Systemic sclerosis (SSc) is a rare autoimmune systemic disorder characterized by inflammation and fibrosis. Fibrinogen and C-reactive protein (CRP) are raised during inflammation. Blood cell-derived inflammatory indices are helpful for monitoring disease activity in several autoimmune diseases. In this study, we aimed to evaluate the levels of fibrinogen, fibrinogen to albumin ratio (FAR), CRP, CRP to albumin ratio (CAR), and blood cell-derived inflammatory indices in SSc patients.
Patients and methodsThis observational case–control study included 31 SSc patients and 20 age- and sex-matched healthy participants. Clinical assessment was performed for SSc patients. Complete blood count, CRP, fibrinogen, and albumin were done for all study subjects with calculation of the following: CAR, FAR, lymphocyte to CRP ratio, neutrophil to lymphocyte ratio (NLR), systemic inflammation response index (SIRI), systemic immune inflammation index (SII), and prognostic nutritional index (PNI).
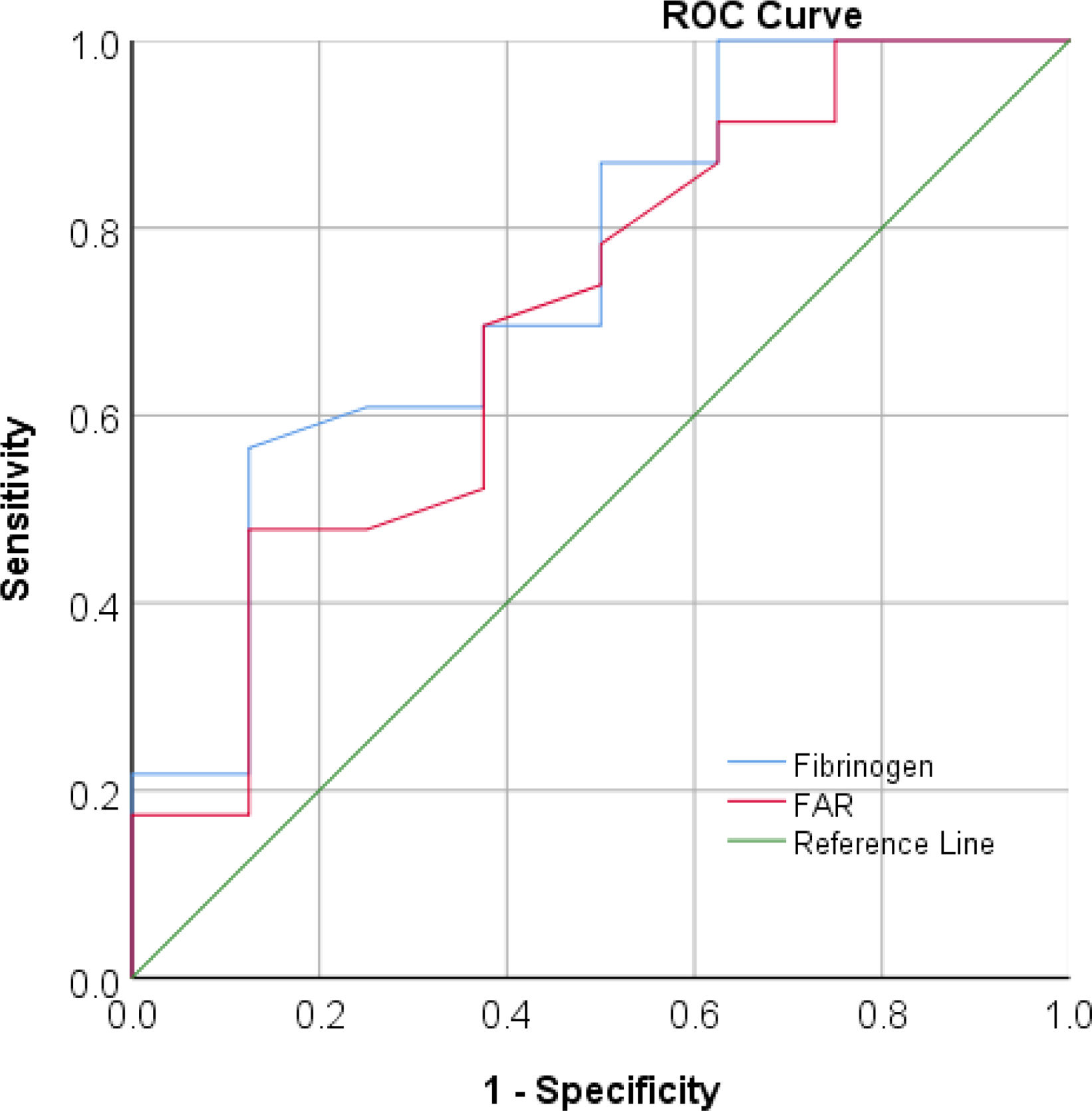
ResultsWe detected significant differences between SSc patients and healthy subjects regarding all studied variables except for albumin and PNI. Both fibrinogen and FAR were positively correlated with disease activity (p-value=0.006 and 0.009, respectively), while NLR, SIRI, and SII were positively correlated with the modified Rodnan Skin Score (p-value=0.04, 0.034, and 0.005, respectively). ROC curve analysis for active disease cutoff point detection revealed that fibrinogen had an area under the curve of 0.75 (p-value=0.04) at a criterion of 3.2 with 70% sensitivity and 62% specificity.
ConclusionsFibrinogen and FAR are reliable indicators of inflammation and disease activity in SSc patients.
La esclerosis sistémica (ES) es un trastorno sistémico autoinmune poco frecuente, que se caracteriza por inflamación y fibrosis. El fibrinógeno y la proteína C reactiva (PCR) aumentan durante la inflamación. Los índices inflamatorios derivados de las células sanguíneas son útiles para controlar la actividad de la enfermedad en varias enfermedades autoinmunes. En este estudio, nuestro objetivo fue evaluar los niveles de fibrinógeno, la relación fibrinógeno-albúmina (FAR), la PCR, la relación PCR-albúmina (CAR) y los índices inflamatorios derivados de las células sanguíneas en los pacientes con ES.
Pacientes y métodosEste estudio observacional de casos y controles incluyó 31 pacientes con ESC y 20 participantes sanos de la misma edad y sexo. Se hizo una evaluación clínica a los pacientes con ESC. Se realizó hemograma completo, PCR, fibrinógeno y albúmina a todos los sujetos del estudio, con el cálculo de lo siguiente: CAR, FAR, relación linfocitos/CRP, relación neutrófilos/linfocitos (NLR), índice de respuesta inflamatoria sistémica (SIRI), índice de inflamación inmunitaria sistémica (SII) e índice nutricional pronóstico (PNI).
ResultadosDetectamos diferencias significativas entre los pacientes con ESC y los sujetos sanos con respecto a todas las variables estudiadas, excepto la albúmina y el PNI. Tanto el fibrinógeno como la FAR se correlacionaron positivamente con la actividad de la enfermedad (valor p=0,006 y 0,009, respectivamente), mientras que el NLR, el SIRI y el SII se correlacionaron positivamente con el índice cutáneo de Rodnan modificado (valor p=0,04, 0,034 y 0,005, respectivamente). El análisis de la curva ROC para la detección del punto de corte de la enfermedad activa reveló que el fibrinógeno tenía un área bajo la curva de 0,75 (valor p=0,04), en un criterio de 3,2, con una sensibilidad del 70% y una especificidad del 62%.
ConclusionesEl fibrinógeno y la FAR son indicadores fiables de la inflamación y la actividad de la enfermedad en los pacientes con ESC.
Systemic sclerosis (SSc) is a rare autoimmune condition characterized by a unique combination of microvascular injury, immune dysregulation, and extensive fibrosis affecting various organs [1,2]. Patients are categorized into two groups based on the extent of skin involvement: limited cutaneous and diffuse cutaneous SSc [3].
Inflammation plays a significant role in the early stages of SSc, occurring before fibrosis develops. Inflammatory infiltrates can be found in multiple organs, alongside elevated levels of circulating inflammatory cytokines and chemokines [4]. The pro-inflammatory substances stimulate hepatic cells to produce acute-phase proteins, such as C-reactive protein (CRP), while reducing albumin synthesis [5,6]. Additionally, fibrinogen, a plasma protein synthesized in the liver, acts as a marker for inflammatory processes at various stages [7].
The value of blood cells derived inflammatory indices has been proved in several autoimmune diseases such as rheumatoid arthritis (RA) and systemic lupus erythematosus (SLE) [8,9]. In this study, we aimed to assess the levels of fibrinogen, fibrinogen to albumin ratio (FAR), CRP, CRP to albumin ratio (CAR), lymphocyte to CRP ratio (LCR), systemic inflammation response index (SIRI), systemic immune inflammation index (SII), and prognostic nutritional index (PNI) in patients with SSc. To the best of our knowledge, this is the first study that explores the relation of fibrinogen, FAR, LCR, PNI, SIRI and SII to disease activity and manifestations in patients with SSc so, our evaluation will provide valuable insights into the significance of these markers in this population.
Patients and methodsStudy design and participantsThis is an observational case–control study that included subjects recruited from inpatient and outpatient settings of Department of Rheumatology, Rehabilitation and Physical Medicine at Assiut University Hospital. The study included 31 SSc patients and 20 healthy subjects. The recruitment and data collection period extended from January 2024 to July 2024.
Ethical approval and consentThis study was approved by the Institutional Review Board of the Faculty of Medicine, Assiut University, Assiut, Egypt in compliance with the Helsinki declaration, IRB No.: 04-2024-300357. A written informed consent was obtained from all study subjects prior to participation in the study.
Inclusion and exclusion criteriaPatients were included in the study if they were at least 18 years old, and had a definite SSc diagnosis according to the American College of Rheumatology (ACR) – European Alliance of Associations for Rheumatology (EULAR) Criteria for the classification of SSc [10]. Exclusion criteria were malignancy, hepatic or renal disease, coagulation disorders, pregnancy, acute infections or other rheumatological diseases.
Clinical assessment- •
Complete history was obtained including demographics, diseases duration and disease manifestations.
- •
Modified Rodnan skin score (mRSS): It is an assessment of patient's skin thickness via clinical palpation utilizing a 0–3 scale (0=normal skin; 1=mild thickness; 2=moderate thickness; 3=severe thickness). Seventeen areas are examined; face, anterior chest, abdomen, (right and left) fingers, forearms, upper arms, thighs, lower legs, dorsum of hands and feet. These individual values are summed to get the total score [11].
- •
European Scleroderma Study Group (EScSG) activity index: It is a 10-items questionnaire. Each item has a constant numerical value from 0.5 to 2. The final score ranges from 0 to 10. The items are mRSS>14, scleredema, changes in skin stiffness during one month, digital necrosis, changes in vascular symptoms during one month, arthritis, diffusion lung capacity<80%, changes in cardiopulmonary symptoms, erythrocyte sedimentation rate (ESR)>30mm/1h and hypocomplementemia [12–14]. A score of ≥3 indicates active disease [15].
- •
Pulmonary artery systolic pressure (PASP) was obtained from patient's records (recent transthoracic echocardiography).
The following tests were performed for all study participants, except ESR, antinuclear antibody (ANA) and complement components C3 and C4 which were conducted only for patients:
- •
Complete blood count (CBC): CBC including hemoglobin level, RBCs indices, total leukocytic count, differential leukocytic count and platelet count were analyzed using the ADVIA 2120i hematology system (Siemens, Germany).
- •
Serum Albumin: It was measured by using Dimension RXL-Max, Siemens Healthineers, Germany.
- •
Plasma Fibrinogen: It was performed on Sysmex CA 1500 coagulation analyzer, Siemens Healthineers, Germany.
- •
CRP: It was measured by nephelometric technique on BN ProSpec analyzer (Siemens, Germany) using Siemen CardioPhase hs CRP (REF OQIYI Siemens, Germany).
- •
ESR: It was performed by standard Westergren method.
- •
ANA: This was performed by indirect immunofluorescence technique on HEP 2 cells using Diasorin ANA Fluoro kits (REF 1660, Diasorin Inc, Germany).
- •
Complement components C3 and C4: They were done by nephelometric technique using BN ProSpec analyzer (Siemens, Germany) using Siemens N Antiserum to Human C3c (REF OSAP09, Siemens, Germany) and Siemens N Antiserum to Human C4 (REF OSA009, Siemens, Germany), respectively.
The following ratios and indices were calculated:
- ∘
FAR=fibrinogen (g/L)/serum albumin (g/L) [16],
- ∘
CAR=CRP (mg/L)/serum albumin (g/L) [17],
- ∘
LCR=lymphocyte count/μL/CRP (mg/dL) [18],
- ∘
NLR=neutrophil absolute count/Lymphocyte absolute count [19],
- ∘
SIRI=neutrophil count/L×monocyte count/L/lymphocyte count/L [20],
- ∘
SII=platelet count/L×neutrophil count/L/lymphocyte count/L [21] and
- ∘
PNI=(10×serum albumin value (g/dl))+(0.005×lymphocyte count/μL in peripheral blood) [22].
Data was analyzed via SPSS version 26 (SPSS Inc, Chicago, IL, USA). Numerical data was expressed as mean±standard deviation or median (range). The Chi square test was used to compare proportions. We used Student's T test to compare means, while Mann–Whitney U test was utilized to compare mean ranks. Spearman's or Pearson's correlation coefficients were applied to assess correlations. Receiver operating characteristic (ROC) analysis was performed to test the ability of fibrinogen and FAR to differentiate between active and inactive disease. The level for statistical significance was set at p<0.05.
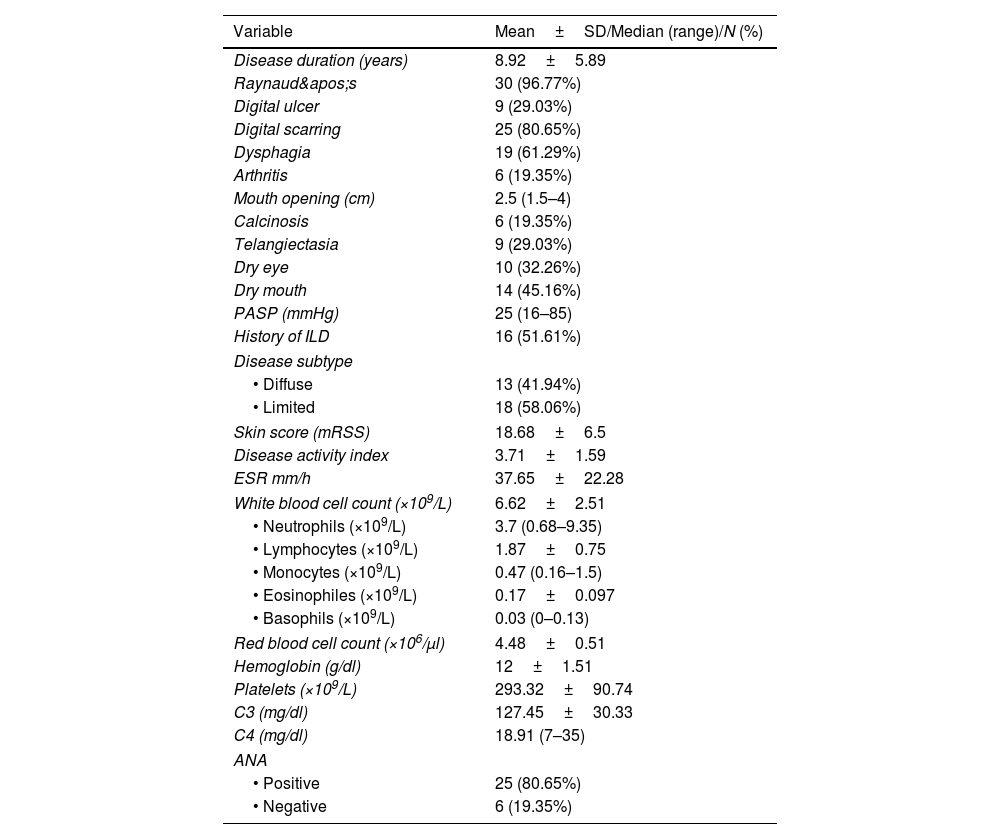
ResultsThe study included 31 SSc patients and 20 healthy participants. The mean age of the included SSc patients was 40.71±10.93 versus 38.2±11.59 years for the control subjects (p-value=0.45). Most of the included subjects were females (90.32% of SSc cases and 85% of the healthy participants, p-value=0.67). Clinical and laboratory features of study patients are illustrated in Table 1.
Clinical and laboratory evaluation of SSc patients (n=31).
| Variable | Mean±SD/Median (range)/N (%) |
|---|---|
| Disease duration (years) | 8.92±5.89 |
| Raynaud's | 30 (96.77%) |
| Digital ulcer | 9 (29.03%) |
| Digital scarring | 25 (80.65%) |
| Dysphagia | 19 (61.29%) |
| Arthritis | 6 (19.35%) |
| Mouth opening (cm) | 2.5 (1.5–4) |
| Calcinosis | 6 (19.35%) |
| Telangiectasia | 9 (29.03%) |
| Dry eye | 10 (32.26%) |
| Dry mouth | 14 (45.16%) |
| PASP (mmHg) | 25 (16–85) |
| History of ILD | 16 (51.61%) |
| Disease subtype | |
| • Diffuse | 13 (41.94%) |
| • Limited | 18 (58.06%) |
| Skin score (mRSS) | 18.68±6.5 |
| Disease activity index | 3.71±1.59 |
| ESR mm/h | 37.65±22.28 |
| White blood cell count (×109/L) | 6.62±2.51 |
| • Neutrophils (×109/L) | 3.7 (0.68–9.35) |
| • Lymphocytes (×109/L) | 1.87±0.75 |
| • Monocytes (×109/L) | 0.47 (0.16–1.5) |
| • Eosinophiles (×109/L) | 0.17±0.097 |
| • Basophils (×109/L) | 0.03 (0–0.13) |
| Red blood cell count (×106/μl) | 4.48±0.51 |
| Hemoglobin (g/dl) | 12±1.51 |
| Platelets (×109/L) | 293.32±90.74 |
| C3 (mg/dl) | 127.45±30.33 |
| C4 (mg/dl) | 18.91 (7–35) |
| ANA | |
| • Positive | 25 (80.65%) |
| • Negative | 6 (19.35%) |
Data expressed as mean±SD/median (range) or frequency (%). PASP: pulmonary artery systolic pressure, ILD: interstitial lung disease, mRSS: modified Rodnan skin score, ESR: erythrocyte sedimentation rate, C3: complement component 3, C4: complement component 4, ANA: antinuclear antibody.
The most frequently administrated drug by SSc patients was nifedipine (taken by 54.84% of the cases). Other drugs included azathioprine, methotrexate, proton pump inhibitors, sildenafil, pentoxifylline, mycophenolate mofetil, prednisolone, cyclophosphamide and hydroxychloroquine.
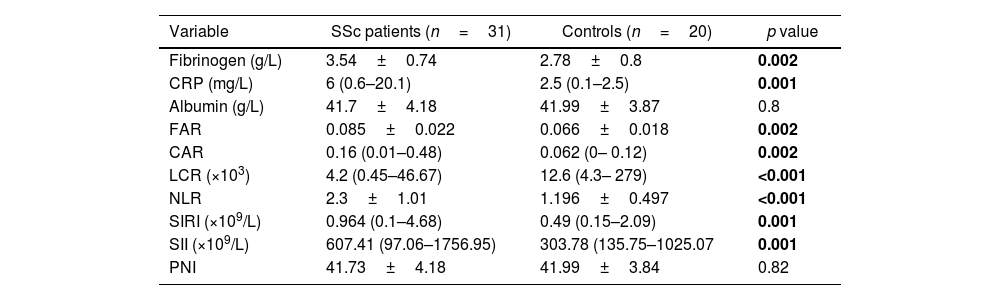
All studied variables showed significant difference between SSc patients and healthy subjects except for serum albumin and PNI as shown in Table 2.
Inflammatory markers among the studied groups.
| Variable | SSc patients (n=31) | Controls (n=20) | p value |
|---|---|---|---|
| Fibrinogen (g/L) | 3.54±0.74 | 2.78±0.8 | 0.002 |
| CRP (mg/L) | 6 (0.6–20.1) | 2.5 (0.1–2.5) | 0.001 |
| Albumin (g/L) | 41.7±4.18 | 41.99±3.87 | 0.8 |
| FAR | 0.085±0.022 | 0.066±0.018 | 0.002 |
| CAR | 0.16 (0.01–0.48) | 0.062 (0– 0.12) | 0.002 |
| LCR (×103) | 4.2 (0.45–46.67) | 12.6 (4.3– 279) | <0.001 |
| NLR | 2.3±1.01 | 1.196±0.497 | <0.001 |
| SIRI (×109/L) | 0.964 (0.1–4.68) | 0.49 (0.15–2.09) | 0.001 |
| SII (×109/L) | 607.41 (97.06–1756.95) | 303.78 (135.75–1025.07 | 0.001 |
| PNI | 41.73±4.18 | 41.99±3.84 | 0.82 |
Data expressed as mean±SD/median (range). The p-value was significant if <0.05. CRP: C reactive protein, FAR: fibrinogen to albumin ratio, CAR: CRP to albumin ratio, LCR: lymphocyte to CRP ratio, NLR: neutrophil to lymphocyte, SIRI: systemic inflammation response index, SII: systemic immune inflammation index, PNI: prognostic nutritional index.
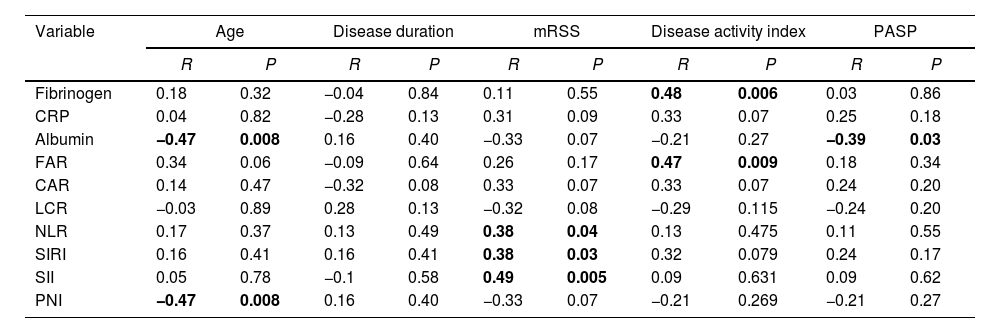
As demonstrated in Table 3, we observed a significant positive correlation of disease activity with fibrinogen and FAR. Serum albumin was negatively correlated with age and PASP. We observed a significant negative correlation between PNI and PASP. SIRI, SII and NLR were positively correlated with mRSS.
Association of clinical characteristics with the studied variables.
| Variable | Age | Disease duration | mRSS | Disease activity index | PASP | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| R | P | R | P | R | P | R | P | R | P | |
| Fibrinogen | 0.18 | 0.32 | −0.04 | 0.84 | 0.11 | 0.55 | 0.48 | 0.006 | 0.03 | 0.86 |
| CRP | 0.04 | 0.82 | −0.28 | 0.13 | 0.31 | 0.09 | 0.33 | 0.07 | 0.25 | 0.18 |
| Albumin | −0.47 | 0.008 | 0.16 | 0.40 | −0.33 | 0.07 | −0.21 | 0.27 | −0.39 | 0.03 |
| FAR | 0.34 | 0.06 | −0.09 | 0.64 | 0.26 | 0.17 | 0.47 | 0.009 | 0.18 | 0.34 |
| CAR | 0.14 | 0.47 | −0.32 | 0.08 | 0.33 | 0.07 | 0.33 | 0.07 | 0.24 | 0.20 |
| LCR | −0.03 | 0.89 | 0.28 | 0.13 | −0.32 | 0.08 | −0.29 | 0.115 | −0.24 | 0.20 |
| NLR | 0.17 | 0.37 | 0.13 | 0.49 | 0.38 | 0.04 | 0.13 | 0.475 | 0.11 | 0.55 |
| SIRI | 0.16 | 0.41 | 0.16 | 0.41 | 0.38 | 0.03 | 0.32 | 0.079 | 0.24 | 0.17 |
| SII | 0.05 | 0.78 | −0.1 | 0.58 | 0.49 | 0.005 | 0.09 | 0.631 | 0.09 | 0.62 |
| PNI | −0.47 | 0.008 | 0.16 | 0.40 | −0.33 | 0.07 | −0.21 | 0.269 | −0.21 | 0.27 |
The p-value was significant if <0.05. R: correlation coefficient, mRSS: modified Rodnan skin score, PASP: pulmonary artery systolic pressure, CRP: C reactive protein, FAR: fibrinogen to albumin ratio, CAR: CRP to albumin ratio, LCR: lymphocyte to CRP ratio, NLR: neutrophil to lymphocyte ratio, SIRI: systemic inflammation response index, SII: systemic immune inflammation index, PNI: prognostic nutritional index.
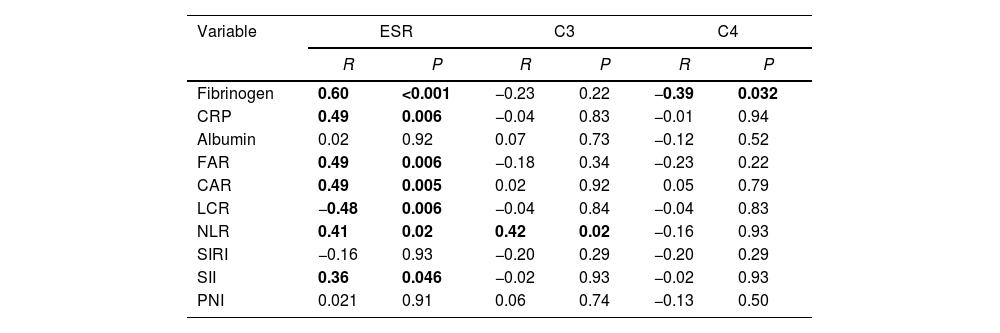
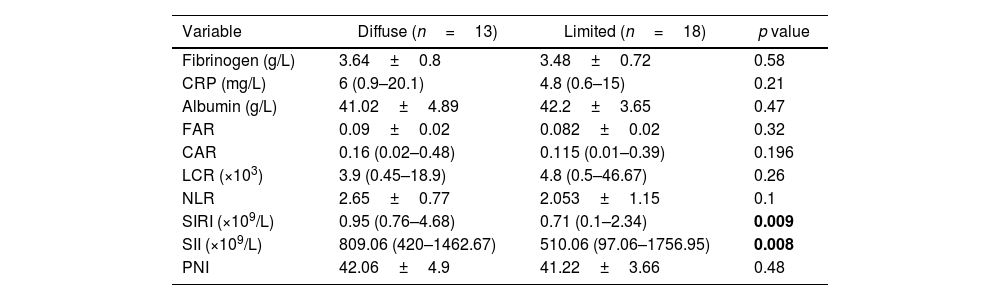
Fibrinogen was negatively correlated with C4. In addition, FAR, CRP, SIRI, CAR and NLR showed significant positive correlation with ESR while LCR was negatively correlated with ESR as shown in Table 4. SIRI and SII were significantly higher in the diffuse disease subtype as shown in Table 5.
Correlation of studied markers with laboratory findings in SSc patients.
| Variable | ESR | C3 | C4 | |||
|---|---|---|---|---|---|---|
| R | P | R | P | R | P | |
| Fibrinogen | 0.60 | <0.001 | −0.23 | 0.22 | −0.39 | 0.032 |
| CRP | 0.49 | 0.006 | −0.04 | 0.83 | −0.01 | 0.94 |
| Albumin | 0.02 | 0.92 | 0.07 | 0.73 | −0.12 | 0.52 |
| FAR | 0.49 | 0.006 | −0.18 | 0.34 | −0.23 | 0.22 |
| CAR | 0.49 | 0.005 | 0.02 | 0.92 | 0.05 | 0.79 |
| LCR | −0.48 | 0.006 | −0.04 | 0.84 | −0.04 | 0.83 |
| NLR | 0.41 | 0.02 | 0.42 | 0.02 | −0.16 | 0.93 |
| SIRI | −0.16 | 0.93 | −0.20 | 0.29 | −0.20 | 0.29 |
| SII | 0.36 | 0.046 | −0.02 | 0.93 | −0.02 | 0.93 |
| PNI | 0.021 | 0.91 | 0.06 | 0.74 | −0.13 | 0.50 |
The p-value was significant if <0.05. R: correlation coefficient, CRP: C reactive protein, FAR: fibrinogen to albumin ratio, CAR: CRP to albumin ratio, LCR: lymphocyte to CRP ratio, NLR: neutrophil to lymphocyte, SIRI: systemic inflammation response index, SII: systemic immune inflammation index, PNI: prognostic nutritional index, ESR: erythrocyte sedimentation rate, C3: complement component 3, C4: complement component 4.
Comparison between diffuse and limited subtypes regarding the studied variables.
| Variable | Diffuse (n=13) | Limited (n=18) | p value |
|---|---|---|---|
| Fibrinogen (g/L) | 3.64±0.8 | 3.48±0.72 | 0.58 |
| CRP (mg/L) | 6 (0.9–20.1) | 4.8 (0.6–15) | 0.21 |
| Albumin (g/L) | 41.02±4.89 | 42.2±3.65 | 0.47 |
| FAR | 0.09±0.02 | 0.082±0.02 | 0.32 |
| CAR | 0.16 (0.02–0.48) | 0.115 (0.01–0.39) | 0.196 |
| LCR (×103) | 3.9 (0.45–18.9) | 4.8 (0.5–46.67) | 0.26 |
| NLR | 2.65±0.77 | 2.053±1.15 | 0.1 |
| SIRI (×109/L) | 0.95 (0.76–4.68) | 0.71 (0.1–2.34) | 0.009 |
| SII (×109/L) | 809.06 (420–1462.67) | 510.06 (97.06–1756.95) | 0.008 |
| PNI | 42.06±4.9 | 41.22±3.66 | 0.48 |
The p-value was significant if <0.05. CRP: C reactive protein, FAR: fibrinogen to albumin ratio, CAR: CRP to albumin ratio, NLR: neutrophil/lymphocyte, LCR: lymphocyte to CRP ratio, SIRI: systemic inflammation response index, SII: systemic immune inflammation index, PNI: prognostic nutritional index,
ROC curve analysis for detection of active disease cutoff point revealed that fibrinogen had an area under the curve of 0.75 (p=0.04) at a criterion of 3.2 with 70% sensitivity and 62% specificity. FAR showed area under the curve of 0.7 (p-value=0.095) at a criterion of 0.075 with 70% sensitivity and 62% specificity. However, the FAR cutoff point should be used cautiously due to insignificant p-value as shown in Fig. 1.
DiscussionSSc is a complex autoimmune inflammatory condition [23]. Systemic inflammation has a harmful impact on vascular endothelial cells by reducing the production of nitric oxide and prostacyclins, which results in vasoconstriction and thrombotic effects [24].
The present study was designed to compare SSc patients and healthy controls regarding systemic inflammation-based ratios and indices (CRP, CAR, fibrinogen, FAR, LCR, NLR, serum albumin, SIRI, SII and PNI) and to assess the association of previous ratios and indices with disease manifestations and activity.
Our study revealed significant difference between SSc patients and healthy subjects regarding fibrinogen, CRP, FAR, CAR, LCR, NLR, SIRI and SII. Serum albumin and PNI showed no significant difference between SSc patients and healthy subjects.
Both CRP and serum albumin are strongly related to inflammatory status; the serum albumin is negatively correlated, while acute-phase protein synthesis is positively correlated with systemic inflammation [5,6]. Muangchan et al. observed elevated CRP levels in 25.7% of SSc patients [25].
Despite the poor nutritional status [26] and the inflammatory process in SSc patients [23], no significant difference was detected between SSc patients and control group regarding serum albumin and PNI. This comes in agreement with Baron et al. who observed that serum albumin is not beneficial for assessment of nutritional status of SSc patients [27]. A previous study observed a fairly little effect of CRP on albumin level [28].
In accordance with our observation that serum albumin is negatively correlated with PASP, a retrospective review of patients with World Health Organization group 1 pulmonary arterial hypertension (PAH) indicated that low serum albumin levels in those patients are associated with advanced disease stage and high mortality rate (p-value=0.008) [29].
Several studies have showed that the inflammation based score, the CAR, is connected to inflammatory conditions [30]. In our study, CRP and CAR levels showed significant positive correlation with ESR. A previous study found that CAR was positively correlated with ESR (r=0.241, p-value=0.01) in SSc patients which agrees with our results [31].
Fibrinogen has been identified as a significant determinant of inflammatory arthritis as in RA; a study detected a significant increase in fibrinogen level in RA patients in comparison to the control group [32]. A significantly higher level of fibrinogen in SLE patients with active disease than those with inactive disease (p-value=0.003) was observed [9].
These accomplishments significantly enhance our interest in integrating fibrinogen and albumin into a possible inflammatory marker in autoimmune diseases. In ankylosing spondylitis (AS) patients, a significantly higher FAR was detected than in the control group (p-value<0.001) [33]. Another study demonstrated that FAR is positively correlated with disease activity, CRP and ESR in RA patients (p-value<0.001) [32]. This aligns with our observation that fibrinogen and the FAR were positively correlated with ESR and disease activity index. Additionally, fibrinogen showed a negative correlation with C4.
There is scarce data about LCR in rheumatological disorders. We observed a significantly higher LCR in SSc patients in comparison to healthy subjects. In addition, LCR showed a significant negative correlation with ESR. LCR was not related to disease activity.
The NLR is a straightforward and inexpensive biomarker derived from the ratio of neutrophil and lymphocyte counts in peripheral blood [34]. A meta-analysis concluded that patients with SSc had significantly higher NLR in comparison to healthy subjects. In addition, it demonstrated that NLR was significantly higher in SSc with ILD and PAH [35]. In our study, NLR showed significant positive correlation with ESR, and mRSS.
SII, an index based on platelet, neutrophil and lymphocyte counts, was initially developed to assess its prognostic value in patients with hepatocellular carcinoma [21]. Its value was proven in assessing disease activity in several autoimmune diseases such as RA [36]. In our study, SIRI and SII were positively correlated with mRSS. Both indices were significantly higher in the diffuse disease subtype.
Conclusions and recommendationsFibrinogen and FAR levels are good indicators of inflammation and disease activity in SSc patients. Consequently, these straightforward, quick, and cost-effective parameters, along with other serum inflammatory markers, can be utilized for initial assessment of inflammatory responses and disease activity in SSc patients. High levels of NLR, SIRI and SII are strongly associated with more severe skin involvement. Serum albumin and PNI are not related to disease activity in SSc. Nonetheless, additional research is needed to confirm these findings in the future.
We recommend studying the relationship between the analyzed ratios and indices, and inflammatory cytokines like tumor necrosis factor α and interleukin-1β, which play key roles in inflammatory conditions as well as monitoring patients to evaluate the possibility of these ratios to be prognostic indicators or a method to gauge treatment response. In addition, studying the association of these markers with active interstitial lung disease in SSc patients is mandatory.
Limitations- •
Small sample size.
- •
The study did not explore the relation of those markers with pulmonary function tests parameters and findings of chest high-resolution computed tomography.
The study conception and design were done by Dr. Maha S.I. Abdelrahman. All authors contributed to the acquisition of data. Dr. Aml A. Rayan and Dr. Fatma M. Helbawi were responsible for data analysis and interpretation. All authors participated in drafting the article and reviewing it critically for important intellectual aspects, and all authors approved the final version to be published.
Ethical considerationsThis study was approved by the Institutional Review Board of the Faculty of Medicine, Assiut University, Assiut, Egypt in compliance with the Helsinki declaration, IRB No.: 04-2024-300357. A written informed consent was obtained from all study subjects prior to participation in the study.
FundingThis study did not receive any financial support.
Conflict of interestNone to declare.
Availability of data and materialsThe date is available from the corresponding author upon reasonable request.
Not applicable.