Forensic physicians should consider the possibility that people who have died from violent or unknown causes may be infected by the virus SARS-CoV-2, or that the diagnosis of the disease has legal implications, which requires adequate knowledge of the epidemiology of the disease, protective measures, adequate sampling and the pathological characteristics.
The practice of autopsies on people who have died from COVID-19 has been limited by the mandatory preventive measures against contagion and by the need for facilities with a level of protection against level-3 biological risk, and therefore series published to date are scarce and partial,with limited approaches (minimally invasive autopsy or needle biopsy).
This article reviews the aspects of the pathophysiology of the disease that have an impact on the infectivity of the body's tissues and fluids, measures for preventing biological risk, taking samples and pathological findings, both macroscopic and microscopic, associated with death caused by infection with the SARS-CoV-2 virus.
La medicina forense debe contemplar la posibilidad de que fallecidos por causas violentas o desconocidas puedan estar infectados por el virus SARS-CoV-2, o que el diagnóstico de la enfermedad tenga implicaciones legales, lo que exige un conocimiento adecuado de la epidemiología de la enfermedad, de las medidas de protección, de la toma de muestras y de las características anatomopatológicas.
La práctica de autopsias en fallecidos por COVID-19 se ha visto limitada por las obligadas medidas preventivas frente al contagio y por la necesidad de disponer de instalaciones con nivel de protección frente a riesgos biológicos de nivel 3, de modo que las series publicadas hasta la fecha son escasas, y parciales, con abordajes limitados (autopsia mínimamente invasiva o biopsia con aguja gruesa).
En este artículo se hace una revisión de los aspectos de la fisiopatología de la enfermedad que tienen repercusión en la infectividad de los tejidos y fluidos del cadáver, de las medidas de prevención del riesgo biológico, de la toma de muestras y de los hallazgos patológicos, tanto macroscópicos como microscópicos, asociados a la muerte provocada por la infección por el virus SARS-CoV-2.
The coronaviruses (Orthocoronavirinae) are a sub-family of positive single-strand RNA virus with a genome size of from 26 to 32 kilonucleotides, making them some of the largest of the RNA viruses, with a diameter of from 120 to 160 nm. They have a characteristic morphology, with spicules on their surface which makes them look “crowned”. They have been known since the mid-twentieth century as viruses which infect domestic and wild animals, especially mammals, and in the human species they cause a large number of trivial infections of the upper respiratory tract. They are divided into 4 subtypes: alpha, beta, gamma and delta.1
In spite of their seeming harmlessness, the betacoronaviruses have caused 2 epidemics this century with major medical repercussions: the epidemic caused by severe acute respiratory syndrome coronavirus (SARS-CoV), which appeared in China in 2002–2003, and the one caused by Middle East respiratory syndrome coronavirus (MERS-CoV), which fundamentally arose in Middle Eastern countries in 2012, with a mortality rate of 35%.2
All of these viruses have a natural reservoir, bats, and they reach the human species through an intermediate reservoir that is usually a mammal. The new virus, which was first isolated in December 2019 in the city of Wuhan, China,3 is a new betacoronavirus, although it has a genomic structure very similar to those of SARS and MERS. It was first denominated 2019-nCoV, but the International Committee on Taxonomy changed this to SARS-CoV-2, using COVID-19 to refer to the disease which it causes. This virus causes a respiratory infection that in some cases develops into pneumonia and has an overall mortality rate of 5%, although there are very large differences between countries and the figures are changing very rapidly.4
Two genes which synthesise polymerase and RNase stand out in its molecular structure (ORF1a and ORF1b), together with gene S, which synthesises the surface spicules. This gene S has 2 subunits: S1, which creates the bond with the AC2 receptor of the cell membrane, and S2, which binds to another coreceptor and produces the fusion with the cell membrane and the entry of the virus into the cell. Once in the cytoplasm, the virus produces polyproteins which are cut by the cellular proteases, giving rise to structural components and the viral RNA which is taken through the Golgi apparatus and the endoplasmic reticule, generating cytoplasmatic vesicles that are then released through the cell membrane, creating thousands of copies of the virus in each vesicle.5
The lung is the most affected organ and is therefore considered to be the target organ of the infection. Nevertheless, some studies underline the multisystemic involvement associated with inflammation and apoptosis of the vascular endothelium.6
The virus binds to AC2 receptors, which are widely distributed throughout the organism and most particularly in the alveolar pneumocytes, and it causes a cytokine storm in which IL-1, IL-6, IL-8 and macrophage migration inhibition factor stand out. In turn these factors attract polymorphonuclear neutrophils, which release enzymes and proteases and aggravate cellular damage, giving rise to adult respiratory stress syndrome, with the formation of the typical hyaline membranes on the internal surface of the alveolar wall, and the resulting alteration of gas exchange and tissue oxygenation.7
The disease is basically transmitted by respiratory secretions, in person to person contact, by Flügge droplets or by deposits on the ground and surfaces. Oral-faecal transmission has also been proven to occur, although it seems to be less important. Transmission while asymptomatic is possible, as is transmission after cure, which is why the WHO recommends isolation during at least 2 weeks after discharge.8 Recent studies show that the SARS-CoV-2 virus lasts in the air for several hours, and that it may last for days on some surfaces such as plastic and metal.9
The average incubation period lasts for 5 days (from 2 to 14 days) and the symptoms are similar to those for a viral infection, with an irritant cough, fever and diarrhoea and vomiting in some cases. Although the majority of cases go on to recover spontaneously, others develop pneumonia and multiple organ failure, these being the most severe cases and the ones with the highest mortality. This higher rate of complications and mortality is associated with an age over 60 years old and certain pre-existing complaints, such as obesity, hypertension, diabetes and pulmonary and cardiovascular diseases. As well as pneumonia and the said adult respiratory distress syndrome, the complications also consist of septic shock, kidney failure, intestinal ischemia,6 disseminated intravascular coagulation and rabdomyolysis.10
As well as the clinical manifestations, CT study seems to be more sensitive than conventional radiology in cases which develop pneumonia to detect alveolar infiltrates that may progress to become adult respiratory distress syndrome, with typical ground glass images. Recently several cutaneous manifestations have been reported, in the form of areas of erythema with vesicles or pustules, urticaria, maculopapular eruptions and necrosis.11
Viral gene amplification is basic in the laboratory using RT-PCR techniques. In general, this is performed in two stages. In the first determination the E gene common to several types of coronavirus is amplified, and once it has been detected, in a second stage the polymerase RNA-dependent RNA gene specific for the SARS-CoV-2 virus is amplified. Other determinations are based on the detection of viral antigens by means of fast tests that are highly specific but low in sensitivity, leading to a high percentage of false negatives. The antibody tests have the particular feature that they may give negative results during the first days of the infection, given that antibodies only appear once a certain level of immunity has developed.12
National and international biosafety and protection recommendationsDeath caused by infection is generally excluded from judicial and forensic interest.13 Nevertheless, infection by SARS-CoV-2 is associated with a high rate of mortality, and many carriers are known to exist who have no symptoms or only mild ones, so that it is possible that some of the corpses that will be subjected to a medical-legal autopsy are infected by this virus. It is therefore important to know what the scientific community has recommended in terms of protection against the biological risks arising from the study of a corpse with disease caused by COVID-19, or the suspicion of this.
There is general agreement, at this time when the pandemic is spreading, that corpses should not be manipulated whenever this is possible. Post-mortem examinations should be limited in number and scope to a specific objective, taking advantage of the options that are legally available in each country.14,15
There is no established scientific evidence regarding the infectious capacity of the corpses of individuals who have died due to SARS-CoV-2, so that protective measures have to be maximised.16 Several international bodies, such as the European Centre for Disease Prevention and Control, report that there is no evidence of SARS-CoV-2 transmission through handling the corpses of people who died due to COVID-19. The potential risk of transmission is considered to be restricted to direct contact with the corpse or its fluids, as well as contaminated fomites. For the same reason, procedures which create aerosols or splashing, as occurs during autopsy, lead to a higher biological risk and make it necessary to use suitable personal protective equipment.17
The main international protocols classify the SARS-CoV-2 virus as a level 3 biological risk. This group includes infectious agents that may cause severe human diseases and are a major risk for the community, although it is possible to establish effective prophylaxis to prevent them. The risk generated by these agents for workers may be considered to be acceptable when the proper preventive measures are applied.18 According to these protocols, there is no reason why a level 3 pathogen cannot be processed in an autopsy room with suitable preventive measures, on condition that it is performed by experienced personnel.19
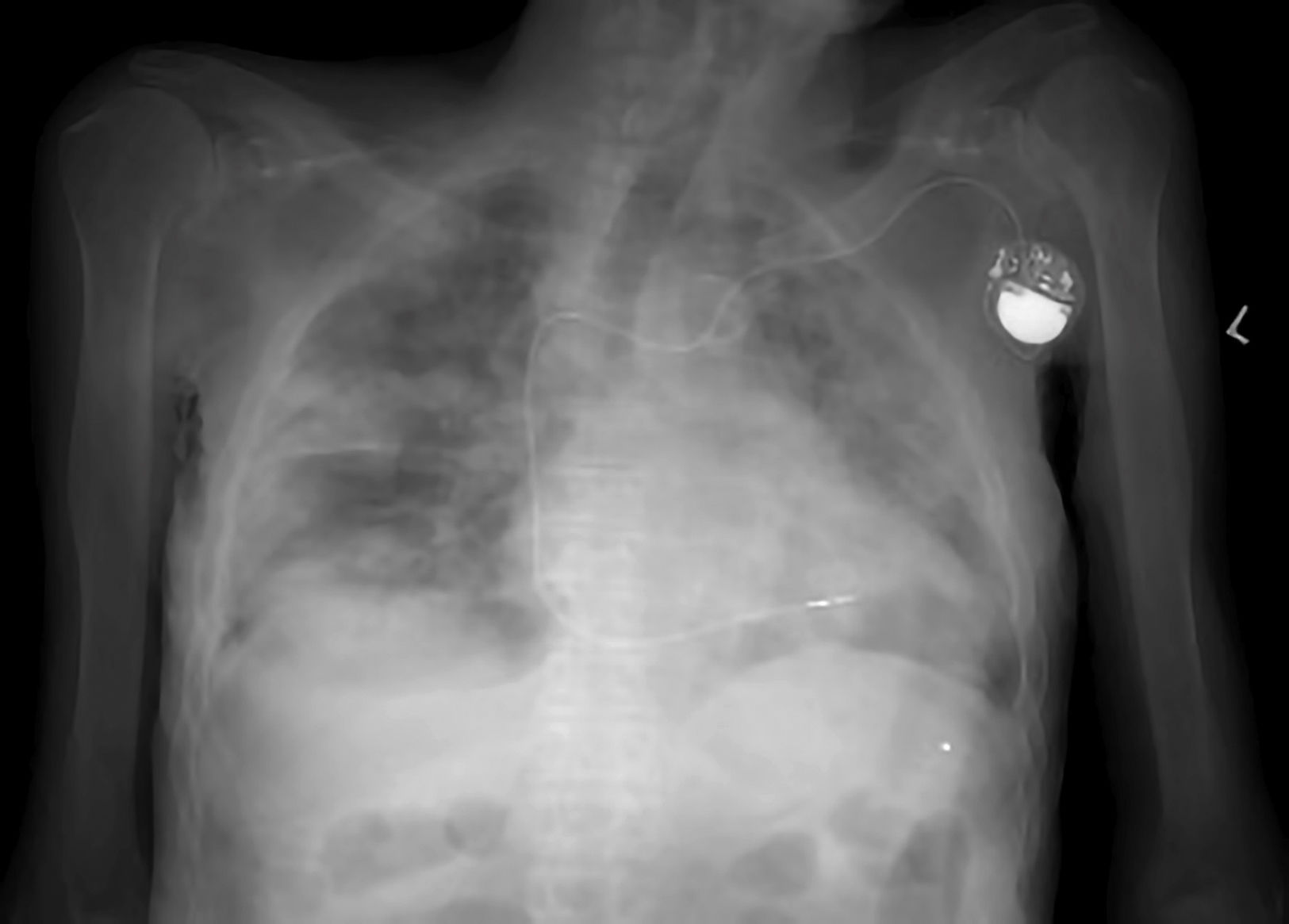
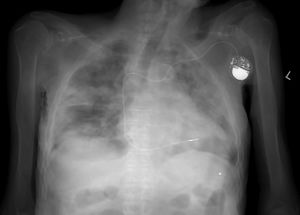
Given the risk that the corpses of individuals who died due to other causes may be carriers of the virus, it is advisable to adopt additional measures. These affect the design of autopsy rooms, the air pressure within them and the occasional use of radiology and CT imaging in suspicious cases (Fig. 1), to avoid handling a corpse.20
Additionally, the following special measures have been suggested for performing autopsies during the epidemic:
- 1.
Rigorously restrict these studies to those institutions with sufficient biosafety conditions and duly experienced personnel.21
- 2.
Restrict the scope of study to taking sufficient samples for diagnosis using external examinations, partial autopsies and core needle biopsies,22 depending on the circumstances.
- 3.
Apply diagnostic tests for the presence of the virus in a corpse prior to performing an autopsy.23 This recommendation may be restricted by the availability of tests or laboratories during the pandemic.
There is a guide to action in Spain24 that covers basic aspects of level 3 biosafety which must be fulfilled by personnel as well as installations, as well as obligatory actions with the corpse and cleaning after performing autopsies, along the line of the other aforementioned international bodies. More specifically, the Spanish Society of Forensic Pathology has published an ad hoc document for performing forensic autopsies.25
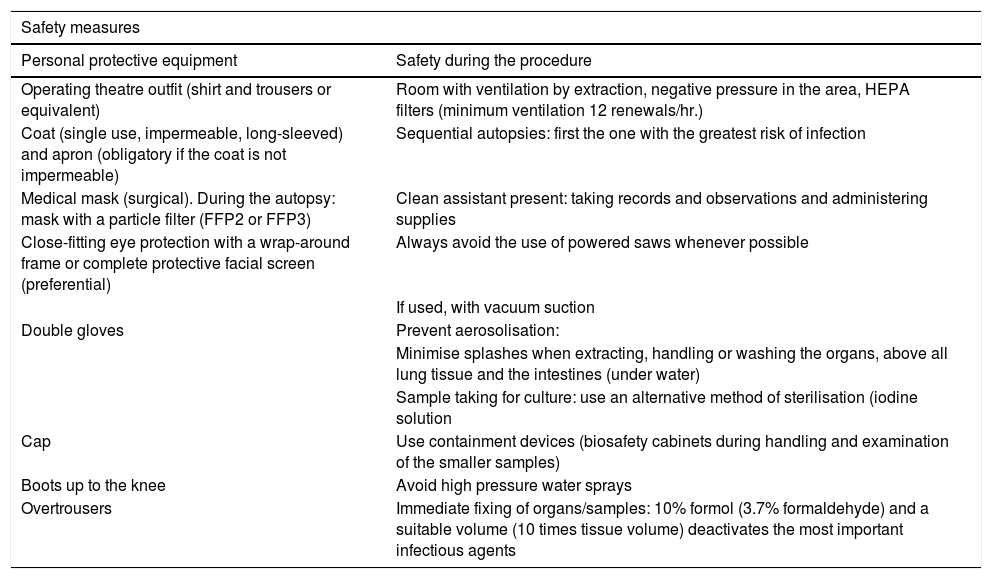
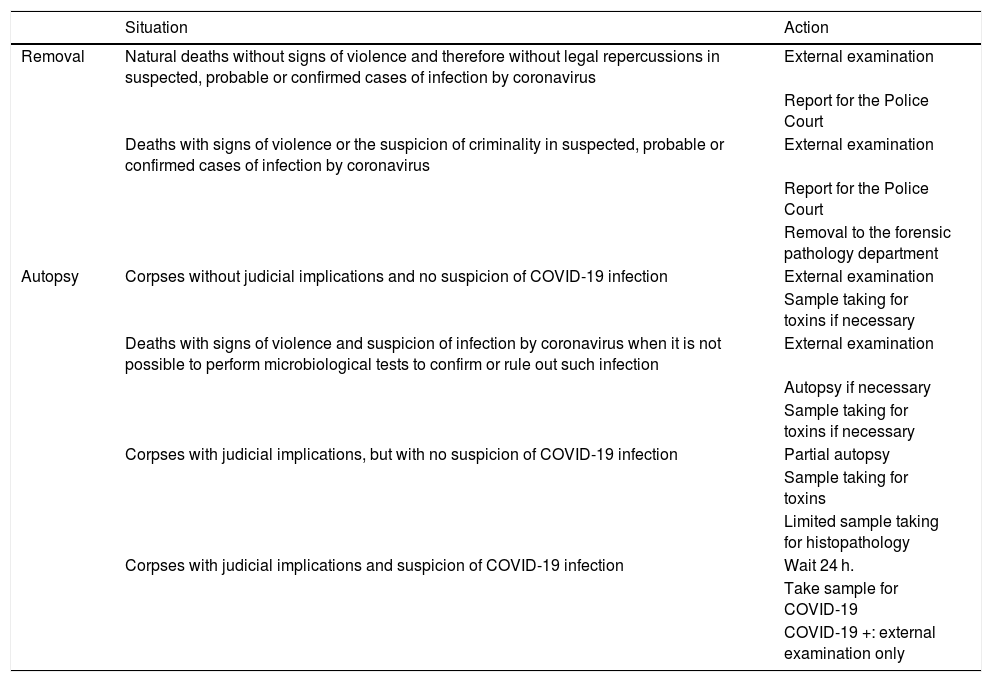
The main recommendations contained in these documents are described n Tables 1 and 2.
Summary of technical document recommendations. Procedure for managing COVID-19 corpses.
| Safety measures | |
|---|---|
| Personal protective equipment | Safety during the procedure |
| Operating theatre outfit (shirt and trousers or equivalent) | Room with ventilation by extraction, negative pressure in the area, HEPA filters (minimum ventilation 12 renewals/hr.) |
| Coat (single use, impermeable, long-sleeved) and apron (obligatory if the coat is not impermeable) | Sequential autopsies: first the one with the greatest risk of infection |
| Medical mask (surgical). During the autopsy: mask with a particle filter (FFP2 or FFP3) | Clean assistant present: taking records and observations and administering supplies |
| Close-fitting eye protection with a wrap-around frame or complete protective facial screen (preferential) | Always avoid the use of powered saws whenever possible |
| If used, with vacuum suction | |
| Double gloves | Prevent aerosolisation: |
| Minimise splashes when extracting, handling or washing the organs, above all lung tissue and the intestines (under water) | |
| Sample taking for culture: use an alternative method of sterilisation (iodine solution | |
| Cap | Use containment devices (biosafety cabinets during handling and examination of the smaller samples) |
| Boots up to the knee | Avoid high pressure water sprays |
| Overtrousers | Immediate fixing of organs/samples: 10% formol (3.7% formaldehyde) and a suitable volume (10 times tissue volume) deactivates the most important infectious agents |
Source: Ministry of Health.24
Summary of the forensic activities included in the Spanish Society of Forensic Pathology document.
| Situation | Action | |
|---|---|---|
| Removal | Natural deaths without signs of violence and therefore without legal repercussions in suspected, probable or confirmed cases of infection by coronavirus | External examination |
| Report for the Police Court | ||
| Deaths with signs of violence or the suspicion of criminality in suspected, probable or confirmed cases of infection by coronavirus | External examination | |
| Report for the Police Court | ||
| Removal to the forensic pathology department | ||
| Autopsy | Corpses without judicial implications and no suspicion of COVID-19 infection | External examination |
| Sample taking for toxins if necessary | ||
| Deaths with signs of violence and suspicion of infection by coronavirus when it is not possible to perform microbiological tests to confirm or rule out such infection | External examination | |
| Autopsy if necessary | ||
| Sample taking for toxins if necessary | ||
| Corpses with judicial implications, but with no suspicion of COVID-19 infection | Partial autopsy | |
| Sample taking for toxins | ||
| Limited sample taking for histopathology | ||
| Corpses with judicial implications and suspicion of COVID-19 infection | Wait 24 h. | |
| Take sample for COVID-19 | ||
| COVID-19 +: external examination only |
Source: Spanish Society of Forensic Pathology.25
Diagnosis of infection by SARS-CoV-2 is confirmed by RT-PCR, as we have seen. Swabs are taken for analysis from the nasopharyngeal region, and smear tests from the oropharyngeal region may be added to these.26
A positive result for SARS-CoV-2 generally confirms the diagnosis. However, well-documented false negative cases exist, so that this possibility must be taken into account in interpreting results.27 The factors that may interfere with the sensitivity of determination are: incorrect sample taking, the type of RT-PCR analysis used, the type of sample, the quality of the same and the phase of the disease at the time the sample was taken.28
Serological tests may be useful complementary tests together with PCR determination. Antigen tests are less sensitive, and they run the risk of not detecting the virus when the viral load is low, if they are used in isolation. On the other hand, antibody determination may be negative in the first 5 days of the infection, so that this test is not very useful in the identification of a corpse with viral load without a PCR test.29 The best option seems to be to combine PCR and serological tests, taking samples as soon post mortem as is possible, as they offer data on the viral load as well as the immunological state of the dead individual.
Samples for histopathological studyBefore opening a corpse all of the containers that will be necessary for complementary studies must be prepared. For histopathological study containers with 4% buffered formol should be available, giving a sample/formal volume ratio of 1/3.30 The recommended samples are: the trachea (proximal and distal, with sections of both bronchial tubes), the lungs (representative areas from both lungs), myocardium, liver, spleen, kidneys, skin, muscle and bone marrow.
7 days fixing in formal is recommended. Very small samples that may be included in a cassette only require 48 h. fixing.18
Samples for other studiesAccording to the said recommendations, for chemical-toxicological analysis peripheral blood must be taken in sodium fluoride and potassium oxalate. It is also advisable to take 2 other tubes of blood in EDTA, as they may be required for toxin studies or genetic study in the context of possible family cardiac pathology. Sample taking for microbiological analysis is broadly covered by another paper in this monograph.
Pathological findingsFew works describe pathological findings in those who have died due to COVID-19. The first ones were published in China, and they are restricted to biopsies pre and post mortem with selective sampling31–33 or minimally invasive autopsies.34 Other works correspond to retrospective studies of surgical samples from 3 patients operated for lung cancer in which COVID-19 was detected subsequently.32,35 Recently, Carsana et al. published their post mortem lung findings in a series of 38 corpses in the north of Italy.36 The only publications we know of describing complete autopsies are from the United States. The first of these studies, in Oklahoma, is forensic,37 with post mortem diagnosis by RT-PRC based on nasopharyngeal and lung swabs in 2 corpses. The second study corresponds to clinical autopsies of 4 patients who died of COVID-19 in New Orleans.38
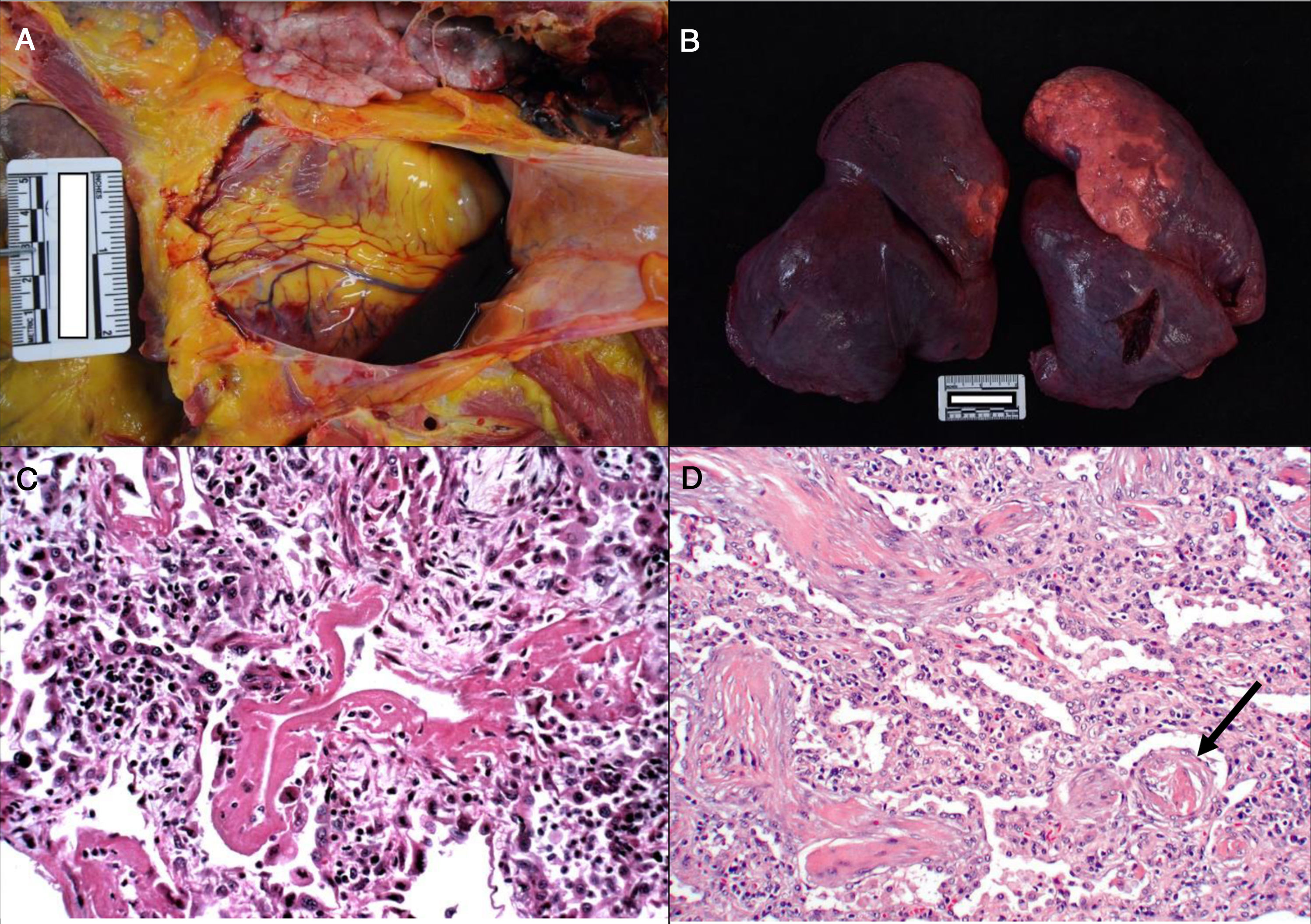
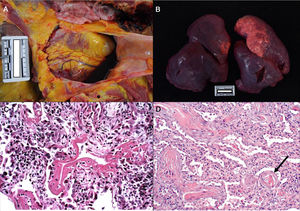
Consistently with the clinical manifestations of COVID-19, the main pathological findings are located in the lungs. Pleural and pericardial bleeding may be seen in the autopsy, generally serous or serosanguineous38 (Fig. 2a), unless there is an additional bacterial infection that causes a purulent exudate.39 Macroscopically the lung are compact, heavy and oedematous (Fig. 2b), with haemorrhagic zones and brownishgrey when consolidated.37–39 Although thrombi are occasionally detected in the peripheral arteries,38 pulmonary thromboembolism has not been seen in any case, even though it has been associated with COVID-19.40 In microscopic study, in early forms of the disease oedema, intra-alveolar exudate that is high in protein, interstitial pneumonitis and the presence of macrophages, giant multinucleated cells and some fibroblasts are observed.35,41
a. Pericardial serosanguinolent bleeding (40 cc) in a 70 year-old man. b. Lungs with a combined weight of 1,550 g. c. A 77 year-old woman who died of COVID disease 6 days after admission. Mechanical ventilation. Histological image of the lung showing diffuse alveolar damage with hyaline membranes, hyperplasia and pneumocyte descaling, fibroblasts and interstitial mononuclear inflammatory infiltrate (HE, ×10). d. 83 year-old male who died 11 days after admission. The image shows expanded walls with slight inflammatory infiltrate and fibrosis. The arrow points to a thrombosed vessel (HE, ×10).
In an advanced phase, as is the case with other coronaviruses, SARS-CoV-2 causes diffuse alveolar damage characterised by the presence of hyaline membranes, fibrin deposits and pneumocyte descaling (Fig. 2c).31,32,34,37,38 The inter-alveolar walls often show inflammatory lymphocytic infiltrate (CD3, CD4 and CD8 positive) of variable intensity31–36 and they may also be seen to form sleeving around the large bronchial tubes and small vessels.38 In some corpses exudate in organisation with a proliferation of fibroblasts and interstitial fibrosis is observed (Fig. 2d),32–34 although the fibrotic phases is infrequent.36
Fibrin-platelet thrombi are repeatedly identified in the small arteries and capillaries.34,36–38 In the autopsies studied by Fox et al. a striking finding was the presence of numerous hyperchromatic intravascular megakaryocytes (CD61+) with atypical nuclei that may be associated with the creation of thrombi.38 These would correspond to native lung megakaryocytes that had been activated by the viral infection. This thrombotic microangiopathy is only seen in the lungs.
Some studies report the detection of viral cytopathic changes in type II pneumocytes and doubtful viral inclusions.38 Immunohistochemical techniques have shown positivity against antigen 2019-nCoV in the alveolar epithelium, descaled pneumocytes and macrophages,33,34 and RNA particles have also been detected in the cytoplasm of numerous multinucleated giant cells.38 Kuang et al. mention the extraction of viral RNA from lung tissue in paraffin.35 Particles of coronavirus have been detected using an electron microscope in the bronchial epithelium and in type II pneumocytes,34,36 as well as in endothelial cells of the glomerular capillaries,6 which would prove the systemic dissemination of the virus.
Neutrophilic inflammatory inflitrate in the lungs was also observed in some cases, with fungal and bacterial abscesses which were interpreted as secondary infections.32,36,38
Chronic pre-existing disease unconnected with COVID-19 is often found in macroscopic examination of the heart.37,38 Microscopic alterations have been minimum: dispersed necrosis, isolated interstitial lymphocytes and small patches of fibrosis.31,32,34,37,38 In spite of the cardiovascular complications observed clinically in these patients,42 acute myocardial infarct has only been described in one dead woman6, although myocarditis was not observed in any case.
The work by Varga et al. stands out, in which they observed inflammatory infiltrate and apoptosis in endothelial cells of the heart, lungs, liver, kidneys and small intestine. These would be the cause of an endothelial dysfunction that leads to organic ischemia, tissue oedema and precoagulation states.6 Hepatic steatosis has been described in several studies,31,32,34,37 although very probably it is not associated with the disease. Lymphocytic depletion has been observed in the spleen, as have degeneration and necrosis of parenchymatous cells, hyaline thrombi in small vessels and chronic disease in other organs.34
Although there are analytical hypercoagulability markers in the physiopathology of COVID-19 disease, with raised D-dimer and indications of disseminated intravascular coagulation, only Yao et al. describe thrombi in the small vessels of several organs (thrombi in glomerular capillaries are visible in a photograph in their original publication in Chinese),34 all other works limit thrombotic microangiopathy to the lungs.
ConclusionsAutopsies have made a significant contribution to knowledge about many diseases. In connection with COVID-19 disease, in spite of its high rate of mortality there have been few studies. The explanation for this lies in its high contagiousness and the lack of suitable installations and equipment. It should be underlined that the first published work describing complete autopsies belongs to the medical-legal context.37 We expect many more necropsies will be performed in the near future under appropriate conditions, and that they will add to the knowledge about this new disease and help to develop relevant therapies that contribute to combating this world emergency.
Please cite this article as: Bañon-Gonzalez R, Carnicero-Caceres S, Suarez-Mier MP, Diaz FJ. Autopsias en casos sospechosos de SARS-CoV-2. Rev Esp Med Legal. 2020;46:93–100.