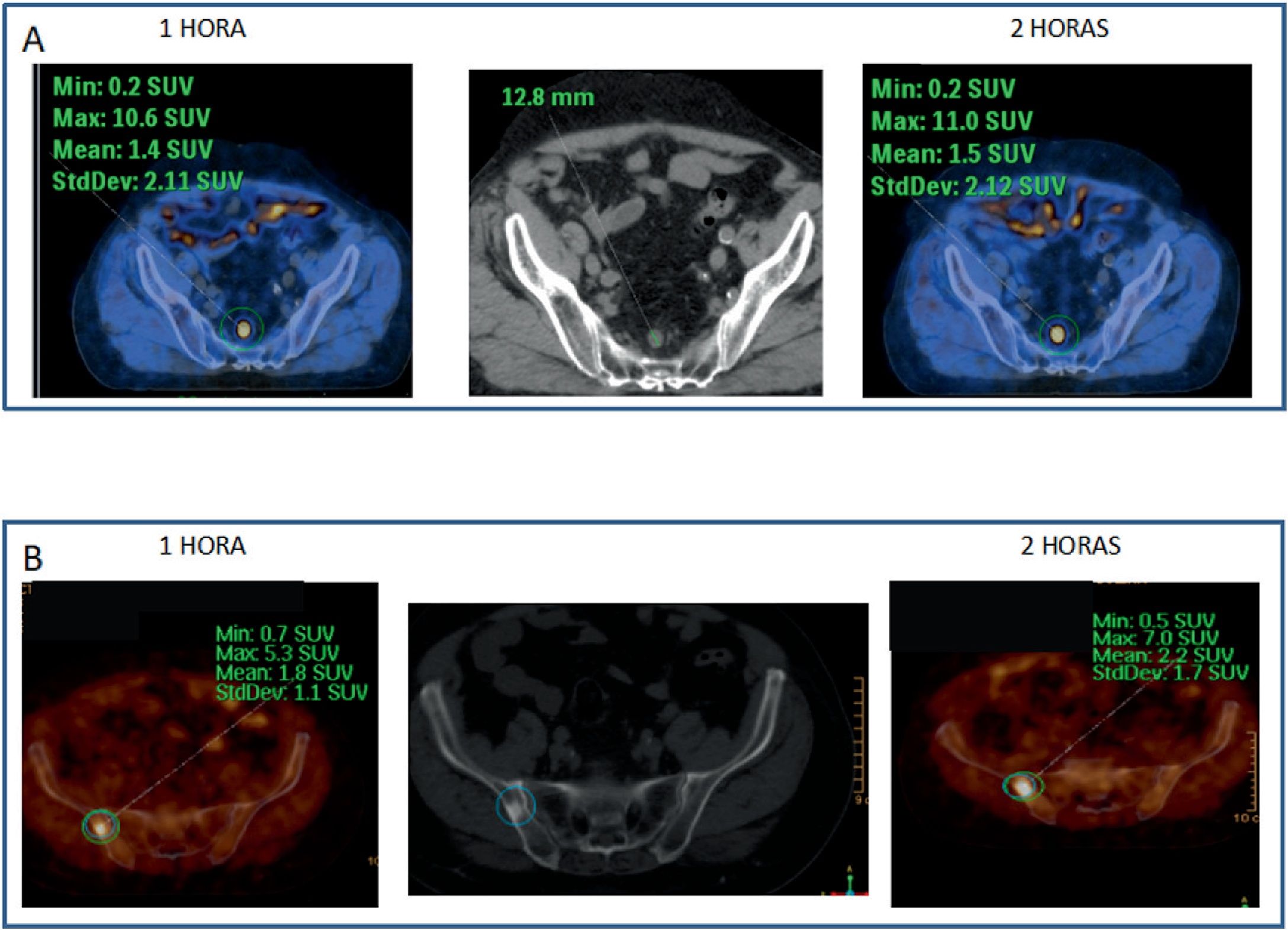
Every bone uptake (n: 18) showed accumulative pattern, with percentage increase: +17.1% (χ2 0.95), all of them depicted on 1h imaging.
ConclusionsAs for prostate assessment is concerned, dual point at 0min/1h proved to be the best procedure. As for supradiaphragmatic lymph-nodes detection, dual point with 1/2h performed best. As for infradiaphragmatic and bone involvement, as well as for inconclusive findings, the 2h imaging increased our diagnostic confidence.
Estandarizar los protocolos de adquisición de 18F-Colina PET/TC, que permitan evitar la interferencia urinaria, evaluar el mejor tiempo del estudio de cuerpo completo y valorar si la «doble fase» permite la diferenciación entre lesiones benignas frente a malignas.
MétodoEstudio prospectivo que incluye 100 pacientes con cáncer de próstata: 31 estadificación y 69 recidiva bioquímica (32 posprostatectomía y 37 posradioterapia). Adquisición pélvica inmediatamente tras inyección de 18F-Colina y estudio de cuerpo completo, 1 y 2h p.i. Cálculo de media SUVmáx por territorios en todos los estudios secuenciales. Análisis de medias (χ2) y porcentaje de cambio del SUV (2/1h; 1h/0min). Patrón de dinámica metabólica: acumulativo frente a aclaramiento. Seguimiento tras tratamiento en todos los pacientes y de forma dirigida, cuando éticamente es posible.
ResultadosCincuenta y tres focos prostáticos, en ninguna de las imágenes precoces actividad urinaria: Patrón acumulativo en 42, porcentaje de aumento: 0min/1h: +16,7% (χ20,94); 1/2h: +10,0% (χ20,83). Patrón aclaramiento en 11, porcentaje de reducción: 0min/1h: 21,4% (χ20,91); 1/2h: −7,7% (χ20,85), correspondiendo en 7 a estadificación y 4 a posradioterapia.
Todos los focos infradiafragmáticos (n: 24) mostraron dinámica acumulativa, porcentaje de aumento: +9,1% (χ20,97), todas ellas visibles en el primer estudio.
De los 12 focos supradiafragmáticos, 8 mostraron aclaramiento, porcentaje de reducción: −13,0% (χ2 0,95). Los otros 4 dinámica acumulativa, porcentaje de aumento: +13,0% (χ2 0,96), siendo valorados invasivamente.
Todos los focos óseos (n: 18) mostraron dinámica acumulativa, porcentaje de aumento: +17,1% (χ20,95), todas ellas visibles en el estudio 1h.
ConclusionesEn la valoración prostática la mejor técnica doble fase es 0min/1h. En la diferenciación de adenopatías supradiafragmáticas es aconsejable la técnica de doble fase: 1/2h. Para la infiltración infradiafragmática y ósea, ante hallazgos dudosos, las imágenes 2h aumentan la confianza diagnóstica.
Article
If you experience access problems, you can contact the SEMNIM Technical Secretariat by email at secretaria.tecnica@semnim.es or by phone at +34 619 594 780.

Revista Española de Medicina Nuclear e Imagen Molecular (English Edition)