To determine the effectiveness of therapy with the radiolabelled somatostatin analogs, 90Y-DOTATOC and 177Lu-DOTATATE, in the treatment of metastatic neuroendocrine tumors with progression to first-line treatment.
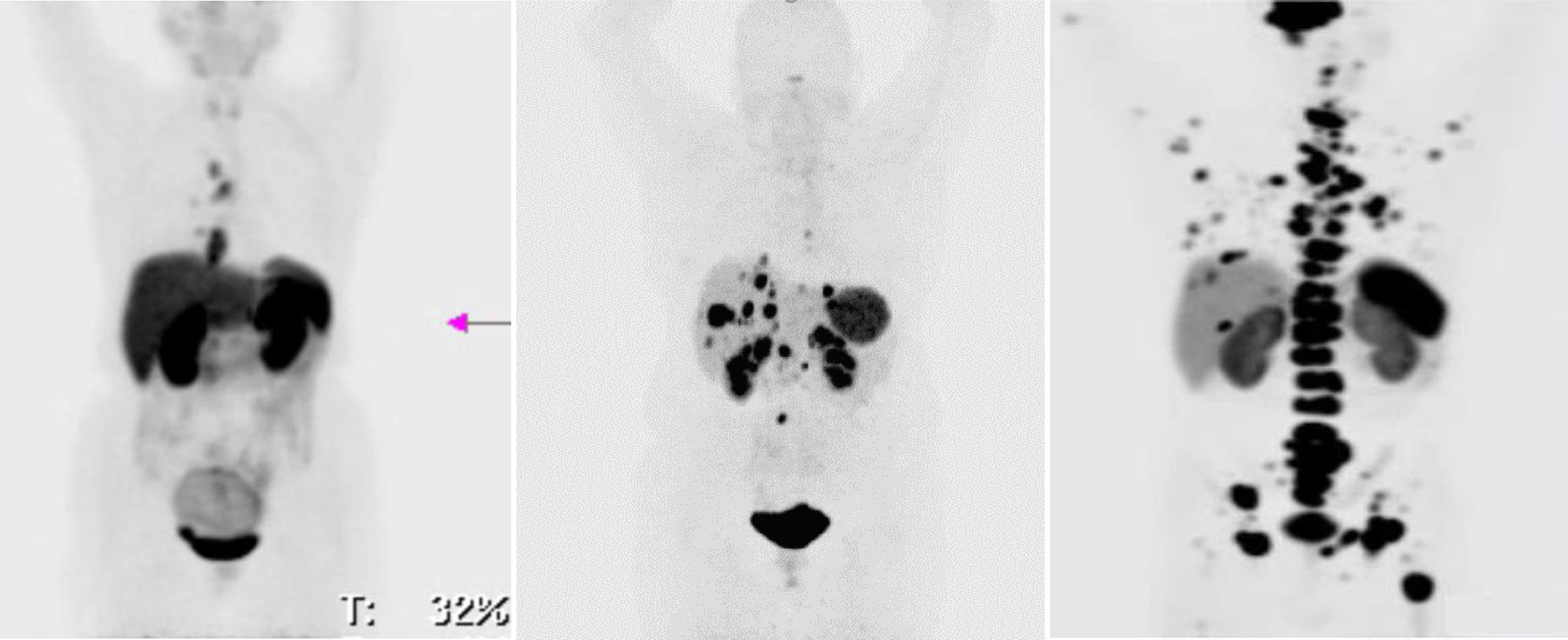
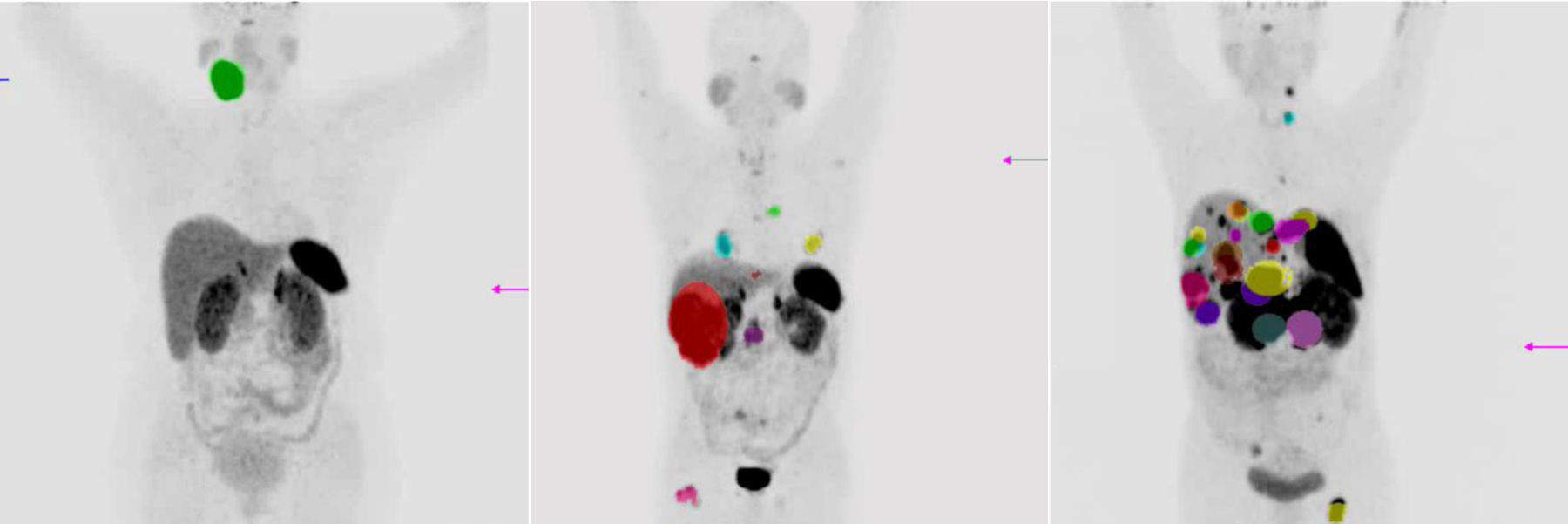
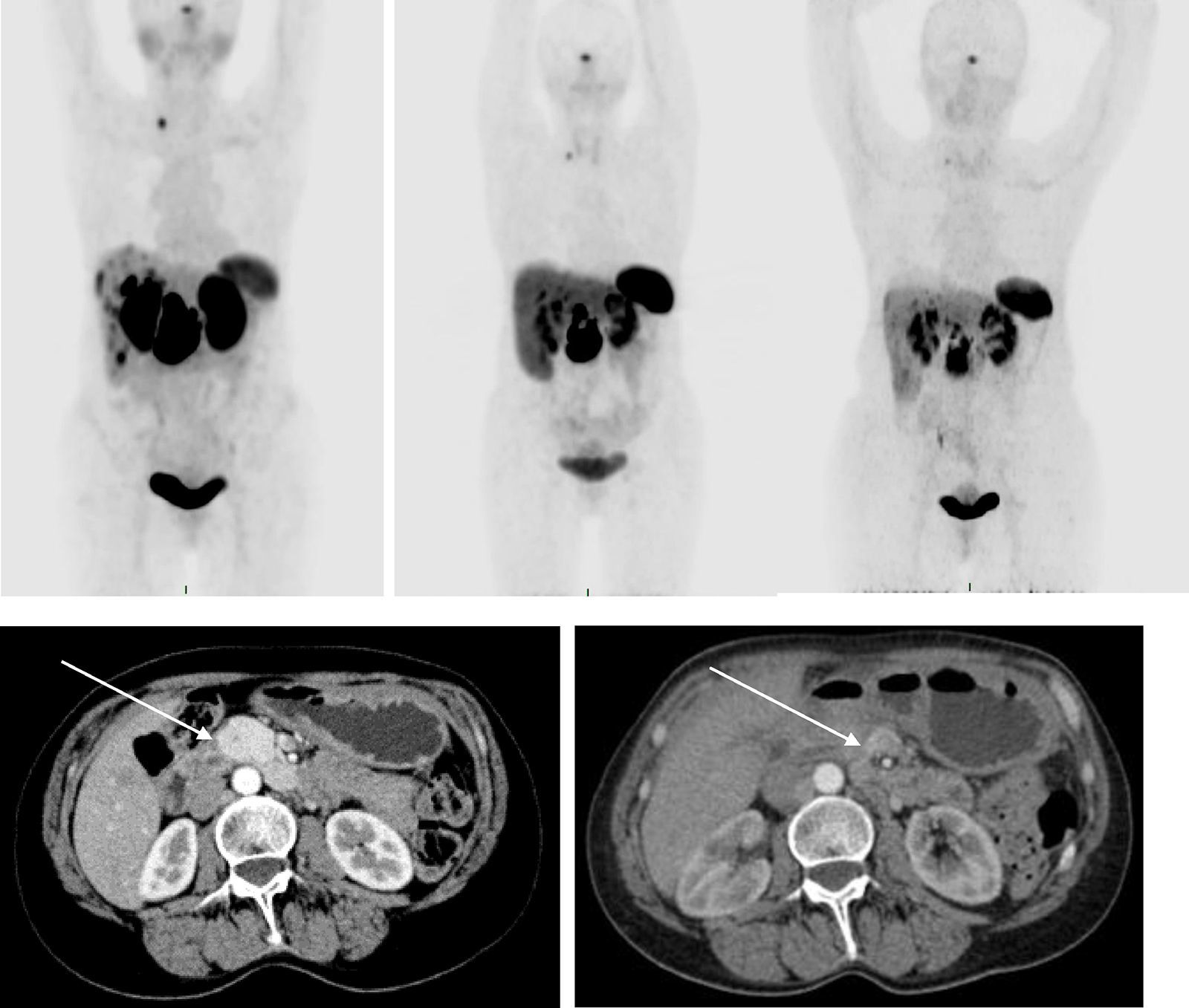
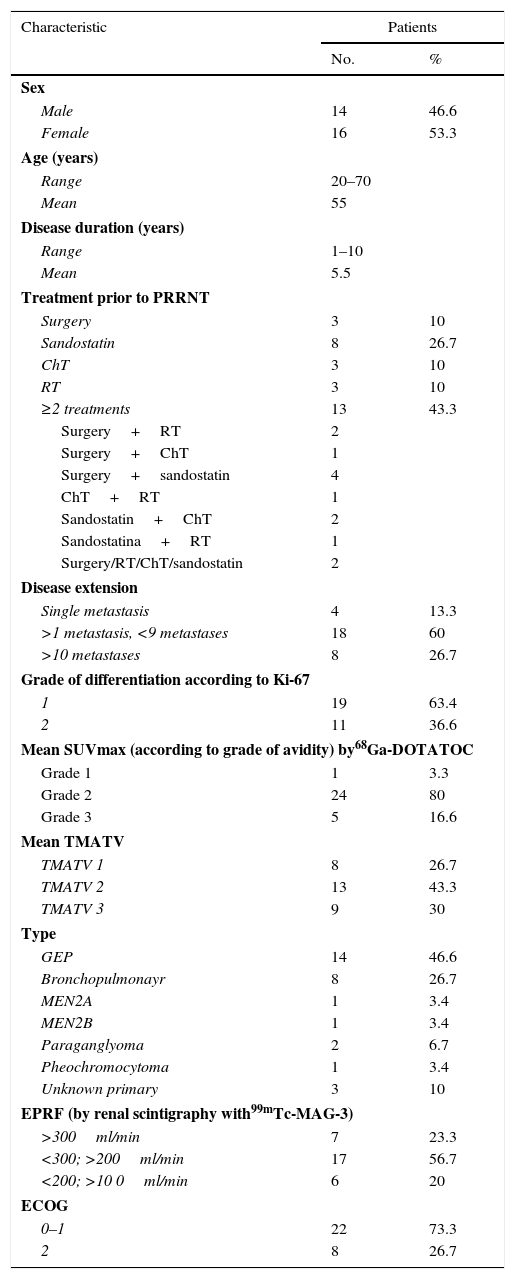
Material and methodsA study was conducted on 30 patients diagnosed with neuroendocrine tumors (gastroenteropancreatic, bronchopulmonary, MEN2A, MEN2B, phaeochromocytoma, and paraganglioma) with metastatic disease diagnosed by the pathology department, with progression to first-line treatment, and recruited from December 2014 to February 2016. Efficacy was analyzed using computed tomography (CT) according RECIST 1.1 criteria, and the molecular changes using the SUVmax of PET/CT with 68Ga-DOTATOC. Safety was carried out with a renal scan with 99mTc-MAG3.
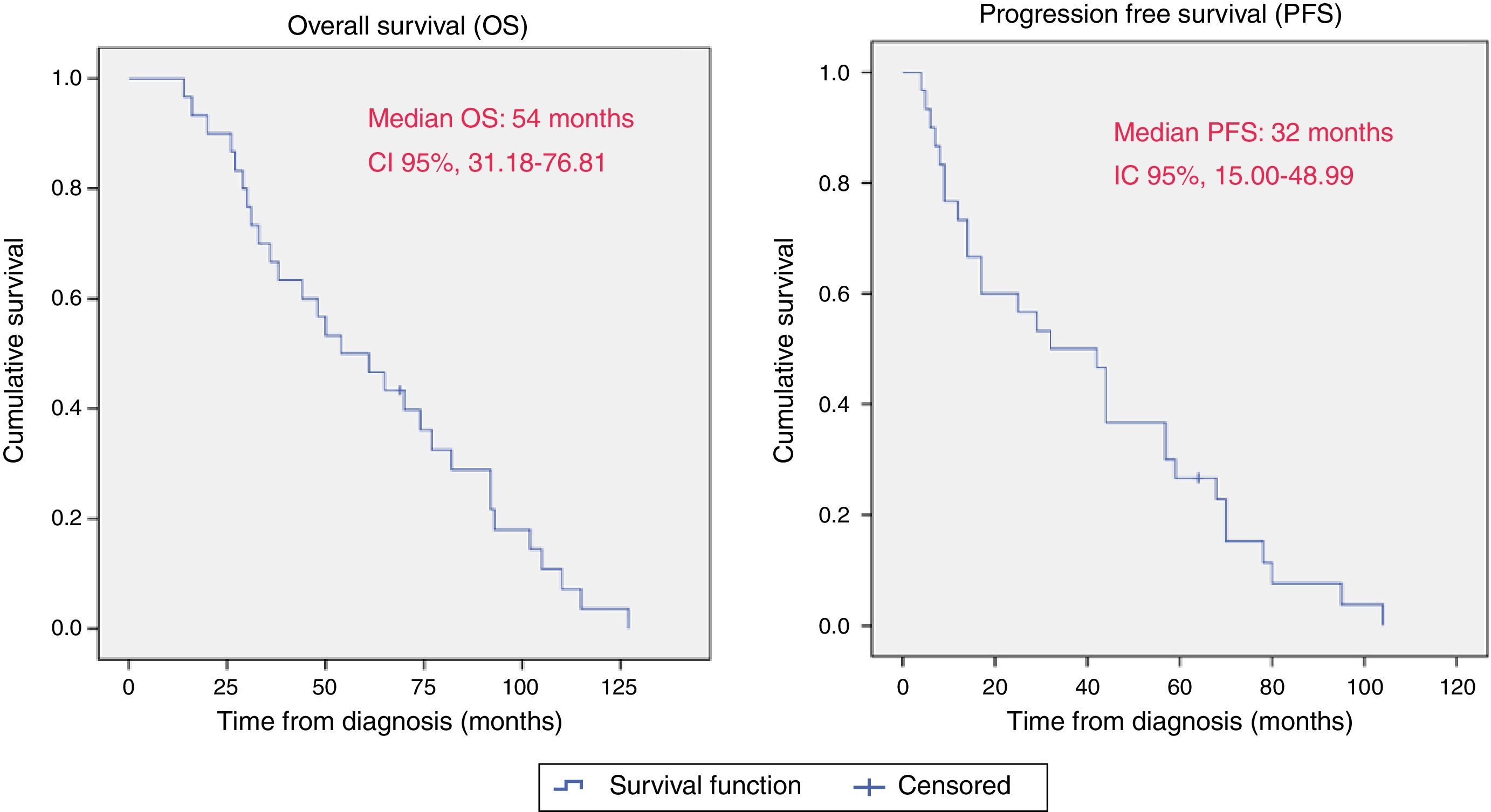
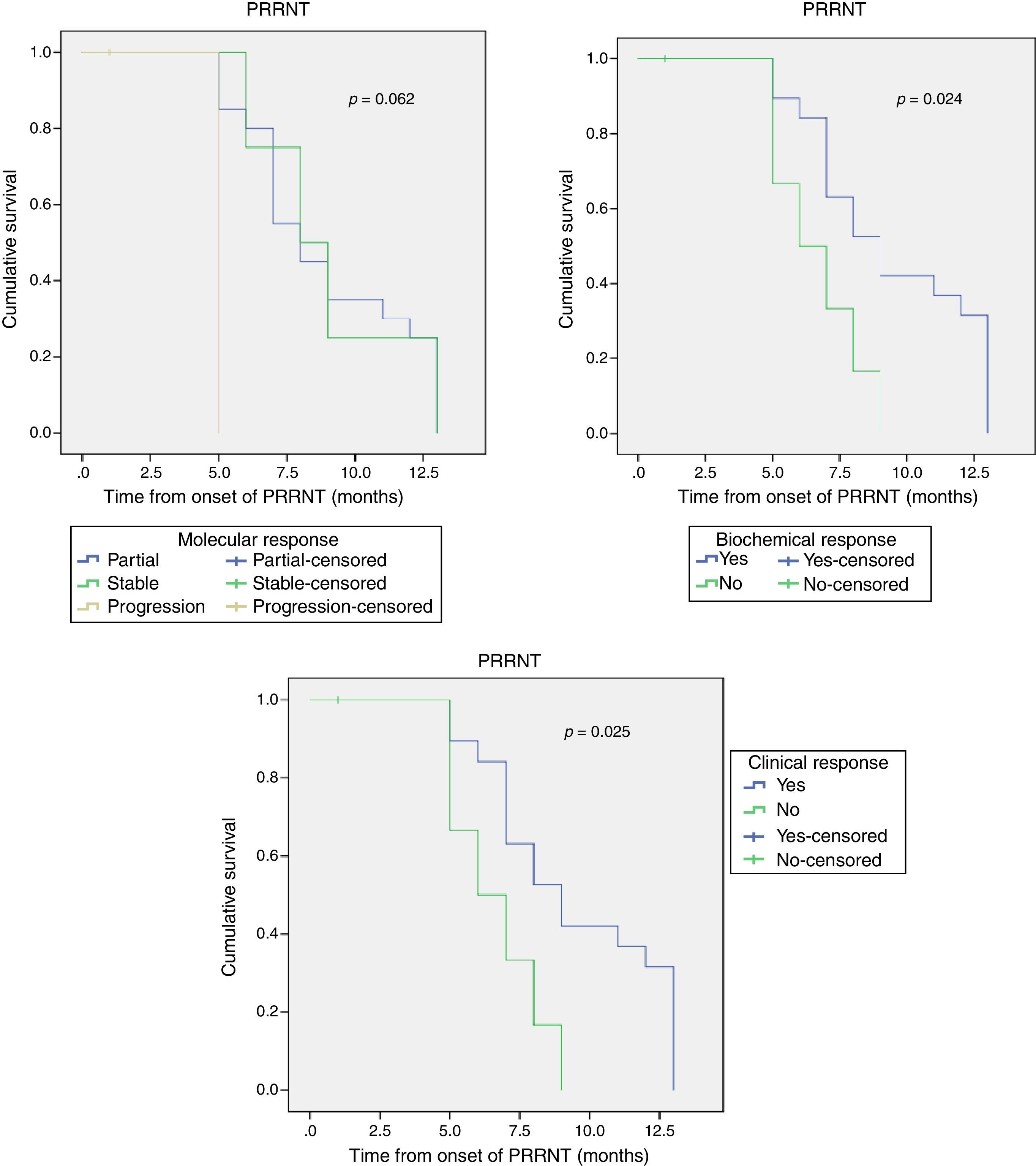
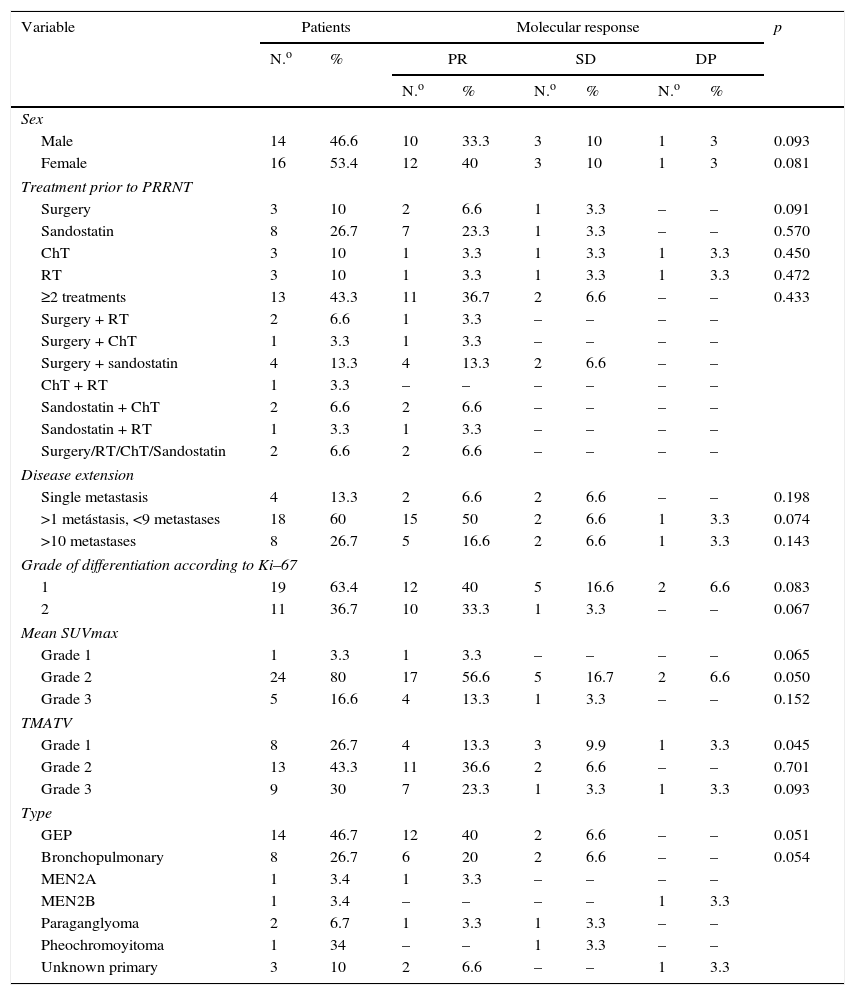
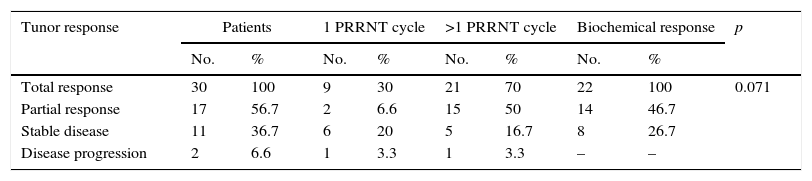
ResultsThe 30 patients received a total of 49 cycles 90Y-DOTATOC (21 doses) and 177Lu-DOTATATE (28 doses), with a mean of 1.5 cycles per patient. Of these, 17 (56.7%) showed a partial morphological response, 22 (73.3%) molecular and biochemical response, and 23 (76.6%) clinical response. One patient died during the median follow-up of 13 months. The median overall survival from diagnosis was 54 months (95% CI; 31.18–76.81), and median progression-free survival was 32 months (95% CI; 15.00–48.99).
ConclusionTherapy with 90Y-DOTATOC and 177Lu-DOTATATE is a promising therapy for patients with well and moderately differentiated neuroendocrine tumors. The efficacy is better the larger the number of cycles administered, inversely proportional to the number of metastases (<10), and is associated with the level of uptake according to the SUVmax by the metastases, regardless of metabolically active tumor volume.
Determinar la eficacia de la terapia con análogos de somatostatina radiomarcados 90Y-DOTATOC y 177Lu-DOTATATE en el tratamiento de tumores neuroendocrinos metastásicos con progresión después de la primera línea de tratamiento.
Material y métodosSe estudiaron 30 pacientes con el diagnóstico de tumor neuroendocrino (gastroenteropancreático, broncopulmonar, MEN2A, MEN2B, feocromocitoma y paraganglioma) con enfermedad metastásica diagnosticados por el departamento de patología, con progresión después de la primera línea de tratamiento, reclutados de diciembre del 2014 a febrero del 2016. La eficacia fue analizada usando la TC siguiendo los criterios RECIST 1.1 y los cambios moleculares en el SUVmáx de la PET/TC con 68Ga-DOTATOC. La seguridad fue valorada mediante el renograma con 99mTc-MAG3.
ResultadosLos 30 pacientes recibieron un total de 49 ciclos de 90Y-DOTATOC (21 dosis) y 177Lu-DOTATATE (28 dosis), con una media de 1,5 ciclos por paciente; 17 (56,7%) mostraron respuesta morfológica parcial; 22 (73,3%) respuesta molecular y bioquímica, y 23 (76,6%) respuesta clínica. Durante la mediana de seguimiento (13 meses) un paciente falleció (3,4%). La mediana de supervivencia global desde el diagnóstico fue de 54 meses (IC 95%, 31,18-76,81), y la mediana de supervivencia libre de progresión fue de 32 meses (IC 95%, 15,00-48,99).
ConclusiónEl tratamiento con 90Y-DOTATOC y 177Lu-DOTATATE es una terapia prometedora para los pacientes con tumores neuroendocrinos bien y moderadamente diferenciados. La eficacia es proporcional al número de ciclos administrados, inversamente proporcional al número de metástasis (<10) y en relación con el grado de captación conforme al SUVmáx por parte de las metástasis, independientemente del volumen tumoral metabólicamente activo.
Article
If you experience access problems, you can contact the SEMNIM Technical Secretariat by email at secretaria.tecnica@semnim.es or by phone at +34 619 594 780.

Revista Española de Medicina Nuclear e Imagen Molecular (English Edition)