Psoriasis (PsO) and psoriatic arthritis (PsA) are immune-mediated inflammatory disorders, in which pro-inflammatory cytokines play a pathogenic role. In addition, sex hormones have been shown to influence the systemic inflammation associated with psoriatic disease.
ObjectiveTherefore, combined assessment of pro-inflammatory cytokines and sex hormones may shed more light on the pathological inflammatory response associated with PsO and PsA.
Materials and methodsIn this case–control study, serum concentrations of three pro-inflammatory interleukins (IL-12, IL-17A, and IL-23) and two sex hormones (testosterone and estrogen) were explored in 88 patients with psoriatic disease (44 PsO and 44 PsA) and 88 controls using enzyme-linked immunosorbent assay kits.
ResultsIL-12, IL-17A, and IL-23 concentrations were significantly greater in PsO and PsA patients than in controls. In addition, IL-12 was significantly higher in PsO than in PsA, while IL-17A was significantly elevated in PsA compared to PsO. IL-23 showed no significant difference between PsO and PsA. Testosterone concentrations were significantly elevated in PsA patients compared to PsO patients or controls, whereas there was no significant difference between PsO patients and controls. Estrogen concentrations were significantly lower in PsO and PsA patients compared to controls. Correlation analysis revealed that IL-12 was positively correlated with IL-17A in PsO, while in PsA it was positively correlated with IL-23. IL-17A and IL-23 were positively correlated with testosterone in PsO, while in PsA, IL-17A and IL-23 were negatively correlated with estrogen and testosterone, respectively.
ConclusionsIL-12, IL-17A, and IL-23 were up-regulated in the serum of PsO and PsA patients. However, IL-12 was more bound to PsO, IL-17A was more bound to PsA, while IL-23 levels were similar in PsO and PsA. Up-regulated testosterone levels were only linked to PsA and not PsO. PsO and PsA shared down-regulated levels of estrogen. Testosterone and estrogen were differentially associated with IL-12, IL-17A, and IL-23 in PsO and PsA.
La psoriasis y la artritis psoriásica son trastornos inflamatorios inmunomediados, en los cuales las citocinas proinflamatorias desempeñan un papel patogénico. Además, se ha demostrado que las hormonas sexuales influyen en la inflamación sistémica asociada con la enfermedad psoriásica.
ObjetivoPor lo tanto, una evaluación combinada de citocinas proinflamatorias y hormonas sexuales puede arrojar más luz sobre la respuesta inflamatoria patológica asociada con PsO y PsA.
Materiales y métodosEn este estudio de casos y controles se exploraron las concentraciones séricas de tres interleucinas proinflamatorias (IL-12, IL-17A e IL-23) y dos hormonas sexuales (testosterona y estrógeno) en 88 pacientes con enfermedad psoriásica (44 con psoriasis y 44 con artritis psoriásica) y 88 controles, para lo cual se utilizaron kits de ensayo inmunoabsorbente ligado a enzimas.
ResultadosLas concentraciones de IL-12, IL-17A e IL-23 fueron significativamente mayores en pacientes con psoriasis y artritis psoriásica que en los controles. Además, la IL-12 fue significativamente mayor en la psoriasis que en la artritis psoriásica, mientras que la IL-17A fue significativamente elevada en la artritis psoriásica en comparación con la psoriasis. IL-23 no mostró diferencias significativas entre las dos enfermedades. Las concentraciones de testosterona estaban significativamente elevadas en los pacientes con artritis psoriásica en comparación con los pacientes con psoriasis o los controles, mientras que no hubo diferencias significativas entre los pacientes con psoriasis y los controles. Las concentraciones de estrógeno fueron significativamente más bajas en pacientes con estas dos enfermedades, en comparación con los controles. El análisis de correlación reveló que la IL-12 se correlacionaba positivamente con la IL-17A en la psoriasis, mientras que en la artritis psoriásica se correlacionaba positivamente con IL-23. IL-17A e IL-23 se correlacionaron positivamente con la testosterona en la psoriasis, mientras que en la artritis psoriásica IL-17A e IL-23 se correlacionaron negativamente con estrógeno y testosterona, respectivamente.
ConclusionesIL-12, IL-17A e IL-23 estaban reguladas positivamente en el suero de pacientes con estas dos enfermedades. Sin embargo, la IL-12 se unió más a la psoriasis, la IL-17A se unió más a la artritis psoriásica, mientras que los niveles de IL-23 fueron similares en ambas enfermedades. Los niveles de testosterona regulados al alza solo se relacionaron con la artritis psoriásica y no con la psoriasis. Las dos enfermedades compartían niveles de estrógeno regulados a la baja. La testosterona y el estrógeno se asociaron diferencialmente con IL-12, IL-17A e IL-23 en la psoriasis y en la artritis psoriásica.
Psoriasis (PsO) is an autoimmune inflammatory condition characterized by red, scaly papules and plaques, which are primarily found on the elbows, knees, and scalp [1,2]. Musculoskeletal inflammatory manifestations (peripheral joints, axial skeleton, and entheses) can occur in approximately 30% of PsO patients due to the development of psoriatic arthritis (PsA) [3]. Although extensive research has been conducted, the mechanisms responsible for psoriatic disease (PsO and PsA) pathogenesis are not well-defined. However, it is well-established that psoriatic disease is characterized by persistent inflammation, keratinocyte hyperproliferation, and immune cell infiltration, which are suggested to be induced by dysregulated expression of immune modulators, especially cytokines belonging to the interleukin (IL)-23/T helper (h) 17 axis [2]. In addition, hormonal dysregulation involving sex hormones such as testosterone (TES) and estrogen (ES) may also contribute to persistent inflammation in patients with psoriatic disease [4].
As signaling low-molecular weight glycoprotein molecules, cytokines have an essential role in the control of various aspects of immune response, innate and adaptive. Convincing evidence suggests that cytokines can influence immunity through their effect on cells involved in mediating inflammation, acting as messengers in promoting the immune system to control infections that can be associated with the initiation and maintenance of inflammation [5]. In the context of psoriatic disease, studies have disclosed that intracellular and extracellular cytokine pathways are major pathogenic factors [6]. In addition, there is evidence to suggest that pro-inflammatory cytokines, including IL-12, IL-17A, and IL-23, play an active role in inducing a cascade of inflammatory responses that stimulate keratinocyte proliferation and neutrophil recruitment [2,7].
IL-12 and IL-23 are heterodimeric cytokines secreted by dendritic cells and play a central role in psoriatic disease by promoting differentiation of naïve T lymphocytes into Th1 or Th17 cells. IL-23 is essential for the survival and development of Th17 cells that promote the production of effector cytokines such as IL-17A. There is increasing evidence suggesting that the IL-23/Th17 related cytokines have a crucial role in PsO and PsA pathogenesis [8]. Furthermore, studies showed that the interaction between cytokines expressed in the skin tissues is critical in the development of PsO clinical features, such as hyperproliferation of keratinocytes and inflammation. Moreover, high levels of pro-inflammatory cytokines can lead to joint pain, swelling, and inflammation in PsA patients [9]. In light of this, cytokine assessments may provide a more precise view of the ongoing process of cellular signaling in PsO and PsA because these disorders are characterized by inflammation-mediated pathogenesis [10].
In patients with psoriatic disease, cytokines can affect sex hormones and fertility, but the exact mechanisms of these effects are unknown. However, IL-17A and IL-23 play an essential role in the multi-organ inflammation detected in psoriatic diseases, which may lead to various systemic complications [11]. These complications include dyslipidemia, obesity, type 2 diabetes, and even cardiovascular disease, which can indirectly affect sex hormones and fertility. However, the role of sex hormones in psoriasis is complex, multifaceted and poorly defined, making it an important area of study with implications for research and clinical practice. Research also shows sex-specific differences in PsA with varying impacts on disease activity, outcomes, and treatment response [12] Besides, studies have shown that sex hormonal changes, such as those that occur during menopause, can trigger PsO flares and worsen PsA symptoms. In addition, sex hormones have been found to have both pro-inflammatory and anti-inflammatory effects on PsO, highlighting the complex role they play in these conditions. [13] Interestingly, evidence has shown that ES can stimulate or reduce the production of pro-inflammatory cytokines depending on its levels in the blood [13,14]. However, it is still not fully understood whether these immune regulatory roles of ES play physiologically important roles in inflammatory diseases such as psoriasis. As for TES, this male hormone has been associated with anti-inflammatory properties, and its concentrations can decrease due to testicular dysfunction under the stimulating effects of pro-inflammatory cytokines, leading to increased inflammation. In men with chronic plaque PsO, low TES levels have been associated with disease severity, but the evidence is not conclusive [13,15].
In this study, the role of three cytokines (IL-12, IL-17A, and IL-23) in the pathogenesis of PsO and PsA was updated in association with the clinical indications of both psoriatic diseases. Particular emphasis has been placed on understanding the influence of two sex hormones (ES and TES) on this association, as evidence highlighting this issue is not well understood.
Materials and methodsPatients and controlsA case–control study was performed on 88 psoriatic patients, 44 with PsO and 44 with PsA (median age (IQR): 35.5 [29.5–46.0] and 41.0 [30.0–52.5] years, respectively) and 88 healthy controls (HC; 40.0 [29.5–52.0] years). Patients were diagnosed by consultant dermatologists and rheumatologists in Dermatology and Rheumatology Units at Baghdad Teaching Hospital within the Medical Education Complex in Baghdad during the period from July 2022 to January 2023. Inclusion criteria for the current study were adult females and males who met the psoriatic disease diagnostic criteria and agreed to participate in the study. Cases younger than 18 years, pregnant women, and patients suffering from other inflammatory and systemic disorders were excluded. The PsO disease activity was assessed by the Psoriasis Area and Severity Index (PASI), and PsO was classified as mild/moderate (n=35) or severe/active (n=9) [16]. The Disease Activity Index for PsA (DAPSA) was used to evaluate disease activity in patients with PsA, and the index was simplified as low disease activity (score≤14; n=23) and moderate/high disease activity (score>14; n=21) [17]. Patients were also tested for white blood cell count (WBC), hemoglobin (Hb), and erythrocyte sedimentation rate (ESR). The HC group included blood donors and university employees without chronic diseases. ESR was adopted as the standard for assessing the general health of HC, and only those with ESR less than 20mm/h were included.
Laboratory methodsWBCs were counted and ESR was measured using an auto-hematology analyzer (BC-700 Series, Mindray, China) following the manufacturer's instructions. Serum levels of IL-12, IL-17A, IL-23, TES, and ES were quantified using enzyme-linked immunosorbent assay (ELISA) kits (Catalog Number: SL0970Hu, SL2334Hu, SL0989Hu, SL3705Hu, and SL0686Hu, respectively) following the manufacturer's instructions (SunLong Biotech Company, China). Briefly, the kits were based on the principles of sandwich ELISA. The provided 96-well plate was pre-coated with an antibody specific to the target cytokine or hormone. Standards or serum samples were dispensed into appropriate wells and combined to the specific antibody and the plate was incubated. After washing, horseradish peroxidase-conjugated antibody specific for the target cytokine or hormone was dispensed into each well and the plate was incubated. The free components were washed off, and then, the TMB substrate solution was added to each well. After color development, the enzymatic reaction was terminated with stop solution. Next, the absorbance was measured spectrophotometrically at a wavelength of 450nm using an ELISA reader (HumaReader HS, Germany). Serum levels of cytokines and hormones were determined using a curve-fitting equation generated from a standard curve. The standard curve range was 0–90pg/mL for IL-12, IL-17A, and IL-23, 0–3nmol/L for TES, and 0–270pg/mL for ES.
Statistical analysisIBM SPSS Statistics 25.0 (Armonk, NY: IBM Corp.) and GraphPad Prism version 9.2.0 (San Diego, CA, USA) were used to perform statistical analysis. Kolmogorov–Smirnov and Shapiro–Wilk tests were used to analyze the normal distribution of continuous variables. Most of these variables were not normally distributed (non-parametric) and were therefore presented as median and interquartile range (IQR: 25–75%) and significance was detected by Mann–Whitney U test. The discriminatory potential of cytokines and hormones between patients and HC was evaluated using receiver operating characteristic (ROC) curve analysis and presented as area under the curve (AUC). Spearman correlation analysis was used to evaluate the strength of association between variables and was presented as a correlation coefficient (rs). A probability (p)<0.05 was taken as significant. Sample size power was calculated using G*power software, version 3.1.9.7 [18].
ResultsSample size powerIn order to assess the statistical validity of the number of patients (PsO and PsA) and HC included in the study, the G*power software was used. The assessment was performed with following inputs: 44 patients, 88 HC, 0.05 one-tailed α-error p, and 0.5 effect size d. The actual power calculated for the sample size was 0.85. The acceptable marginal power is 0.8 [18].
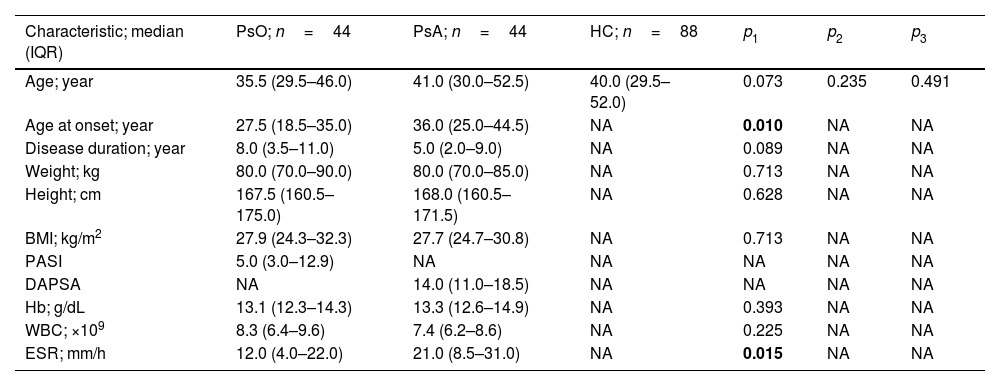
Baseline dataData representing clinical and laboratory characteristics of patients (PsO and PsA) and HC are shown in Table 1. PsO and PsA patients were age-matched to HC as there were no statistically significant differences (35.5 [29.5–46.0] and 41.0 [30.0–52.5] vs. 40.0 [29.5–52.0] years; p>0.05). The age at onset of PsA patients was significantly higher compared to PsO patients (36.0 [25.0–44.5] vs. 27.5 [18.5–35.0] years; p=0.01). Additionally, ESR levels were significantly elevated in PsA compared to PsO (21.0 [8.5–31.0] vs. 12.0 [4.0–22.0]mm/h; p=0.015). When other laboratory and clinical indicators of RA were compared between PsO and PsA patients, no significant differences were found (Table 1).
Clinical and laboratory characteristics of patients with psoriasis and psoriasis arthritis.
| Characteristic; median (IQR) | PsO; n=44 | PsA; n=44 | HC; n=88 | p1 | p2 | p3 |
|---|---|---|---|---|---|---|
| Age; year | 35.5 (29.5–46.0) | 41.0 (30.0–52.5) | 40.0 (29.5–52.0) | 0.073 | 0.235 | 0.491 |
| Age at onset; year | 27.5 (18.5–35.0) | 36.0 (25.0–44.5) | NA | 0.010 | NA | NA |
| Disease duration; year | 8.0 (3.5–11.0) | 5.0 (2.0–9.0) | NA | 0.089 | NA | NA |
| Weight; kg | 80.0 (70.0–90.0) | 80.0 (70.0–85.0) | NA | 0.713 | NA | NA |
| Height; cm | 167.5 (160.5–175.0) | 168.0 (160.5–171.5) | NA | 0.628 | NA | NA |
| BMI; kg/m2 | 27.9 (24.3–32.3) | 27.7 (24.7–30.8) | NA | 0.713 | NA | NA |
| PASI | 5.0 (3.0–12.9) | NA | NA | NA | NA | NA |
| DAPSA | NA | 14.0 (11.0–18.5) | NA | NA | NA | NA |
| Hb; g/dL | 13.1 (12.3–14.3) | 13.3 (12.6–14.9) | NA | 0.393 | NA | NA |
| WBC; ×109 | 8.3 (6.4–9.6) | 7.4 (6.2–8.6) | NA | 0.225 | NA | NA |
| ESR; mm/h | 12.0 (4.0–22.0) | 21.0 (8.5–31.0) | NA | 0.015 | NA | NA |
IQR: interquartile range; PsO: psoriasis; PsA: psoriatic arthritis; HC: healthy controls; BMI: body mass index; PASI: psoriasis area and severity index; DAPSA: disease activity in psoriatic arthritis; Hb: hemoglobin; WBC: white blood cell count; ESR: erythrocyte sedimentation rate; NA: not applicable; p: probability (significant p-value is indicated in bold); p1: PsO vs. PSA; p2: PsO vs. HC; p3: PsA vs. HC. Significance was detected using Mann–Whitney U test.
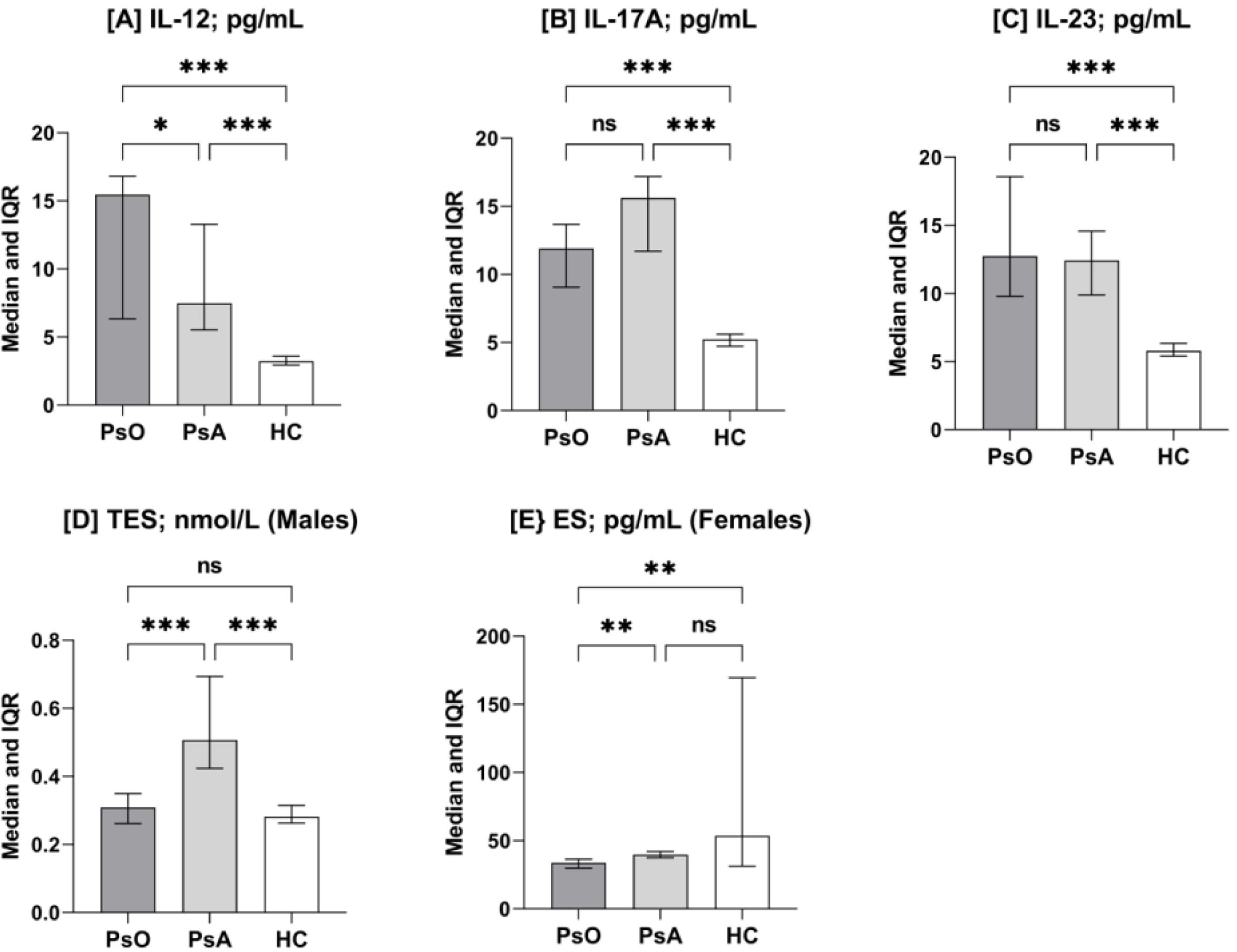
IL-12 concentrations (median and IQR) were significantly increased in PsO and PsA patients compared to the HC group (15.46 [6.3–16.8] and 7.46 [5.5–13.2] vs. 3.22 [2.9–3.5]pg/mL; p<0.001). IL-12 concentrations were also significantly increased in PsO patients compared to PsA patients (p=0.03). For IL-17A, its concentrations were also significantly increased in PsO and PsA patients compared to HC (11.91 [9.0–13.7] and 15.61 [11.7–17.1] vs. 5.24 [4.7–5.6]pg/mL; p<0.001). Although IL-17A concentrations were higher in PsA patients than in PsO patients, the difference was not significant (p=0.08). Likewise, IL-23 concentrations were significantly increased in PsO and PsA patients compared to HC (12.47 [9.7–18.5] and 12.42 [9.8–14.5] vs. 5.79 [5.4–6.3]pg/mL; p<0.001), while there were no significant differences between PsO and PsA patients (p=0.58) (Fig. 1).
Column-bar plots of interleukin (IL)-12 (A), IL-17-A (B), IL-23 (C), testosterone (TES; D), and estrogen (ES; E) in patients with psoriasis (PsO) and psoriatic arthritis (PsA) and healthy controls (HC) Columns represent median. Bar represent interquartile range (IQR: 25–75%). Significance was detected using Mann–Whitney U test (*p<0.05; **p<0.01; ***p<0.001; ns: not significant).
Regarding sex hormones, TES was examined only in males, while ES was detected only in females. The median concentrations (IQR) of TES were significantly elevated in PsA patients compared to PsO patients or HC (0.51 [0.42–0.69] vs. 0.31 [0.26–0.35] and 0.28 [0.26–0.32]nmol/L; p<0.001), while the difference was not significant between PsO patients and HC (p=0.27). In contrast, ES concentrations were significantly reduced in PsO patients compared to PsA patients or HC (33.7 [29.9–36.4] vs. 39.7 [37.4–42.0] and 53.5 [31.2–169.6]pg/mL; p=0.005 and 0.003, respectively), while the difference was not significant between PsA patients an HC (p=0.99) (Fig. 1).
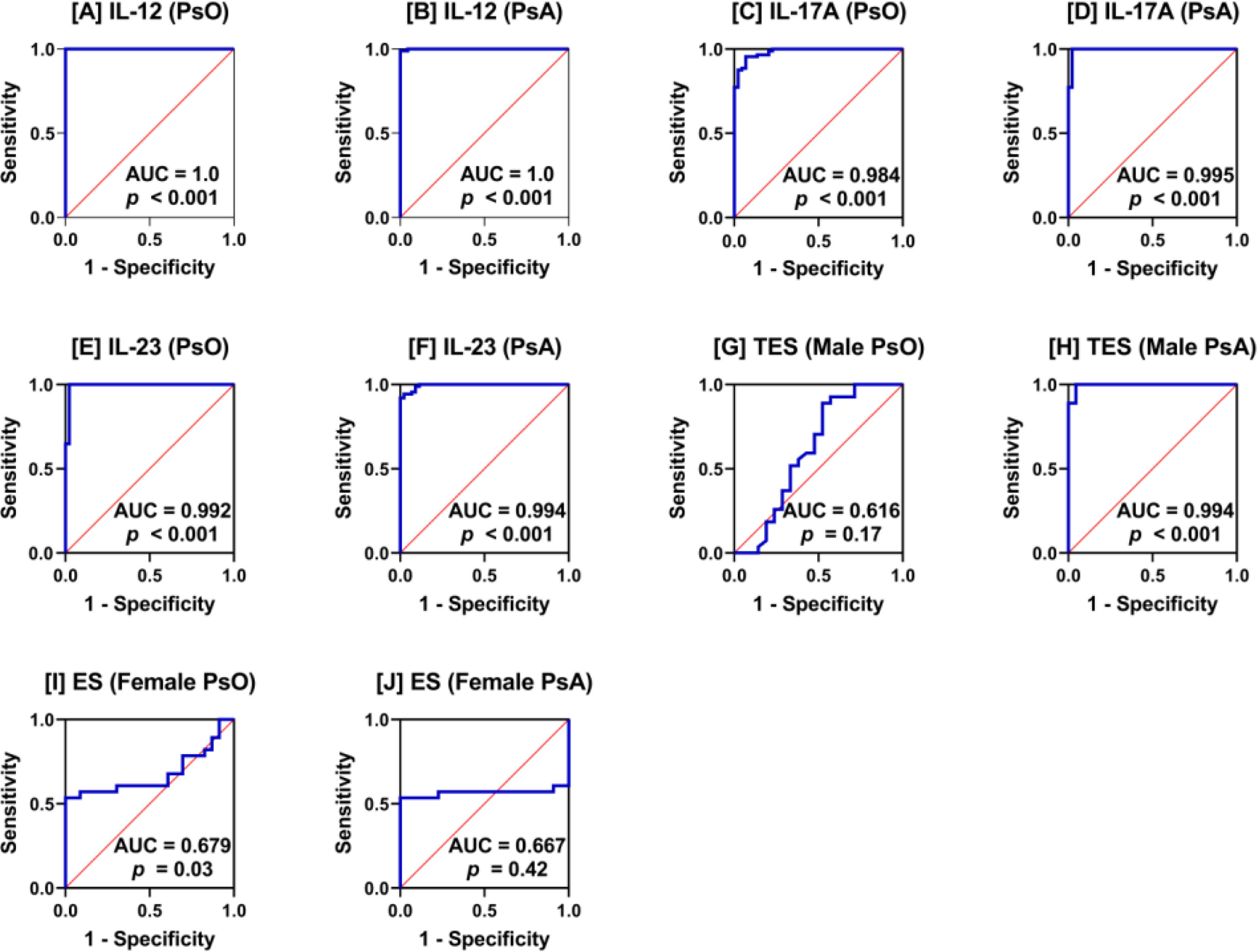
ROC curve analysisROC curve analysis indicated that IL-12, IL-17A, and IL-23 were excellent biomarkers in distinguishing PsO and PsA patients from HC (AUC>0.9; p<0.001). TSE was also an excellent discriminatory biomarker but between PsA and HC males (AUC=0.994; p<0.001) and not between PsO and HC males (AUC=0.616; p=0.17). For ES, the discriminatory potential of this sex hormone between PsO or PsA and HC females was less reliable as the AUC value was <0.7 (AUC=0.679 and 0.667), but the p-value was significant in PsO (p=0.03) (Fig. 2).
Receiver operating characteristic (ROC) curve analysis interleukin (IL)-12 (A and B), IL-17-A (C and D), IL-23 (E and F), testosterone (TES; G and H), and estrogen (ES; I and J) in patients with psoriasis (PsO) and psoriatic arthritis (PsA) versus healthy controls. Area under the curve (AUC) and probability (p) are shown.
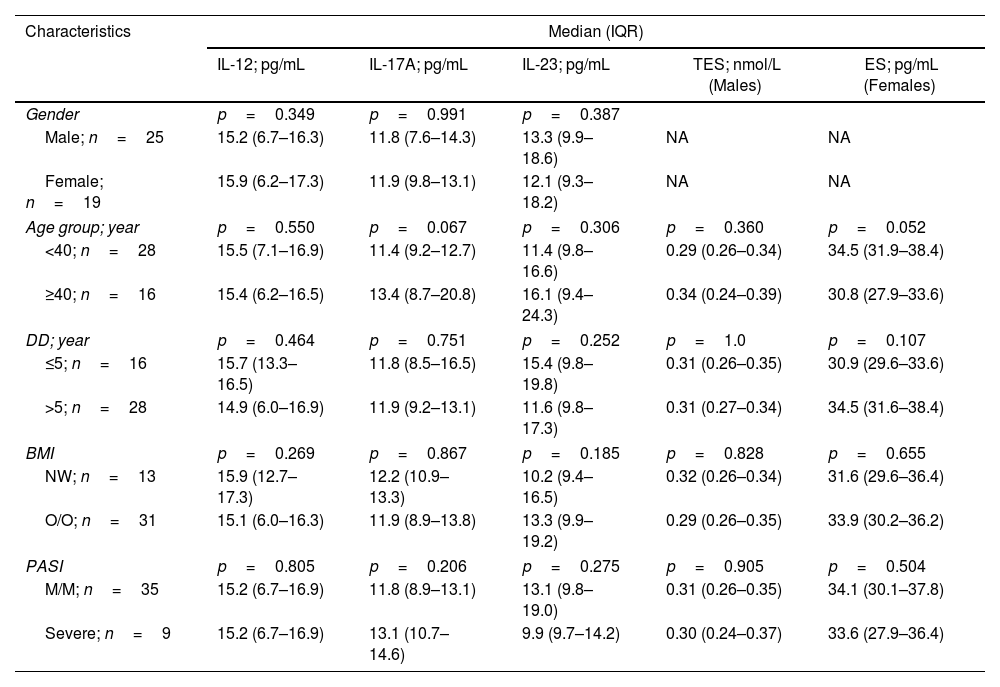
Serum concentrations of cytokines (IL-12, IL-17A, and IL-23) and sex hormones (TES and ES) were stratified by PsO characteristics, including gender, age group (<40 and ≥40 years), disease duration (≤5 and >5 years), BMI (NW and O/O), and PASI (M/M and severe). There were no significant differences in each of these strata (Table 2).
Serum concentrations of interleukins (IL-12, IL-17A, and IL-23) and sex hormones (testosterone and estrogen) stratified by characteristics of patients with psoriasis.
| Characteristics | Median (IQR) | ||||
|---|---|---|---|---|---|
| IL-12; pg/mL | IL-17A; pg/mL | IL-23; pg/mL | TES; nmol/L (Males) | ES; pg/mL (Females) | |
| Gender | p=0.349 | p=0.991 | p=0.387 | ||
| Male; n=25 | 15.2 (6.7–16.3) | 11.8 (7.6–14.3) | 13.3 (9.9–18.6) | NA | NA |
| Female; n=19 | 15.9 (6.2–17.3) | 11.9 (9.8–13.1) | 12.1 (9.3–18.2) | NA | NA |
| Age group; year | p=0.550 | p=0.067 | p=0.306 | p=0.360 | p=0.052 |
| <40; n=28 | 15.5 (7.1–16.9) | 11.4 (9.2–12.7) | 11.4 (9.8–16.6) | 0.29 (0.26–0.34) | 34.5 (31.9–38.4) |
| ≥40; n=16 | 15.4 (6.2–16.5) | 13.4 (8.7–20.8) | 16.1 (9.4–24.3) | 0.34 (0.24–0.39) | 30.8 (27.9–33.6) |
| DD; year | p=0.464 | p=0.751 | p=0.252 | p=1.0 | p=0.107 |
| ≤5; n=16 | 15.7 (13.3–16.5) | 11.8 (8.5–16.5) | 15.4 (9.8–19.8) | 0.31 (0.26–0.35) | 30.9 (29.6–33.6) |
| >5; n=28 | 14.9 (6.0–16.9) | 11.9 (9.2–13.1) | 11.6 (9.8–17.3) | 0.31 (0.27–0.34) | 34.5 (31.6–38.4) |
| BMI | p=0.269 | p=0.867 | p=0.185 | p=0.828 | p=0.655 |
| NW; n=13 | 15.9 (12.7–17.3) | 12.2 (10.9–13.3) | 10.2 (9.4–16.5) | 0.32 (0.26–0.34) | 31.6 (29.6–36.4) |
| O/O; n=31 | 15.1 (6.0–16.3) | 11.9 (8.9–13.8) | 13.3 (9.9–19.2) | 0.29 (0.26–0.35) | 33.9 (30.2–36.2) |
| PASI | p=0.805 | p=0.206 | p=0.275 | p=0.905 | p=0.504 |
| M/M; n=35 | 15.2 (6.7–16.9) | 11.8 (8.9–13.1) | 13.1 (9.8–19.0) | 0.31 (0.26–0.35) | 34.1 (30.1–37.8) |
| Severe; n=9 | 15.2 (6.7–16.9) | 13.1 (10.7–14.6) | 9.9 (9.7–14.2) | 0.30 (0.24–0.37) | 33.6 (27.9–36.4) |
IQR: interquartile range; IL: interleukin; TES: testosterone; ES: estrogen; DD: disease duration; BMI: body mass index; NW: normal-weight; O/O: overweight/obese; PASI: psoriasis area and severity index; M/M: mild/moderate; NA: not applicable; p: probability of Mann–Whitney U test.
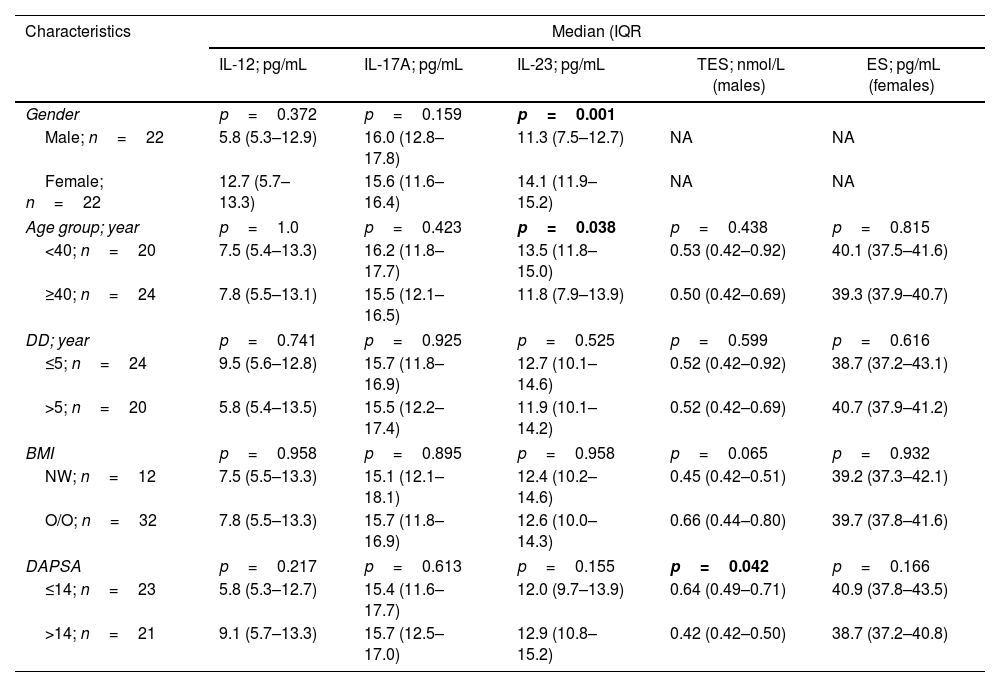
In the case of PsA, three significant differences were recorded. IL-23 concentrations were elevated in females than in males (14.1 [11.9–15.2] vs. 11.3 [7.5–12.7]pg/mL; p=0.001) and the age group<40 years compared the age group≥40 years (13.5 [11.8–15.0] vs. 11.8 [7.9–13.9]pg/mL; p=0.038, respectively). TES concentrations were also significantly elevated in male patients with DAPSA≤14 compared to male patients with DAPSA>14 (0.64 [0.49–0.71] vs. 0.42 [0.42–0.50]nmol/L; p=0.042) (Table 3).
Serum concentrations of interleukins (IL-12, IL-17A, and IL-23) and sex hormones (testosterone and estrogen) stratified by characteristics of patients with psoriatic arthritis.
| Characteristics | Median (IQR | ||||
|---|---|---|---|---|---|
| IL-12; pg/mL | IL-17A; pg/mL | IL-23; pg/mL | TES; nmol/L (males) | ES; pg/mL (females) | |
| Gender | p=0.372 | p=0.159 | p=0.001 | ||
| Male; n=22 | 5.8 (5.3–12.9) | 16.0 (12.8–17.8) | 11.3 (7.5–12.7) | NA | NA |
| Female; n=22 | 12.7 (5.7–13.3) | 15.6 (11.6–16.4) | 14.1 (11.9–15.2) | NA | NA |
| Age group; year | p=1.0 | p=0.423 | p=0.038 | p=0.438 | p=0.815 |
| <40; n=20 | 7.5 (5.4–13.3) | 16.2 (11.8–17.7) | 13.5 (11.8–15.0) | 0.53 (0.42–0.92) | 40.1 (37.5–41.6) |
| ≥40; n=24 | 7.8 (5.5–13.1) | 15.5 (12.1–16.5) | 11.8 (7.9–13.9) | 0.50 (0.42–0.69) | 39.3 (37.9–40.7) |
| DD; year | p=0.741 | p=0.925 | p=0.525 | p=0.599 | p=0.616 |
| ≤5; n=24 | 9.5 (5.6–12.8) | 15.7 (11.8–16.9) | 12.7 (10.1–14.6) | 0.52 (0.42–0.92) | 38.7 (37.2–43.1) |
| >5; n=20 | 5.8 (5.4–13.5) | 15.5 (12.2–17.4) | 11.9 (10.1–14.2) | 0.52 (0.42–0.69) | 40.7 (37.9–41.2) |
| BMI | p=0.958 | p=0.895 | p=0.958 | p=0.065 | p=0.932 |
| NW; n=12 | 7.5 (5.5–13.3) | 15.1 (12.1–18.1) | 12.4 (10.2–14.6) | 0.45 (0.42–0.51) | 39.2 (37.3–42.1) |
| O/O; n=32 | 7.8 (5.5–13.3) | 15.7 (11.8–16.9) | 12.6 (10.0–14.3) | 0.66 (0.44–0.80) | 39.7 (37.8–41.6) |
| DAPSA | p=0.217 | p=0.613 | p=0.155 | p=0.042 | p=0.166 |
| ≤14; n=23 | 5.8 (5.3–12.7) | 15.4 (11.6–17.7) | 12.0 (9.7–13.9) | 0.64 (0.49–0.71) | 40.9 (37.8–43.5) |
| >14; n=21 | 9.1 (5.7–13.3) | 15.7 (12.5–17.0) | 12.9 (10.8–15.2) | 0.42 (0.42–0.50) | 38.7 (37.2–40.8) |
IQR: interquartile range; IL: interleukin; TES: testosterone; ES: estrogen; DD: disease duration; BMI: body mass index; NW: normal-weight; O/O: overweight/obese; DAPSA: disease activity in psoriatic arthritis; NA: not applicable; p: probability of Mann–Whitney U test (significant p-value is indicated in bold).
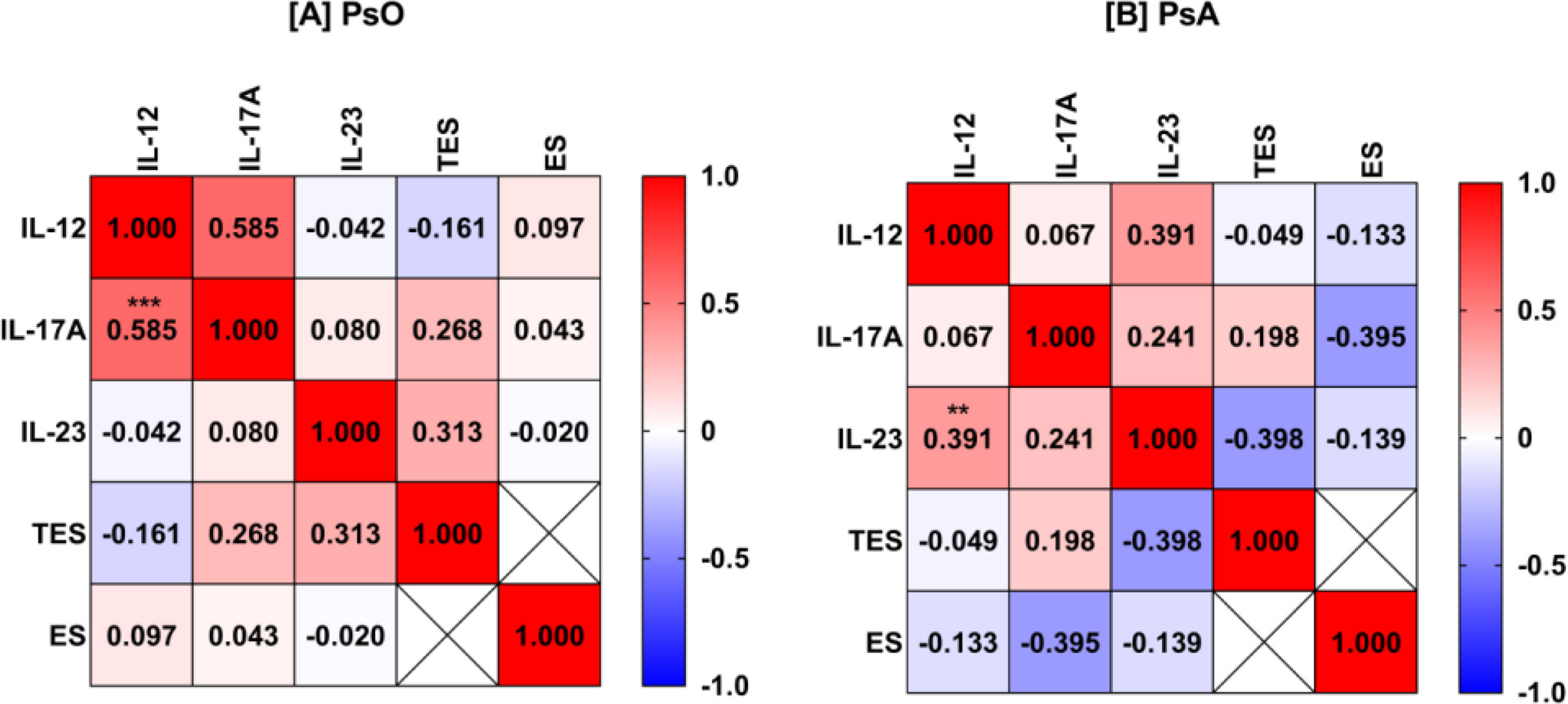
Bivariate Spearman rank correlation analysis was performed between five variables, IL-12, IL-17A, IL-23, TES, and ES, in PsO and PsA patients. Significant positive correlations included IL-12 and IL-17A in PsO patients (rs=0.585; p<0.001) and IL-12 and IL-23 in PsA patients (rs=0.391; p=0.01). It is also interesting to note that IL-17A and IL-23 were positively correlated with TES in PsO (rs=0.268 and 0.313; p=0.24 and 0.17, respectively). In PsA, the correlation pattern of sex hormones was different and IL-17A and IL-23 were negatively correlated with ES and TES, respectively (rs=−0.395 and −0.398; p=0.07 and 0.07, respectively). However, the p-values for these correlations were not significant perhaps due to the relatively low numbers of patients included (Fig. 3).
Heat-map matrix of Spearman's correlation analysis of interleukin (IL)-12, IL-17-A, IL-23, testosterone (TES), and estrogen (ES) in patients with psoriasis (PsO; A) and psoriatic arthritis (PsA; B). The value inside the square represents the correlation coefficient. Red describes a positive correlation. Blue describes a negative correlation. The asterisk indicates a significant correlation (**p<0.01; ***p<0.001).
In this study, three pro-inflammatory cytokines, IL-12, IL-17A, IL-23, and two sex hormones, TES and ES, were evaluated simultaneously in two groups of patients with psoriatic disease, PsO and PsA. The focus was on understanding their interconnected role in the pathogenesis of PsO and PsA and the characteristics of psoriatic disease (age, gender, disease duration, BMI, PASI, and DAPSA) because the data in this regard are not overwhelming. Although IL-12, IL-17A, and IL-23 were significantly up-regulated in the serum of both groups of patients, IL-12 was more associated with PsO, IL-17A was more associated with PsA, and IL-23 shared a similar association with PsA and PsO. In addition, the levels of the three cytokines were not affected by the characteristics of PsO, whereas in PsA, age and gender had an effect. Regarding sex hormones, up-regulated TES was exclusively associated with PsA and was more pronounced in male patients with DAPSA≤14. ES was oppositely down-regulated in females with psoriatic disease, especially those with PsO. Moreover, the correlation pattern of cytokines to sex hormones was different in PsO and PsA.
Pro-inflammatory cytokines and their receptors are known to be fundamentally involved in complex immune pathways that mediate the onset and progression of inflammatory and autoimmune diseases, such that they are being promoted as a promising therapeutic target [19,20]. In line with this view, the results of the present study indicated that IL-12, IL-17A, and IL-23 were involved in the development of PsO and PsA. Moreover, the three cytokines showed excellent potential in differentiating patients from HC (AUC>0.9). IL-12 and IL-23 are heterodimeric cytokines (IL-12p35/IL-12p40 and IL-23p19/IL-12p40, respectively) belonging to the IL-12 family of cytokines that have emerged as important regulators of host immunity [21]. Both cytokines are critical in the differentiation of naïve T lymphocytes into interferon (IFN)-γ-producing Th1 cell and IL-17A-producing Th17 cell [22]. IL-12 and IL-23 share the IL-12p40 subunit, and functionally it is now well established that the two cytokines play a key role in the formation of psoriatic plaques through their effects on various components of the chronic inflammatory episode associated with the development of PsO [23]. The presence of Th1 cells and IFN-γ expression in psoriatic lesions supports the association between IL-12 and the pathogenesis of psoriatic disease [24]. However, data from animal model studies of PsO suggest that IL-12 may also attenuate skin inflammation in PsO by modulating IL-23-mediated inflammatory events and reducing skin invasion by Th17 cells along with promoting an anti-inflammatory genetic program in keratinocytes [25]. However, there are conflicting results for serum IL-12 levels in PsO and PsA patients. Some studies report a higher, lower, or no difference in IL-12 levels in psoriatic patients compared to controls [26,27]. In a mouse model of PsO, administration of IL-12 with bacterial products enhanced the severity of psoriatic lesions. Whereas when IL-12 was neutralized, the expression of other pro-inflammatory cytokines such as TNF-α and INF-γ decreased and was associated with inhibition of PsO development [28]. This may indicate that the contribution of IL-12 to the inflammatory processes observed in PsO and PsA occurs through the induction of other pro-inflammatory cytokines, particularly through the IL-23/IL-17A axis, which has been shown to be fundamental to the pathogenesis of both psoriatic diseases [8].
The pathogenic role of the IL-23/IL-17A axis and the effectiveness of therapies targeting this pathway in psoriatic disease have been confirmed for the first time in cutaneous PsO [29]. Information obtained from circulating and tissue cytokine investigations and genome-wide association studies have confirmed the involvement of IL-23 and IL-17A in mediating the inflammatory response in PsO and PsA [30]. In psoriatic lesions, IL-23 expression has been shown to be significantly up-regulated compared to unlesioned tissue. Studies have also shown that increased IL-23 expression in animal models contributes to the development of PsO and PsA. It was found that mice with high levels of IL-23 expression in the skin developed features of PsO skin inflammation, followed by joint inflammation [31]. Furthermore, IL-23 inhibitors have been shown to significantly reduce joint inflammation in PsA patients compared to IL-12/IL-23 inhibitors. In addition, IL-23 has been shown to effectively promote Th17 proliferation and enhance its ability to produce IL-17A, and inhibition of IL-23 signaling has been associated with decreased IL-17A production [32,33]. IL-17A is the most prominent member of the IL-17 family of cytokines. While it has a role in enhancing immune responses against pathogens, its excessive production has been linked to inflammatory diseases such as PsO and PsA [29]. IL-17A is mainly produced through Th17 activation, which can directly or indirectly mediate inflammatory responses by promoting a variety of cells to secrete inflammatory factors [34]. This study found that IL-17A levels were significantly elevated in patients with PsO and PsA. Other studies have also reported over-expressed levels of IL-17A in skin lesions of PsO patients and synovial fluid of PsA patients, contributing to tissue inflammation and bone remodeling [26,29]. This over-expression is believed to play an integral role in psoriatic disease development, making IL-17A a key target for therapeutic interventions [29]. Together, these data suggest that the IL-12/IL-23/IL-17A axis plays a contributing and complementary pathogenic role in PsO and PsA, and each cytokine may have a specific physiological role in the development and progression of the disease. For example, it has been indicated that IL-23 has an essential role in initiating disease progression, while IL-17A is primarily responsible for promoting disease progression [35]. Indeed, clinical studies have shown that biological inhibitors of this cytokine axis can have satisfactory therapeutic outcomes in PsO and PsA [8,36].
The next and most important focus of this study was to understand the association of two sex hormones (TES and ES) with the pathogenesis of PsO and PsA in the context of three pro-inflammatory cytokines (IL-12, IL-23, and IL-17A), as studies targeting this axis of association are limited. Exceptionally high serum TES levels were associated with PsA, especially in patients with DAPSA≤14, whereas this pattern of association was not detected in PsO. Indeed, TES was an excellent biomarker in differentiating between PsA patients and HC (AUC=0.994). TES, the predominant male androgen, is potentiated with immunomodulatory effects due to its anti-inflammatory functions. Decreased TES levels have been associated with an increase in pro-inflammatory cytokines, such as IL-1β, IL-6, and TNF-α, leading to systemic inflammation. In contrast, TES replacement therapy reduced pro-inflammatory cytokines and enhanced the production of anti-inflammatory cytokines such as IL-10 [37]. A compelling body of evidence has indicated that systemic inflammation can be suppressed by TES in a number of inflammatory diseases such as prostate cancer, coronary artery disease, and diabetes [38]. In PsO, studies have also shown that TES levels are significantly decreased, and there has been some, although not conclusive, evidence depicting a significant inverse relationship between TES and PASI regardless of age [13]. In addition, symptoms of hypogonadism associated with PsO were improved by TES replacement therapy, suggesting that sex hormonal imbalances may contribute to the pathogenesis of the disease [39]. In current PsO patients, these findings were not confirmed, and serum TES levels did not differ significantly from HC. Furthermore, TES levels were not affected by whether the disease was M/M or severe as measured by the PASI. When it comes to PsA, the present results showed that TES levels were significantly elevated in male patients especially those with low disease activity (DAPSA≤14). The elevated levels of TES in existing PsA patients can be explained on the basis of its anti-inflammatory effects to counteract the increased levels of pro-inflammatory cytokines such as IL-12, IL-23 and IL-17A. In this context, there is only one study that showed that serum TES levels were negatively correlated with PsA disease activity, an observation that may support the association of TES with disease activity [40]. However, inconsistent observations have been reported regarding the association of TES with arthritis. A recent cross-sectional study of 10,439 adults showed lower TES levels in the arthritis group compared to the non- arthritis group [41]. Therefore, it is necessary to further study the relationship between TES and arthritis in general and PsA in particular to clarify the mechanism of action of serum TES on arthritis.
In contrast to TES, ES levels were significantly decreased in female PsO and PsA patients compared to healthy females. Therefore, this female sex hormone may have immune regulatory functions and low ES levels may be associated with an increased risk of PsO and PsA due to dysregulated inflammatory responses. Functional signaling of ES is mediated primarily through binding to ES receptors expressed on immune cells involved in the pathogenesis of psoriatic disease such as neutrophils, monocytes/macrophages, dendritic cells, and T lymphocytes [42]. It has been indicated that the interaction of ES with its receptors is associated with the induction of transcriptional activities that down-regulate the expression of several pro-inflammatory cytokine genes, such as IL1B, IL12, IL23, IL17A, and TNFA, and this may explain the influential role of ES in immune regulation [42]. More evidence suggests that during pregnancy, where elevated levels of ES appear, PsO symptoms improve significantly, while skin lesions worsen during the postpartum period [43]. Recently, it has been experimentally demonstrated that mice lacking endogenous ovarian hormones showed exacerbation of PsO inflammation associated with up-regulated levels of the pro-inflammatory cytokines IL-17A and IL-1β. These PsO-associated inflammatory events were reversed upon administration of exogenous ES. Furthermore, the suppressive effect of ES on IL-1β and IL-17A production by murine neutrophils and macrophages lacking ES receptors was abolished [44]. Similar effects of ES have also been suggested in the pathogenesis of PsA and ES has been associated with improvement in disease symptoms [45]. Regardless of these results, the exact mechanism by which ES affects PsO and PsA needs to be clarified. More research is needed to determine whether modifying ES could have therapeutic potential in these conditions.
Correlation analysis revealed that the pattern of association between the five variables examined, IL-12, IL-17A, IL-23, TES, and ES, depended on whether the psoriatic disease was PsO or PsA. IL-12 showed a positive correlation with IL-17A in PsO, while in PsA, it showed a positive correlation with IL-23. The same variability is applied to sex hormones. IL-17A and IL-23 showed a positive correlation with TES in PsO, while in PsA, IL-17A and IL-23 were negatively correlated with ES and TES, respectively. Although the observed associations between sex hormones and cytokines were not significant (this may be due to low sample size), the signaling pathway interactions of sex hormones with cytokines in the pathogenesis of PsO and PsA may be different. In fact, the results of this study indicated that the contribution of IL-12, IL-17A, IL-23, TES, and ES in both psoriatic diseases was not similar and there were some differences. Therefore, profiling of sex hormones should be included in uncovering and understanding the mechanistic clues behind the association of inflammatory markers with PsO and PsA.
The study faced some limitations. First, other sex hormones, sex hormone receptors, and related hormones, such as progesterone, luteinizing hormone, follicle-stimulating hormone, prolactin, and cortisol, were not examined. In addition, TES was only examined in males and ES only in females. Therefore, it may be interesting to examine the two sex hormones in patients of both sexes. Second, anti-inflammatory cytokines, such as IL-10, were not explored. Third, although the sample size power assessment validated the number of patients and HC, the PsO and PsA patient groups were still relatively small. However, compared with other relevant studies, the number of patients included was similar. For example, Pirowska and colleagues evaluated serum levels of four cytokines (IL-12, IL-23, IL-17, and TNF-α) in 26 patients with PsO, 34 patients with PsA, and 30 HC [46]. In addition, Michalak-Stoma and coworkers examined levels of multiple cytokines in 52 patients with PsO and 24 HC [47].
ConclusionsIL-12, IL-17A, and IL-23 were up-regulated in the serum of PsO and PsA patients. However, IL-12 was more bound to PsO, IL-17A was more bound to PsA, while the profile of IL-23 was similar in PsO and PsA. Up-regulated TES levels were only linked to PsA and not PsO. PsO and PsA shared down-regulated levels of ES. TES and ES were differentially associated with IL-12, IL-17A, and IL-23 in PsO and PsA. However, a better understanding of the role of cytokines (IL-12, IL-17A, and IL-23) and sex hormones (TES and ES) in the pathogenesis of PsO and PsA can be enriched after overcoming the limitations of this study.
Authors’ contributionRanda R. Ghamyes: Writing-Reviewing and Editing, Writing-original draft, Visualization, Validation, Methodology, Investigation, Formal analysis, Data curation, Conceptualization.
AbdulKareem A. Alkazaz: Writing-Reviewing and Editing, Writing-original draft, Visualization, Validation, Supervision, Software, project administration, Methodology, Investigation, Formal analysis, Data curation, Conceptualization.
Ethical considerationsThis study obtained approval from the Ethics Committee at the Department of Biotechnology, College of Science, University of Baghdad (College of Science Ethics Committee [CSEC]; Reference No.: CSEC/0223/0022 on February 5, 2023). The committee issued approval after filling out and signing the consent form prepared in accordance with the Declaration of Helsinki. Each participant provided written consent after being informed of the study objectives.
Declaration of generative AI and AI-assisted technologies in the writing processNone of them have been used.
FundingThis research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Conflict of interestThe authors declare no conflict of interest.
The cooperation of the medical staff of the dermatology and rheumatology units at Baghdad Teaching Hospital is highly appreciated. We also thank dermatologist Ali F. Alsaadi and rheumatologist Mohammed H. Alosami for their role in diagnosing PsO and PsA.