Lupus nephritis (LN) is one of the most prevalent and severe complications of systemic lupus erythematosus (SLE), requiring reliable urine and serum biomarkers to evaluate it. Anti-nucleosome and anti-C1q antibodies are associated with LN in several geographic regions. Also, southwest Colombia has a heterogeneous ethnicity, which motivated the evaluation of the frequency and relationship of such markers with LN in this region.
MethodsA cross-sectional study was conducted in a health centre in south-west Colombia in 84 patients diagnosed with SLE (57 without LN; 27 with LN) between 2016 and 2018. Demographic and clinical and laboratory features, including anti-dsDNA, complement, and anti-C1q and anti-nucleosome antibodies were compared in these patients. ELISA immunoassays were performed to measure the antibodies of interest in blood samples. Statistical analysis was carried out using STATA14 software (StataCorp, College Station, Texas, USA). Quantitative variables were summarised as means or medians and compared with Mann–Whitney or Two-sample t test. Categorical variables were shown as proportions, and compared with Chi-squared or Fisher's exact test. Correlation analysis between quantitative variables was calculated using Spearman's correlation.
ResultsOf all 84 patients, 27 patients had LN, of which 16 (59.2%) had a positive test for anti-nucleosome antibodies and 10 (37%) for anti-C1q antibodies. An association was found between anti-C1q and proliferative forms of LN and newly diagnosed LN. A correlation was found between anti-nucleosome and anti-C1q antibodies, and anti-dsDNA and low serum complement concentrations.
ConclusionAlthough both markers were found in variable percentages in SLE patients and seem not to be specific markers of LN in our population, anti-C1q was associated with proliferative forms of LN and de novo LN.
La nefritis lúpica (NL), una de las complicaciones más frecuentes y graves del lupus eritematoso sistémico (LES), requiere biomarcadores confiables de orina y suero para su evaluación. Los anticuerpos anti-nucleosoma y anti-C1q se asocian con la NL en varias regiones geográficas. En el suroccidente colombiano se asienta una etnia heterogénea, lo que motivó la evaluación de la frecuencia y la relación de dichos marcadores con NL en dicha región.
MétodosRealizamos un estudio transversal en un centro de salud en el suroccidente de Colombia, con 84 pacientes diagnosticados con LES (57 sin NL; 27 con NL) entre los años 2016 y 2018. Se compararon las características demográficas, clínicas y de laboratorio, incluidos los anticuerpos anti-dsDNA, complemento, anti-C1q y anti-nucleosomas entre estos pacientes. Se realizaron inmunoensayos ELISA para medir los anticuerpos de interés en muestras de sangre. El análisis estadístico se llevó a cabo con el software Stata v.14 (StataCorp, College Station, Texas, EE. UU.). Las variables cuantitativas se resumieron como medias o medianas y se compararon con la prueba t de Mann-Whitney o Two-sample t test; las variables categóricas se mostraron como proporciones y se compararon con Chi-cuadrado o con la prueba exacta de Fisher. Para el análisis de correlaciones entre variables cuantitativas se calculó el coeficiente de correlación de Spearman.
ResultadosEntre los 84 pacientes, 27 presentaban LN, de los cuales 16 (59,2%) tuvieron una prueba positiva para anticuerpos anti-nucleosoma y 10 (37%) para anticuerpos anti-C1q. Se encontró una asociación entre anti-C1q y formas proliferativas de NL, así como formas recientemente diagnosticadas de NL. Hubo una correlación entre los anticuerpos anti-nucleosoma y anti-C1q y el anti-dsDNA y las bajas concentraciones de complemento sérico.
ConclusiónAunque los 2 marcadores se encontraron en porcentajes variables de pacientes con LES y no parecen ser marcadores específicos de NL en nuestra población, la presencia de anti-C1q se asoció con formas proliferativas de NL y NL de novo.
Systemic Lupus Erythematosus (SLE) is a systemic, autoimmune disease characterized by the presence of autoantibodies and damage to multiple organs and tissues. It has a wide spectrum of clinical manifestations and is heterogeneous in its presentation.1 Renal involvement is one of the most frequent features. Up to 70% of patients with SLE may present lupus nephritis (LN),1 which is reportedly apparent in 25–50% of patients at the time of SLE diagnosis.2 LN portends a poor prognosis and is associated with high morbidity and mortality.2 Up to 20% of patients with LN progress to end-stage renal disease after 10 years, even with treatment.1,2
LN is manifested by changes in the urinalysis, mainly proteinuria and hematuria, and there may or may not be a rise in serum creatinine.1 In most cases monitoring and follow-up of patients with LN is complex. In clinical practice, high levels of anti-dsDNA antibodies and low levels of serum complement components (C3 and C4) are used as serologic markers of SLE systemic activity, although the value of these tests to identify an organ-specific manifestation of SLE is limited.3 That is why several serum and urinary biomarkers are currently being studied for their value in identifying the presence of LN. Among these markers are anti-nucleosome and anti-C1q antibodies.4,5
Anti-C1q antibodies are directed against the C1q complement molecule. Under normal conditions, the classical pathway starts with the activation of C1q,6 which is responsible for inhibiting the production of type I interferon and promoting the clearance of immune complexes, explaining how autoantibodies to C1q may contribute to the pathophysiology of SLE.5 For this reason, investigators, including the European League Against Rheumatism, are studying these antibodies as a diagnostic tool for SLE and for an ability to differentiate among patients who do or do not have LN.3 However, their value as a useful biomarker remains controversial.7
Anti-nucleosome antibodies are directed against complexes produced by histones and double-stranded DNA and have an important role in the pathophysiology of SLE and, more specifically, of LN.4 Their role as diagnostic markers of the disease has also been studied.4,8 They are reportedly even more specific and sensitive than other recognized markers, such as anti-dsDNA antibodies.4
Some cohorts have evaluated them together, finding there is relation with manifestations other than renal damage, for example vasculitis, photosensitivity, low complement levels and high scores in disease activity indexes.9
The presentation of SLE is variable, as is the expression of these antibodies based on demographic characteristics.10,11 A study carried out in Caucasians, African descent and Asian, identified that anti-C1q antibodies were more frequent in Asians than in others.12 As the heterogeneity of SLE depends on factors such as ethnicity, our aim was to evaluate the behavior of anti-C1q and anti-nucleosome antibodies in patients with SLE, including their frequency and relationship with LN, in southwest Colombia; a region known for its ethnic mixture of white, black, and indigenous peoples.13 This non-Caucasian ancestry seen in our region is a characteristic that although it is not exclusive for this region, confers a susceptibility to a higher incidence of LN and earlier age of onset of SLE.11,14,15
MethodsWe conducted a cross-sectional study of patients diagnosed with SLE based on the 1997 American College of Rheumatologists classification criteria.16 Patients meeting the criteria for LN (defined as persistent proteinuria>0.5 grams per day or cellular casts) had undergone renal biopsy for classification (according to the International Society of Nephrology and Renal Pathology criteria17) with their activity and chronicity indexes (according to Austin, et al.18). Proliferative LN was defined as either class III or class IV, as well as these combinations: III+V, IV+V, IV+VI; also renal transplantation was included in this category. Whereas, non-proliferative comprised class I, II, V, VI and the combination II+V.
All patients were treated at the Rheumatology Unit of Fundación Valle del Lili Hospital in Cali-Colombia between February 2016 and January 2018. We did not have exclusion criteria regarding ethnicity. Furthermore, our population has a heterogeneous one given the genetic ancestry.
A total of 84 subjects were enrolled. Blood samples for anti-C1q and anti-nucleosome antibodies were drawn after the Ethics Committee approved the study (protocol number 1138) and every subject signed informed consent to participate. We collected data on participants’ laboratory test results (ANA, ENA, Crithidia luciliae immunofluorescence test – anti-dsDNA, complement, blood count, and urinalysis) and histological reports already recorded in their medical records. Enzyme-linked immunosorbent assay (ELISA) was performed to measure serum anti-C1q and anti-nucleosome antibodies using the ORG 249 and ORG 228 essay Alegria kits (Mainz, Germany), respectively. The measurement of both antibodies was carried out following the provider's instructions with a cut-off for anti-C1q of ≥10U/ml, and anti-nucleosome ≥20U/ml. All the serum samples were collected either in the inpatient stay or during an outpatient visit and the rest of laboratory tests results were taken from the clinical records of the same day. SLEDAI was calculated to measure disease activity at admission, with active disease defined by scores of ≥3.19
Patients were classified regarding the presence of LN, into SLE without LN and SLE with LN. Then, demographical data, anti-dsDNA positivity, anti-nucleosome, anti-C1q, and complement consumption were evaluated. Additionally, patients were categorized into five different groups, as follows (LN activity was considered if proteinuria>500mg/24h or>3+or presence of urinary casts):
- -
Patients who did not have LN at the moment of the serum sampling, but developed LN by the last control consultation. For these patients we revised the last time they attended to a control consultation with a rheumatologist in our institution, which varied from months to years after the serum sampling in every case.
- -
Patients who did not have LN at the moment of the serum sampling, and did not develop LN by the last control consultation either. For these patients we revised the last time they attended to a control consultation with a rheumatologist in our institution, which varied from months to years after the serum sampling in every case.
- -
Patients with history of LN, that was active at the moment of the serum sampling.
- -
Patients who had inactive LN at the moment of the serum sampling.
- -
Patients who were newly diagnosed with LN at the moment of the serum sampling.
Statistical analysis was carried out using STATA14 software (StataCorp, College Station, Texas, USA). Quantitative variables were summarized as means or medians (with standard deviation [SD] or interquartile range [IQR]) and were compared with Mann–Whitney or Two-sample t test, according to normality depending on Shapiro–Wilk test. Categorical variables are shown as proportions and were compared with Chi-squared or Fisher's exact test as appropriate. Correlation analysis between quantitative variables was calculated with Spearman's correlation. A p value<0.05 was considered statistically significant.
ResultsPatient characteristicsOf the 84 patients with an SLE diagnosis, 73 were women and the mean age was 34 years (SD 15.95). The median SLEDAI was 10 (IQR 4–10) and 73 (86.9%) patients had SLE activity. Twenty-seven patients had LN (32.1%) and 57 had SLE without LN (67.8%). In LN patients, anti-nucleosome antibodies showed a frequency of 59.2%, while it was of 37% for anti-C1q. Demographic features, histological and laboratory findings in patients with and without LN are shown in Table 1.
Demographic features and histological and laboratory findings according to the presence of LN.
| Lupus nephritis | p value | ||
|---|---|---|---|
| Variables | No, n=57 | Yes, n=27 | |
| Agea | 35 (21–47.5) | 30 (23–42) | 0.6744 |
| Sex, n (%)c | |||
| Male | 5 (8.7) | 6 (22.2) | 0.273 |
| Female | 52 (91.2) | 21 (77.7) | |
| Positive anti-dsDNA, n(%)c | |||
| EIA, n (%) | 29 (50.9) | 13 (48.1) | 0.81 |
| IIF, n (%) | 31 (54.4) | 10 (37) | 0.165 |
| Anti-nucleosome antibodies, n(%)c | |||
| Positive | 39 (68.4) | 16 (59.2) | 0.465 |
| Negative | 18 (31.6) | 11 (40.7) | |
| Anti-C1q antibodies, n(%)c | |||
| Positive | 14 (24.6) | 10 (37) | 0.302 |
| Negative | 43 (75.4) | 17 (63) | |
| Complement | |||
| C3 (value)b | 75.4 (34.7) | 70.1 (32.1) | 0.4718 |
| C4 (value)a | 11.5 (7.1–19.2) | 11.9 (6.9–19.2) | 0.5892 |
| Patients with low serum values, n(%)c | 35 (61.4) | 15 (55.6) | 0.64 |
EIA, ELISA immunoassay; IIF, indirect immunofluorescence.
Among all 84 patients, 18 (21.4%) had triple positivity for anti-nucleosome, anti-C1q, and anti-dsDNA (either by EIA, IIF or both) antibodies. Of these 18, 7 had LN, which was classified as type III in 3, type IV in 3, and type III+V in 1.
Frequency of anti-nucleosome and anti-C1q antibodiesOf the 84 patients, 55 (65.4%) had a positive test for anti-nucleosome antibodies and 24 (28.5%) had a positive test for anti-C1q antibodies. There were no differences in terms of age between those with and without the antibodies, whereas a significant frequency of positive anti-C1q antibodies was found in women (p=0.011).
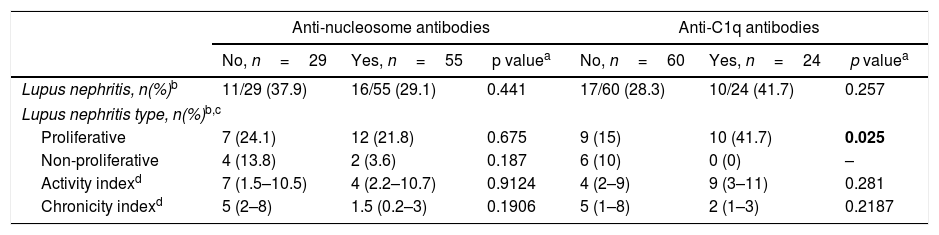
Anti-nucleosome and anti-C1q antibodies expression in LN patientsOf the 27 patients with LN, there were two patients without histological classification of their LN, reason why only 25 are classified into the proliferative and non-proliferative types. Of these patients, sixteen (29.1%) were positive for anti-nucleosome, while 10 (41.7%) were positive for anti-C1q antibodies. The two autoantibodies were not significantly associated with indexes of activity or chronicity, but patients with proliferative LN had a higher incidence of positivity for anti-C1q antibodies (p=0.025) (Table 2).
Anti-nucleosome and anti-C1q antibody testing regarding LN status.
| Anti-nucleosome antibodies | Anti-C1q antibodies | |||||
|---|---|---|---|---|---|---|
| No, n=29 | Yes, n=55 | p valuea | No, n=60 | Yes, n=24 | p valuea | |
| Lupus nephritis, n(%)b | 11/29 (37.9) | 16/55 (29.1) | 0.441 | 17/60 (28.3) | 10/24 (41.7) | 0.257 |
| Lupus nephritis type, n(%)b,c | ||||||
| Proliferative | 7 (24.1) | 12 (21.8) | 0.675 | 9 (15) | 10 (41.7) | 0.025 |
| Non-proliferative | 4 (13.8) | 2 (3.6) | 0.187 | 6 (10) | 0 (0) | – |
| Activity indexd | 7 (1.5–10.5) | 4 (2.2–10.7) | 0.9124 | 4 (2–9) | 9 (3–11) | 0.281 |
| Chronicity indexd | 5 (2–8) | 1.5 (0.2–3) | 0.1906 | 5 (1–8) | 2 (1–3) | 0.2187 |
LN activity or its development and the relation with anti-nucleosome or anti-C1q results are shown in Table 3. The number of patients in some groups were so small to be compared, although a significance was found in patients with newly diagnosed LN who had positive anti-C1q antibodies versus those with a negative result (p=0.022). Additionally, three patients developed LN in time, two of them had positive results for both antibodies, while one had negative anti-C1q and positive anti-nucleosome.
LN activity and its development in positive anti-nucleosome and anti-C1q tests.
| Anti-nucleosome antibodies | Anti-C1q antibodies | |||||
|---|---|---|---|---|---|---|
| Patients | Positive,n=55N (%) | Negative, n=29N (%) | p value | Positive,n=24N (%) | Negative,n=60N (%) | p value |
| SLE without LN, who developed LN by the last follow-up | 3 (5.4) | 0 (0) | – | 2 (8.3) | 1 (1.6) | – |
| SLE without LN, who did not develop LN by the last follow-up | 34 (61.8) | 16 (55.2) | 0.6421 | 11 (45.8) | 39 (65) | 0.141 |
| Active LN at the moment of serum sampling | 6 (10.9) | 7 (24.1) | 0.125 | 6 (25) | 7 (11.7) | 0.180 |
| Inactive LN at the moment of serum sampling | 5 (9) | 3 (10.3) | 1 | 0 (0) | 8 (13.3) | – |
| Newly diagnosed LN at the moment of serum sampling | 5 (9) | 0 (0) | – | 4 (16.6) | 1 (1.6) | 0.022 |
Apart from the association between anti-C1q and proliferative forms of LN and its presence in newly diagnosed cases of LN; we evaluated the implications of conventional markers of SLE activity in relation to these antibodies.
We carried out correlation measurements between anti-dsDNA antibodies by IIF, anti-dsDNA antibodies by EIA, low serum levels of the C3, and low serum levels of the C4 with the expression of anti-nucleosome and anti-C1q antibodies, independently. We found positive correlations for anti-dsDNA by IIF and EIA with both antibodies. On the contrary, negative correlations were seen for low serum levels of C3 and C4 with both antibodies. Correlation coefficients were variable; the highest, 0.5386, was presented between anti-dsDNA by EIA and anti-nucleosome antibodies. However, a p value<0.05 was found in every case (Table 4).
Correlations of anti-nucleosome and anti-C1q antibodies with anti-dsDNA antibodies and low complement levels.
| Positive for anti-nucleosome antibodies (r2) | p value | Positive for anti-C1q antibodies (r2) | p value | |
|---|---|---|---|---|
| Anti-dsDNA (EIA) | 0.5386 | 0 | 0.4694 | 0.0001 |
| Anti-dsDNA (IIF) | 0.3319 | 0.0036 | 0.4882 | 0 |
| Complement-C3 | −0.3378 | 0.0022 | −0.311 | 0.005 |
| Complement-C4 | −0.3193 | 0.0039 | −0.2732 | 0.0142 |
EIA, ELISA immunoassay; IIF, indirect immunofluorescence.
Ho: ro=0.
p<0.05.
Reject Ho.
The southwestern region of Colombia has an heterogeneous ethnicity and thus, diverse presentations of SLE, including the likelihood of developing LN, reason why it is important to define biomarkers that serve to identify LN in this population. Our study showed that anti-nucleosome antibodies were more frequently positive than were anti-C1q antibodies in patients with LN (59.2% vs. 37%), similar to previous observations.5,20
Our aim was to establish an association between LN and the presence of these two antibodies, and our findings suggest that anti-C1q can be useful in identifying proliferative forms and also in facilitating the diagnostic approach in cases of recent development of LN, where, for instance, may be difficulties in accessing renal biopsy. Other authors have pointed the utility of these markers towards the recognition of active forms of LN.20–22 Metwally et al. who studied an Egyptian cohort of patients and found an association between positive anti-C1q antibodies in patients with LN and low serum complement, but anti-nucleosome antibodies alone were not associated to clinical nor serological characteristics.9 Moura et al. found anti-C1q antibodies in patients with proteinuria in Salvador, Brazil,5 same finding of Simón et al. in México, but with anti-nucleosome antibodies.23 That study identified a great usefulness for anti-nucleosome antibodies as diagnostic markers of SLE in absence of anti-dsDNA antibodies, SLE activity and renal involvement.23 These differences might be attributable to the heterogeneous presentations of SLE in the Latin American population.14 Apart from that foreign studies, Vásquez et al. from Colombia, evaluated anti-C1q antibodies in patients with similar ethnicity to ours, finding a frequency of 55%, of which 71% had LN.24 Another reason for their association with findings other than LN might be the fact that these antibodies are involved in the pathogenesis of the disease rather than exclusively organ-specific damage.25
Interestingly, beyond LN or organ-specific implications, it was clear that classical disease activity markers do have relation with anti-nucleosome and anti-C1q, demonstrated by a significant correlation between each antibody with anti-dsDNA antibodies either by IIF or EIA, and low C3 and C4 serum levels. Additionally we saw a big proportion of patients with active SLE based on SLEDAI scores. This suggests that anti-nucleosome and anti-C1q antibodies might indicate SLE relapse and, therefore have possible wider applications in our population, as has been noted by other authors.6,23,24,26,27
In Metwally's cohort, patients who had positive tests for both anti-C1q and anti-nucleosome antibodies presented low C3 serum levels, while C3 and C4 were diminished in those who had positive tests for anti-C1q only.9 Additionally, the significant association we noted between proliferative types of LN and positive anti-C1q antibodies suggests that this marker might indicate the severity and class of LN. This is comparable to the Colombian group that described that of twenty patients of whom a renal biopsy was available, high levels of anti-C1q antibodies were mostly found in LN types III and IV.24
Even though triple positivity for anti-nucleosome, anti-C1q, and anti-dsDNA antibodies was present in a small number of subjects with LN, they all had the more severe classes of LN. Further study of this issue may help determine whether testing for these antibodies will be applicable in our population specifically in relation to the type of LN.
We evaluated the presence or absence of LN and its activity in our patients in relation to the positivity to anti-C1q and anti-nucleosome antibodies, and whether they developed the complication in time (months-years), after the sampling was done. It is to remark, that LN activity was distributed in active, inactive and newly diagnosed, regardless the antibody that was present. It is worth mentioning of those who had positive anti-C1q, in first place, none of them presented an inactive LN at that time and secondly, a significance was seen in patients who were newly diagnosed with LN compared to patients with absence of anti-C1q antibodies, what might be associated not only to the renal impairment but to an active SLE as has been cited previously. On the other hand, there were three cases of positive anti-nucleosome and two cases of positive anti-C1q antibodies, that developed LN afterwards. Therefore, additional roles different from acute phase biomarkers should be taken into account; in fact, it has been proposed that anti-C1q antibodies represent an OR of 3.60 to present LN,9 as well as an elevation of anti-C1q 2.3 months before a renal relapse occurs, has been described.6,28
Conversely, we only found one patient who had negative results for anti-C1q tests, who developed LN in time. Treatment has also shown to lower or normalize antibodies titers.29
Finally, our results suggest that anti-C1q antibodies may be useful to identify proliferative and early forms of LN. Besides, both anti-C1q and anti-nucleosome antibodies keep a relation with other known markers of SLE and renal flares, pointing their utility towards the identification of increased systemic and renal disease activity. Hence, diagnosing LN at other stages different to those of recent development remains dependent on conventional clinical and laboratory information.
Study limitationsThis study is cross-sectional, so that there may be patients who develop LN in time that are not taken into account; also, presence or absence of autoantibodies might be affected by treatment. Additionally, the sample could be comparatively small to establish that although we did not find an association, it definitely does not exist.
FundingThe authors received no financial support for the research, authorship, and/or publication of this article.
Conflict of interestsThe authors declare that there is no conflict of interest.