Rhodococcus is a pathogen that is known to cause infections in animals and humans, mainly in cases of immunocompromised patients. A case of a pediatric cancer patient suffering from a bloodstream infection caused by Rhodococcus corynebacterioides was described in this work. Gram positive rods were isolated from blood cultures. The target bacterium was identified using a combination of biochemical tests, the MALDI-TOF mass spectrometry technique, and the analysis of the 16S rRNA sequence. Moreover, an antimicrobial susceptibility test was performed using the E-test. The isolated bacterium was identified as R. corynebacterioides. The 3-year-old patient was successfully treated with vancomycin and meropenem. This is the first published report of R. corynebacterioides in a pediatric patient diagnosed with retinoblastoma that developed a bloodstream infection. R. corynebacterioides should be considered among the opportunistic infectious agents affecting pediatric cancer patients.
Rhodococcus es un patógeno conocido por causar infecciones en animales y humanos, principalmente en pacientes inmunocomprometidos. En este trabajo se describe el caso de un paciente pediátrico con cáncer que presentó una infección del torrente sanguíneo causada por Rhodococcus corynebacterioides. A partir de hemocultivos, se aislaron bacilos gram positivos. La bacteria diana fue identificada usando una combinación de pruebas bioquímicas, por espectrometría de masas MALDI-TOF y por el análisis de la secuencia del gen 16S ARNr. Además, se realizó una prueba de sensibilidad a los antimicrobianos utilizando E-test. La cepa bacteriana se identificó como R. corynebacterioides. El paciente, de 3 años, fue tratado con vancomicina y meropenem, exitosamente. Este es el primer reporte de R. corynebacterioides como agente causal de una infección del torrente sanguíneo en un paciente pediátrico con retinoblastoma. R. corynebacterioides debe considerarse entre los agentes infecciosos oportunistas que afectan a los pacientes pediátricos con cáncer.
Rhodococcus spp. is a genus of gram positive, intracellular, aerobic, non-spore-forming bacteria, whose nonmotile and microscopic morphology in the gram stain can range from coccoid to bacillary. The genus Rhodococcus has had different changes and updates in its taxonomic history, generating reclassifications. Currently this microorganism belongs to the phylum Actinobacteria, subclass Actinobacteridae, order Actinomycetales, suborder Corynebacterineae, and family Nocardiaceae1,7,11.
Rhodococcus spp. is widely distributed in various sources of the environment and plays a role in a large variety of infections in humans and a wide diversity of animals (wild birds, cats, dogs, farm animals such as pigs and ruminants). Rhodococcusequi is the main and most described causative agent of rhodococcal infections in humans and animals4, being pneumonia, abscesses in the lungs and various extrapulmonary infections the most common infections in susceptible hosts11,14. In human, Rhodococcus spp. infections are associated with patients with acquired immune deficiency syndrome (AIDS), transplant recipients, diabetes mellitus, chronic kidney disease, cancer, and other states of immunodeficiency. Immunosuppressed patients may develop diseases such as pneumonia, lung abscesses, pleural malakoplakia, mediastinitis and bloodstream infection by Rhodococcus spp.2,11,13. Infections in immunocompetent patients are rare but have been reported, especially in children11.
Different species of Rhodococcus genus (other than R. equi) are considered pathogens, including Rhodococcus erythropolis, Rhodococcus rubber, Rhodococcus gordonia, Rhodococcus facsians, Rhodococcus ruber, Rhodococcus globerulus and recently, Rhodococcus hoagii2,6,11. However, there are few confirmed case reports of human infections caused by Rhodococcus corynebacterioides1,3,7,15.
The present work describes a case of a bloodstream infection caused by R. corynebacterioides in a pediatric patient diagnosed with retinoblastoma.
This case describes a 3-year-old male infant, who had previously been diagnosed with high-risk left eye retinoblastoma stage IV-b (International Retinoblastoma Staging System) at the Teleton Oncology Children's Hospital (HITO) in April 2017, which is a specialized care hospital in Querétaro City, México. The patient was in treatment with the COG ARET 0321 Protocol. He was attended to at the emergency room, due to fever, hyaline respiratory secretion, nasal congestion, productive cough, poor appetite and irritability. Eight days before, he had received the fourth chemotherapy induction cycle with vincristine, cyclophosphamide, cysplatine and etoposide. Physical examination showed an axillary temperature of 38.5°C, tachycardia (144 beats/min), normal blood pressure (104/63mmHg), hyperemic pharynx and abundant hyaline secretions in the upper respiratory tract. Pancytopenia was detected (hemoglobin 9g/dl, neutrophils 0cells/μl, lymphocytes 60cells/μl, platelets 80000cells/μl), reactive C protein (RCP) was recorded at 25mg/l. Samples of 3ml of blood were obtained from the central venous catheter and from a peripheral venous puncture and then placed into two individual blood culture bottles (BD Bactec® Peds plus/F), and processed in the Instrumented Blood Culture BD Bactec® FX Top system (BD Diagnostics, Sparks, MD) for five days (this first set of blood cultures remained negative by day 5). The diagnosis was fever and neutropenia (FN), and the initial antibiotic treatment was chosen in accordance with our institutional FN protocol with cefepime 150mg/kg/day every 8h and started. Chest X-rays were normal.
On day two the patient continued displaying two fever episodes (38–38.3°C), and, by the third day, the patient's fever rose to 39°C, with diarrhea and mucus, but no blood presence, tachycardia (142bpm) and delayed distal capillary refill (4s). Severe sepsis in the neutropenic patient was diagnosed. He received two loads of crystalloid solution, red blood cells and platelet transfusions. A new set of blood cultures was obtained from the central venous catheter and from a peripheral venous puncture. Antibiotic treatment was escalated in accordance with our institutional FN protocol (FN and sepsis or septic shock), to intravenous meropenem 60mg/kg/day every 8h in addition to intravenous vancomycin 40mg/kg/day every 6h. Cefepime was discontinued. Pancytopenia persisted and a drop in hemoglobin was detected (hemoglobin 6.4g/dl, neutrophils 0cells/μl, lymphocytes 20cells/μl, platelets 17000cells/μl), RCP increased to 61mg/l, stool cytology showed 3–5 leukocytes per field, mucin, neutral fats and non-pathogenic microbiota in the stool culture.
On the fourth day, bacterial growth was detected by the Bactec® FX Top system with a detection time of 43h 34min in the peripheral blood culture and 54h 7min in the central line blood culture. The aliquot of broth was taken for the gram stain smear and subculture. Gram positive rods in palisade arrangements and V forms were visualized after microscopic analysis. Blood culture broths were sub-cultured onto sheep blood agar (Columbia Agar with 5% Sheep Blood, bioMérieux, Marcy l’Etoile, Francia) and incubated at 35°C under aerobic conditions.
On the fifth day, the patient had a favorable clinical evolution, with fever remittance, improved food intake, and no respiratory symptoms or diarrhea. A pure culture of orange, non-hemolytic colonies was obtained in the laboratory. Gram stain, Ziehl–Neelsen staining was performed from the growth in the culture medium. Non-acid fast, gram positive rod-shaped bacilli were observed. Subsequent biochemical tests (citrate, catalase, oxidase, mobility, nitrate–nitrite reduction and urea test) and two automated microbial identification systems were used. The microorganism produced an alkaline reaction in the Simmons Citrate Agar, reacted positively to catalase and negatively to oxidase, nitrate-nitrite reduction and urea. The strain was initially tested with Vitek® 2 compact using the ANC ID card (bioMérieux, Sa., Marcy l’Etoile, France), identifying the bacterium as Turicella otitidis (probability level, 95%); however, the identification failed because this result did not match the colony morphology of the strain.
On the sixth day, a new set of blood cultures was taken, which tested negative (including from the central venous catheter).
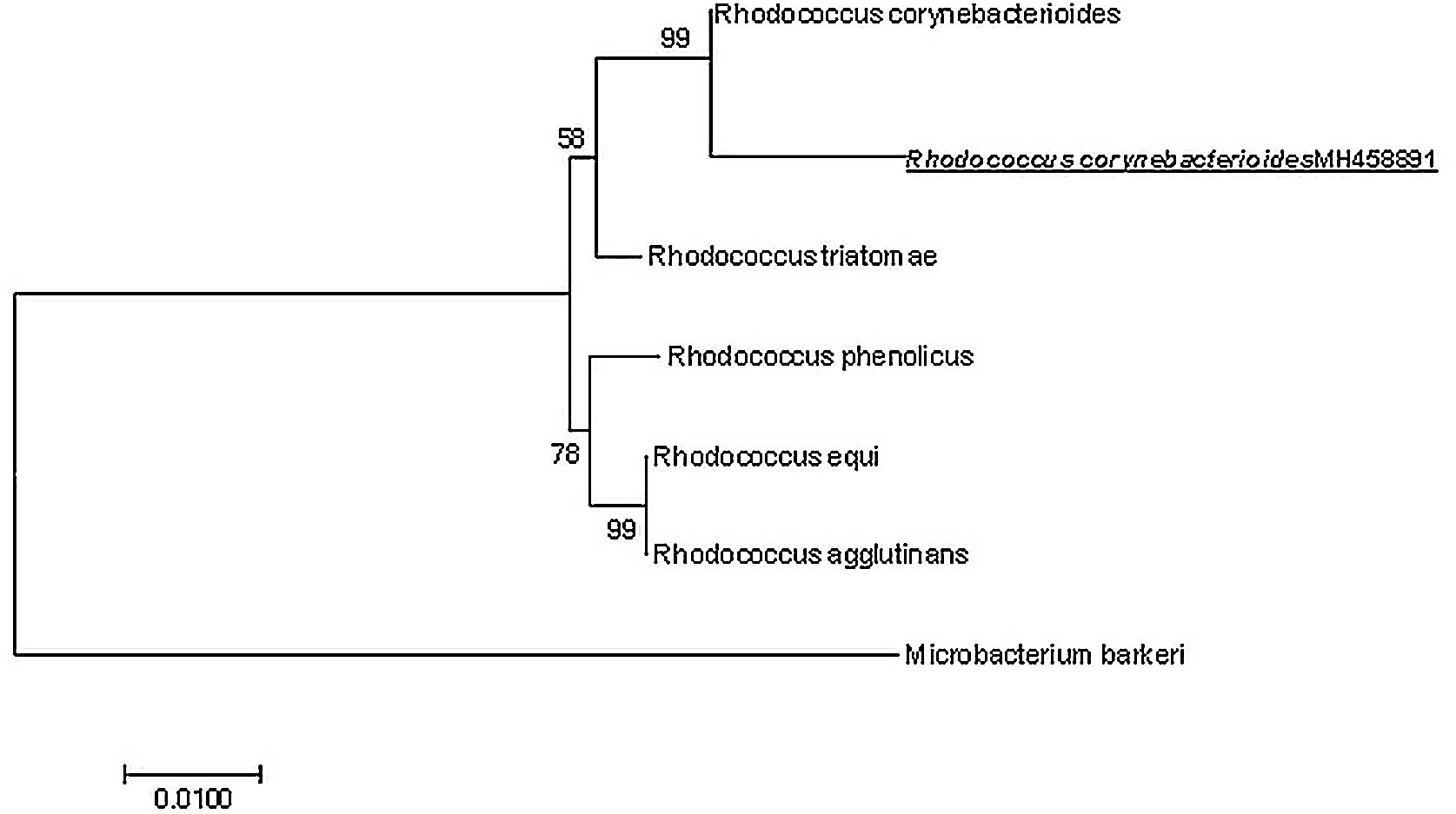
Subsequently on day 7, the microorganism was identified as genus Rhodococcus sp. using matrix-assisted laser desorption ionization–time of light MALDI-TOF (Biotyper: Bruker® Daltonics GmbH, Bremen, Germany) and spectra were compared using the Bruker BioTyper database (score value 1832); however, it failed to confidently identify the isolate to the species level. Further species identification was confirmed by the partial sequencing of the 16S ribosomal RNA (rRNA) gene. DNA was extracted using the method described by Morales-Estrada and co-workers12. A PCR assay of the 16S rRNA gene was performed in a Biorad thermocycler. The reaction contained 0.1μM of each primer 8F (5′-AGAGTTTGATCCTGGCTCAG-3′) and 1492r (5′-TACGGYTACCTTGTTACGACTT-3′) respectively.9 1U of Taq DNA polymerase (Promega), 1X colorless of GoTaq Flexi buffer, 0.5mM of Nucleotide mix, 2.5mM of MgCl2 and ∼50ng of chromosomal DNA. 20μl PCR reaction was subjected to the following thermal conditions: initial denaturation at 95°C for 5min, followed by 30 cycles consisting of a denaturation step at 95°C for 1min; primer annealing at 50°C for 1min and an extension step at 72°C for 1min. Lastly, a final extension at 72°C for 5min. The amplicons were purified using the ExoSAP-IT kit (Thermo Fisher Scientific) and the purified amplicons were sequenced. The sequence obtained was compared with the sequences available in the GenBank database (http://www.ncbi.nlm.nih.gov/blast). The most similar sequences were used for a subsequent phylogenetic analysis. The Clustal W program10 was used to align the sequences and phylogenetic distances were calculated by the Maximum Likelihood method conducted in MEGA78. This sequence was uploaded into the GenBank under accession number MH458891.1. The sequence obtained was compared using BLAST and the results demonstrated 99% similarity to the 16S rRNA gene sequence of R. corynebacterioides. In addition, the phylogenetic relationship of the obtained sequence against sequences from other members of the Rhodococcus genus is shown in Figure 1, including Microbacterium barkery as an outgroup.
Maximum likelihood phylogenetic tree showing the taxonomic affiliation of the strain isolated from the blood samples (accession number MH458891). The tree was constructed with 16S rDNA gene sequences. Numbers at nodes represent the percentages of occurrence of nodes in 1000 bootstrap trials. The 16S rDNA gene from Microbacterium barkeri strain served as outgroup.
The antimicrobial susceptibility testing was determined by the epsilometer test (E test®) (Liofilchem, Inc., Waltham, MA). MICs were read after incubation (48h of incubation at 35±2°C in ambient air). On the eighth day, results were interpreted according to the breakpoints provided by the Clinical and Laboratory Standards Institute (CLSI) guidelines (M24-A2, 2nd edition, 2011). The isolated bacterium was susceptible to all beta-lactam antimicrobials (except benzylpenicillin, which was previously documented in vitro resistance), levofloxacin and vancomycin (Table 1). With this result, therapy with meropenem was discontinued as from this day, and the patient completed treatment with vancomycin for 10 days.
R. corynebacterioides, like other species of the genus Rhodococcus, is a poorly understood pathogen and not a common cause of infection; to date only approximately six cases have been reported, including the present case1,3,6,7,15.
It was previously established that Rhodococcus spp. bacteremia in immunocompromised patients was secondary to pneumonia or associated with concomitant cavitary lung infection1,15, and it has even been documented that these bloodstream infections entailed a high mortality rate, for which reason prolonged antimicrobial therapy was recommended2,4,13. Recent case reports of bacteremia by Rhodococcus spp. described that bloodstream infection by this microorganism is not always accompanied by respiratory symptoms and lung involvement1–4. In this case, the initial symptoms exhibited by this child involved upper airway and gastrointestinal compromise.
Although these episodes of bacteremia due to R. equi were precedently related to animals4,11,14, there are currently several reports of bacteremia due to Rhodococcus spp. where it is established that such exposure is not always present2,3,6,7,13. In the present case, the patient's family denied contact with animals. The source of this bacteremia was primary. However, we do not know how the infection was acquired, we only recorded the symptoms at the time the patient was admitted to hospital; thus, this case cannot be considered a healthcare-associated infection.
Most bacteremia episodes in cancer patients by Rhodococcus spp. are related to central venous catheters. In a study of seventeen cancer patients with bacteremia by Rhodococcus, the majority of them (94%, 16 patients) had central venous catheter-related bloodstream infection1, which is due to its ability to form biofilms on inert surfaces1,3,4,13,15. Unlike in previous reported cases, the patient did not have central venous catheter-related bacteremia because of the following reasons: the patient's initial symptoms were present on the date the patient was admitted to hospital, the bacterium in blood cultures was isolated with a differential time positivity greater than 2h (peripheral blood culture first) and, the bacterium was not present in blood cultures after the treatment.
In clinical microbiology laboratories, the identification of gram positive bacilli by conventional phenotypic methods is difficult, in addition to the fact that the performance of these manual methods is not always sufficient to identify microorganisms, even using automated identification equipment5. We encountered these difficulties because the Vitek® 2 compact automated equipment had wrongly identified the microorganism. This misidentification has previously been reported for this microorganism7. The MALDI-TOF system has become a widely used technique in recent years for the rapid identification of aerobic gram positive rods. In this work, the isolated strain was identified at a genus level using the MALDI-TOF system. This result was expected due to the current limitations of the system database; only 80% of Rhodococcus species are correctly identified to species level (score values, 2.0) by the Bruker® Biotyper system5. To obtain a reliable identification of the microorganism the 16S rRNA gene sequencing analysis was done. Therefore, through the bioinformatic analysis of the 16S rRNA sequence it was possible to identify R. corynebacterioides as the causative agent of the blood stream infection in the pediatric patient. Different reports have used this gene to identify some Rhodococcus species11. However, the analysis of the 16S rRNA sequence of an isolated gram positive rod from a clinical case of bacteremia and oligoarthritis was not enough to discriminate between R. corynebacterioides and Rhodococcus kroppenstedtii species6. Bacteremia caused by Rhodococcus spp. is underreported and probably misdiagnosed because of a conjunction of factors that include the difficulty of identifying genus and species by traditional techniques2, and because it can also be mistaken for a contaminant4,13,14. The aforementioned reaffirms the concept that gram positive bacilli in blood cultures should not always be ruled out as contaminating diphtheroids.
Due to the rarity of bacteremia caused by Rhodococcus spp. there is a lack of clinical experience, and the optimal antibiotic therapy is still undetermined. Different authors suggest combining at least two antibacterial agents to treat infection by Rhodococcus spp. because there is evidence that monotherapy may lead to the emergence of antimicrobial resistance6,11, especially in systemic infections15 and the management of central venous-catheter related bacteremia4. Initial treatment with cefepime was not clinically effective since the patient continued with fever, tachycardia, diarrhea, retarded capillary refill and sepsis. When the treatment was escalated to meropenem and vancomycin, we observed hemodynamic stability and remission of all the patient's symptoms. The negative blood cultures (obtained at the end of the treatment with vancomycin and meropenem) proved the success of the treatment with these two antibiotics. We also attributed the therapeutic success to the fact that the bacteremia was not related to the central venous-catheter, there was an absence of damage to a specific organ and because of the antibiotic susceptibility of the isolated bacterium. We found that the MICs for most of the antibiotics tested (except for vancomycin) were higher than those reported by Kitamura et al. and Eri Fukao et al. In the case of vancomycin, the MIC we found was similar to the one reported by Kitamura et al., but higher than that reported by Eri Fukao et al., even though the different MIC values for vancomycin all fell within the intervals to be considered susceptible. While the MIC for benzylpenicillin was considered resistant, other species of Rhodococcus spp., including R. equi, have been reported resistant to this antibiotic1,4,6,15. We attribute this variation in MICs of the different antibiotics to the existence of resistance mechanisms described for the different species of the genus Rhodococcus spp. and to the previously described variation of resistance patterns by geographic regions11.
In the present clinical report, a case of R. corynebacteriodes bacteremia in a pediatric cancer patient was described. Publishing clinical cases caused by R. corynebacterioides, including microbiological identification techniques, clinical picture and treatment can greatly contribute to better understanding the clinical management and diagnosis of the infections caused by this microorganism.
FundingThis case report has not received any specific grants from public sector, commercial, or non-profit agencies.
Conflict of interestThe authors declare that they have no conflicts of interest.
We thank the Fundación Teletón Vida IAP for all the support provided for the publication of this article.