Rhupus syndrome, characterized by overlapping clinical features of rheumatoid arthritis (RA) and systemic lupus erythematosus (SLE), presents significant therapeutic challenges due to its complex pathogenesis and limited treatment options. This case report evaluates the efficacy of filgotinib, a selective JAK1 inhibitor, in a patient with refractory rhupus syndrome.
Case presentationA 42-year-old male, initially diagnosed with SLE in 2013, presented with persistent polyarthritis, cutaneous manifestations, and constitutional symptoms. Conventional treatments including hydroxychloroquine, methotrexate, and belimumab failed to achieve adequate disease control.
Diagnosis and treatmentIn October 2022, the diagnosis was revised to rhupus based on the evolution of clinical manifestations and laboratory findings, leading to the initiation of filgotinib, a JAK1 inhibitor. Disease activity was monitored over 24 months using standardized assessment tools.
ResultsSignificant clinical improvement was observed within weeks of filgotinib initiation, enabling prednisone dose reduction and maintaining low disease activity throughout the 24-month follow-up period.
ConclusionThis case highlights the therapeutic potential of filgotinib in managing rhupus syndrome but further research and larger clinical trials are necessary to establish filgotinib's therapeutic potential and safety profile in the management of SLE.
El síndrome de Rhupus, caracterizado por manifestaciones clínicas superpuestas de artritis reumatoide (AR) y lupus eritematoso sistémico (LES), presenta desafíos terapéuticos significativos debido a su patogénesis compleja y opciones de tratamiento limitadas. Este reporte de caso evalúa la eficacia de filgotinib, un inhibidor selectivo de JAK1, en un paciente con síndrome de Rhupus refractario.
Presentación del casoUn paciente masculino de 42años, inicialmente diagnosticado con LES en 2013, presentó poliartritis persistente, manifestaciones cutáneas y síntomas constitucionales. Los tratamientos convencionales, incluyendo hidroxicloroquina, metotrexato y belimumab, no lograron alcanzar un control adecuado de la enfermedad.
Diagnóstico y tratamientoEn octubre de 2022 el diagnóstico fue revisado a Rhupus, con base en la evolución de las manifestaciones clínicas y hallazgos de laboratorio, lo que llevó al inicio del tratamiento con filgotinib, un inhibidor de JAK1. La actividad de la enfermedad fue monitoreada durante 24meses utilizando herramientas de evaluación estandarizadas.
ResultadosSe observó una mejora clínica significativa dentro de las semanas posteriores al inicio de filgotinib, lo que permitió que se redujera la dosis de prednisona y mantener una baja actividad de la enfermedad durante todo el período de seguimiento de 24meses.
ConclusiónEste caso destaca el potencial terapéutico de filgotinib en el manejo del síndrome de Rhupus, pero se necesitan más investigaciones y ensayos clínicos más amplios para establecer el potencial terapéutico y el perfil de seguridad de filgotinib en el manejo del LES.
Rhupus syndrome is a rare condition and has lesser visceral organ involvement as compared with systemic lupus erythematosus (SLE). The syndrome is manifested by patients coincidentally sharing clinical features and classification criteria of both rheumatoid arthritis (RA) and SLE. Still, it cannot be combined as a unique clinical, pathologic, or immunologic syndrome [1,2]. The precise etiology and triggers of rhupus remain elusive thus far, with scant research indicating the collective influence of genetic, immunological and hormonal. The natural history of rhupus follows a similar pattern to that of RA and can progress from inflammation with deformities and disability to sometimes erosions. However, the definition and positioning of SLE in the context of inflammatory, beyond autoimmune, diseases remain controversial. The rarity of rhupus disease has made it difficult to understand its prevalence, pathophysiology, natural history, and radiological and immunological profiles [3]. Current therapy is based on clinical experience and limited study results.
The Janus kinase (JAK)-signal transducer and activator of transcription (JAK-STAT) pathway is a key driver of RA by way of mediating the response to multiple pro-inflammatory cytokines and cellular growth factors. Blocking the JAK-STAT pathway has proven effective in treating conditions mediated by the immune system and has emerged as an innovative therapeutic focus for managing RA [4]. Specifically targeting JAK1 can influence a specific group of inflammatory proteins in the JAK-STAT pathway, distinct from those affected by JAK2 or JAK3 inhibition. This targeted approach may offer a more favorable balance between the benefits and risks than broad-spectrum JAK inhibitors [5].
Filgotinib, a second-generation selective inhibitor of JAK1 exhibits rapid onset of action, maintains effectiveness over time, and possesses a well-established safety profile in patients with moderate-to-severe RA who have responded inadequately to methotrexate, are treatment-naïve to biologics, or have prior biologic experience [6]. Baricitinib, an oral JAK-STAT inhibitor already used in other rheumatological conditions. Phase 3 results showed partial improvement: the drug reached the primary endpoint with a higher proportion of patients responding to SRI-4 criteria, especially in subgroups with highly active disease or on high-dose glucocorticoid treatment [7].
Upadacitinib at the 30mg dose, administered as monotherapy, achieved the primary endpoint (SRI-4 response with glucocorticoid dose ≤10mg per day) and several secondary endpoints. The study demonstrated significant improvements in lupus disease activity, achieving a low disease activity state, reducing disease flares, and delaying the time to first flare compared to placebo. The treatment's efficacy remained consistent throughout a 48-week evaluation period. Exposure-response analyses highlighted higher response rates with increased upadacitinib plasma concentrations, suggesting that the 30mg dose is predicted to be an effective dose for treating patients with SLE with moderate to severe disease activity [8]. The primary objective of this case report is to evaluate the clinical and immunological outcomes of filgotinib therapy in a patient diagnosed with rhupus syndrome, focusing on its effects on disease activity, glucocorticoid-sparing potential, and safety profile.
Case reportA 42-year-old male patient was diagnosed with SLE in 2013, initially presenting with polyarthralgia, photophobia, asthenia, butterfly-like rash, and chest pain. Blood tests revealed ANA positivity 1:1280 granular, the presence of anti-DNA 100IU/mL (normal values (nv) <20), and a reduction in C3 79mg/dl (nv 90–180), C4 12.9mg/dl (nv 15–53) and 24-h proteinuria 400mg. He was initially treated with hydroxychloroquine and prednisone (high dose), initially and then associated with azathioprine, both with poor response (SLEDAI 12, DAS28 5.7). As of April 2019, treatment with belimumab was started with an improvement in the SLEDAI score: 8. However, the patient could not reduce the prednisone dose (25mg/day) due to persistent articular disease activity.
The patient developed recurrent purulent ulcers on the lower limbs and elbows, under belimumab treatment, with consequent septic arthritis that induced long hospitalization. Due to intense pain and the progression of arthritis, despite treatment with hydroxychloroquine and methotrexate, the patient went to the emergency room numerous times, between January 2020 and September 2022, and resorted, independently, to excessive doses of prednisone (up to 50mg/die).
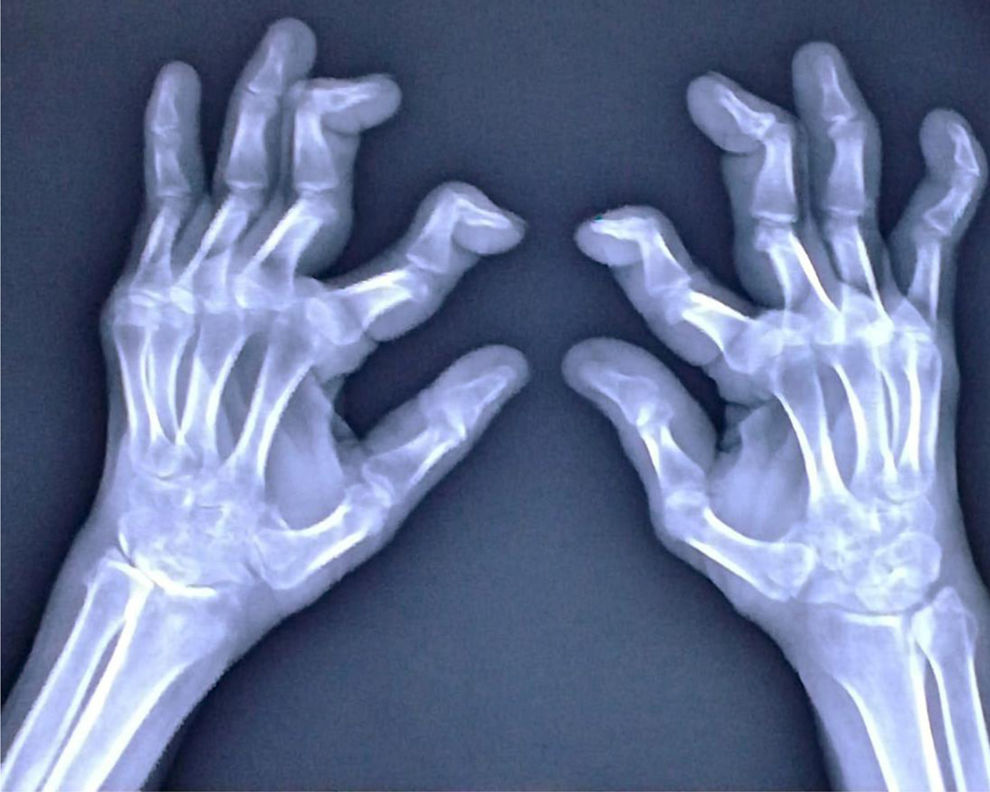
In October 2022, re-evaluating the diagnosis, based on the new positivity of the rheumatoid factor >3xULN 95IU/mL (nv <30), tenderness and swelling in both knees, hands and foot joints, and particularly in the heels, erosions and reduction of the joint rim at the level of the carpal bones, with a notable reduction in calcium tone at X-ray images (Fig. 1) and the presence of butterfly-style erythema on the forehead, nose, the diagnosis of rhupus was made and it was decided to add filgotinib to the therapeutic regimen.
The radiographic images of the patient's hands demonstrate the presence of swan neck deformity. In the images, there is observed hyperextension of the proximal interphalangeal joint (PIP) and marked flexion of the distal interphalangeal joint (DIP) in multiple fingers of the hands. Furthermore, erosions and reduction of the joint rim are observed at the level of the carpal bones, with a notable reduction in calcium tone.
We do not use rituximab because in patients with moderate-to-severe SLE with increased glucocorticoid doses rituximab and belimumab have similar serious infection risks [9] and we do not use anifrolumab, because it was not commercially available at the time.
The choice of filgotinib was based on its lower interaction with the patient's polypharmacy and lower risk profile of infections [5,6]. The patient was started on filgotinib at a dose of 200mg/day.
After 3 months the patient reported significantly improving his joint pain and swelling, erythema, normalization of C3 (129.8mg/dl), C4 (35mg/dl), decrease in anti-dsDNA, and proteinuria (0mg); his SLEDAI score decreased to 2, and his DAS28 score decreased to 3.3. He was able to taper the prednisone (to 5mg/day) and continue with filgotinib. At 18 months follow-up, the patient remained in low disease activity (SLEDAI 2) with progressive reduction of the prednisolone (PDN) dose until discontinuation.
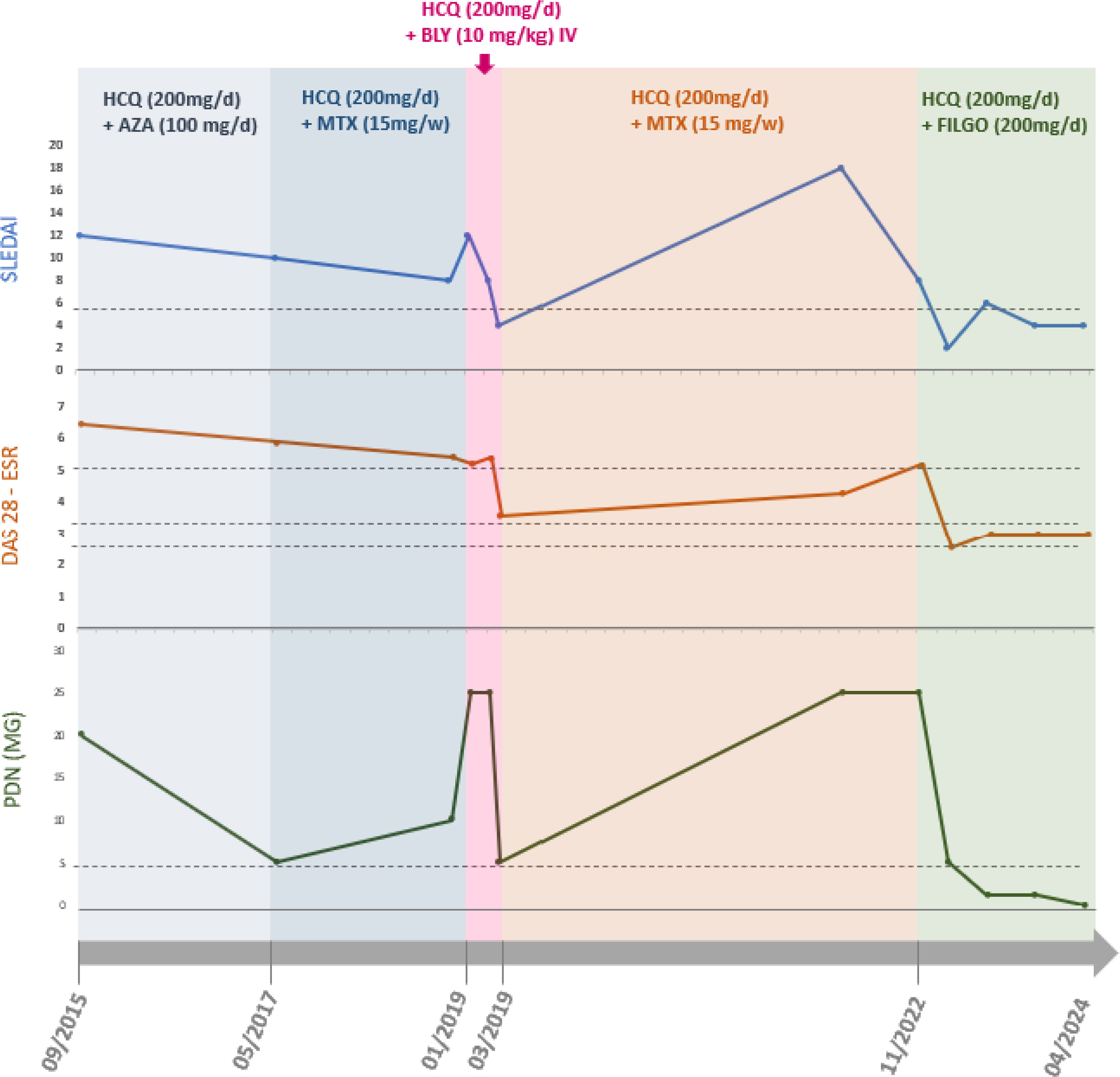
He had no joint pain or swelling, and his laboratory tests, including ESR, CRP, complements, 24-h proteinuria, anti-dsDNA, blood count, GOT, GPT, GammaGT were stably normal. He also reported no side effects from filgotinib nor reactivation of infective ulcer. A summary graphic representation of the clinical course, the dose of PDN and the therapies carried out is shown in Fig. 2. The examination of the patient was conducted in line with the declaration of Helsinki principles. The patient was diagnosed with SLE and RA overlap disease according to the American College of Rheumatology (ACR) classification criteria [1,2].
Graphic representation of clinical parameters collected at the visit times, as lupus disease activity (SLEDAI), arthritis disease activity with the erythrocyte sedimentation rate (DAS28 ESR), and dose of prednisone (PDN) in milligram/die; which shows the typical relapsing/remitting of SLE course. The different colours evidenced the various treatment regimens in the period. Hydroxychloroquine (HCQ) was administered at 200mg/die; azathioprine (AZA) at the dose of 100mg/die; methotrexate (MTX) at 15mg once a week; belimumab (BLY) 10mg/kilo as indicated in the technical data sheet; and filgotinib (FILGO) at 200mg/die. Although belimumab therapy was promptly effective (on clinimetric indexes and in reduction of steroid dose), it was discontinued due to severe septic arthritis episodes with hospitalisations. From March 2019 to November 2022 the patients underwent few outpatient check-ups due to several hospitalisations for sepsis and infected ulcers and the advent of the COVID-19 era, reporting autonomous management of the PDN dose (up to 50mg/die) based on joint pain.
SLE is a chronic autoimmune condition driven by a complex interplay of pro-inflammatory and anti-inflammatory cytokines.
The 2019 EULAR/ACR classification criteria for SLE mandate positive antinuclear antibodies (ANA) as an obligatory entry criterion. The diagnostic algorithm comprises additive weighted criteria distributed across two primary domains: seven clinical domains (constitutional, hematological, neuropsychiatric, mucocutaneous, serosal, musculoskeletal, and renal) and three immunological domains (antiphospholipid antibodies, complement proteins, and SLE-specific antibodies). Each criterion is assigned a weighted score ranging from 2 to 10 points. Patients are classified as having SLE when they accumulate a total score of ≥10 points [10].
Our patient had a malar rash, arthritis, ANA positivity, anti-dsDNA positivity, proteinuria and low C3 and C4 and arthritis. Although therapy with belimumab has a good infectious safety profile, in our patient we observed a severe joint infection probably due to the immunosuppression caused by the high doses of steroid that the patient self-administered for pain.
At initial disease presentation (2013), the patient exhibited a SLEDAI score of 12 and a DAS28 score of 5.7, demonstrating poor therapeutic response to hydroxychloroquine, high-dose prednisone, and azathioprine. In 2019, the initiation of belimumab therapy resulted in improved disease activity with SLEDAI decreasing to 8; however, the patient remained unable to reduce the prednisone dosage (25mg/day) due to persistent articular manifestations. Between January 2020 and September 2022, clinical deterioration was observed, characterized by frequent emergency department presentations for severe pain and self-initiated escalation of prednisone dosing (up to 50mg/day).
Following the initiation of filgotinib (October 2022), significant clinical improvement was observed. At the 3-month assessment, the SLEDAI score decreased to 2 and DAS28 to 3.3, accompanied by normalization of complement levels (C3: 129.8mg/dl; C4: 35mg/dl), reduction in anti-dsDNA antibodies, resolution of proteinuria, and successful tapering of prednisone to 5mg/day.
At 18-month follow-up, the patient maintained low disease activity (SLEDAI 2) and achieved complete prednisone discontinuation. Laboratory parameters, including ESR, CRP, complement levels, 24-h urinary protein, anti-dsDNA antibodies, complete blood count, and liver function tests, remained consistently within normal ranges. No adverse effects from filgotinib administration or recurrence of previous infectious ulcers were observed.
JAKs, intracellular enzymes crucial for cytokine signaling, play a pivotal role in this process. The JAK signal transducers and activators of transcription (STAT) pathways, comprising four JAK kinases and seven STAT family members, are central to immune regulation. Dysregulation of these pathways is implicated in the pathogenesis of SLE, highlighting the potential therapeutic significance of targeting specific signaling cascades. Ongoing phase II and III trials aim to address this uncertainty, with JAK inhibitors poised to emerge as a promising therapeutic avenue in the management of SLE [11]. The analysis of available data on PubMed highlights a limited number of studies regarding the use of filgotinib in the treatment of SLE compared to other JAK inhibitors such as baricitinib [12,13].
The limited availability of data concerning filgotinib may reflect its lesser utilization in clinical and research settings related to SLE, suggesting the need for further investigation to fully assess its therapeutic potential in this autoimmune condition. Filgotinib is a JAK inhibitor specifically engineered to achieve increased selectivity towards JAK1. This enzyme plays a crucial role in IFNAR signal transmission and intervenes in multiple relevant biological pathways in the context of SLE. In particular, JAK1 performs a modulation function for several important immunological mechanisms, including type II interferon signals and transmission pathways related to various interleukins, including IL-2, IL-4, IL-6, IL-10, and IL-15 [5,14]. By inhibiting interferon signaling, filgotinib may help modulate the dysregulated immune response characteristic of SLE, potentially leading to disease remission with a reduction of glucocorticoid (GC) dosage [14].
Ethical considerationsNo funding was received, and there are no conflicts of interest in the writing of this case report. Informed consent was obtained from the patients to receive the treatment or to participate in the described research. The research (or work) complies with the current regulations on bioethical research and has obtained the authorization of the institution's ethics committee (if the author deems that this step was not necessary, an explanation will be provided). If the patient can be recognized or identified through the images or data present in the article, the author will declare that they have the patient's informed consent for the publication of their data/images. If the author is certain that the patient's consent for the publication of the article is not necessary because their anonymity has been completely preserved, this must also be recorded (e.g., the authors declare that this article does not contain personal information that would allow the identification of the patient). The work does not involve animal experimentation.
ConclusionFilgotinib, a selective JAK1 inhibitor, demonstrated potential in managing rhupus syndrome by reducing inflammation, improving symptoms, and lowering glucocorticoid dependency. While this case highlights its therapeutic promise, further research and larger trials are needed to confirm its efficacy and safety in this rare overlap syndrome and maybe consider filgotinib for the treatment of SLE.
We would like to inform you that the case report has received approval from the institutional ethics committee, in accordance with current ethics and privacy for approval and peer review.
CRediT authorship contribution statementConceptualization: R.B., R.C.; methodology: C.R., A.C., F.P.C.; software: V.R.; validation: C.R., A.C. and F.P.C.; writing – original draft preparation: R.B.; writing – review and editing: R.B., C.R.; project administration: C.R. All authors have read and agreed to the published version of the manuscript.
FundingNone declared.
Conflict of interestNone declared.