Listeria monocytogenes is a foodborne pathogen. The recent alert for L. monocytogenes in vegetables from Argentina warns about the importance of reinforcing its isolation, characterization and subtyping in food, clinical and environmental samples. The aim of the present study was to compare the discriminatory power of enterobacterial repetitive intergenic consensus polymerase chain reaction (ERIC-PCR), automated ribotyping and pulsed-field gel electrophoresis (PFGE) to subtype strains of L. monocytogenes isolated from Argentine meat and environmental samples. Simpson's Diversity Index (DI) was calculated on the basis of based on the dendrograms obtained in the by cluster analysis, showing the following discriminatory power: ApaI-PFGE (0.980), AscI-PFGE (0.966), ribotyping (0.912), ERIC-PCR (0.886). The ID values between ApaI- and AscI-PFGE and between ribotyping and ERIC-PCR were not significantly different. Of the three techniques evaluated, PFGE showed the highest discriminatory power. However, the subtyping techniques should be accompanied by effective food monitoring strategies and reliable clinical and epidemiological studies.
Listeria monocytogenes es un patógeno alimentario. La reciente alerta por la presencia de L. monocytogenes en vegetales en Argentina advierte sobre la importancia de reforzar el aislamiento, la caracterización y la subtipificación de esta bacteria en muestras clínicas de alimentos y ambientales. El objetivo del presente estudio fue comparar el poder discriminatorio de enterobacterial repetitive intergenic consensus polymerase chain reaction (ERIC-PCR), la ribotipificación automatizada y la pulsed-field gel electrophoresis (PFGE) para subtipificar cepas de L. monocytogenes aisladas de carne y de muestras ambientales en Argentina. El índice de diversidad (ID) de Simpson, calculado a partir de los dendrogramas obtenidos en el análisis de agrupamiento, mostró los siguientes resultados: ApaI-PFGE (0,980), AscI-PFGE (0,966), ribotipado (0,912), ERIC-PCR (0,886). Los valores obtenidos no fueron significativamente diferentes al comparar entre ApaI- y AscI-PFGE, ni entre ribotipado y ERIC-PCR. De las técnicas evaluadas, la PFGE presentó el mayor poder discriminatorio. Sin embargo, las técnicas de subtipificación deberían acompañarse de estrategias de control de los alimentos efectivas y de estudios clínicos y epidemiológicos confiables.
Listeria monocytogenes is a foodborne pathogen that has wide distribution in the environment and high prevalence in a broad range of foodstuff. Infection with L. monocytogenes can cause from mild, febrile illness to systemic illness in susceptible populations, accompanied by more severe symptoms such as meningitis, meningoencephalitis, septicemia, and abortion as well as high hospitalization and case fatality rates. The cause of illness has been traditionally associated with the intake of contaminated ready-to-eat food, although recent outbreaks have recognized many other transmission vehicles such as crustaceans, shellfish, mollusks and products thereof, dairy products, meat and meat products, vegetables and products thereof, fruits and juices2.
In Argentina, no outbreaks of L. monocytogenes associated with contaminated food have been reported so far. A total of 310 cases of listeriosis have been registered by the Argentinean National Institute of Infectious Diseases of the National Administration of Laboratories and Institutes of Health “Dr. Carlos Malbrán” (INEI-ANLIS, for its Spanish acronym) from 1985 to the present, all of them corresponding to invasive disease, mainly sepsis and meningitis. These data suggest that the disease is underreported and its prevalence and real impact on public health is unknown13.
In the global context, a massive outbreak of invasive L. monocytogenes linked to frozen vegetables has been affecting Austria, Denmark, Finland, Sweden and the United Kingdom since 2015, with 47 reported cases including nine deaths as of June 15, 20186. In Argentina, as a result of the warning issued by the European Rapid Alert System for Food and Feed, the Argentinean National Administration of Medicines, Food and Medical Technology (ANMAT, for its Spanish acronym) ordered the preventive withdrawal of 128 batches of frozen products based on corn and other vegetables made with raw material presumably contaminated with L. monocytogenes1.
This situation warns about the importance of reinforcing the isolation, characterization and subtyping of L. monocytogenes from food, clinical and environmental samples. In this sense, molecular typing is fundamental for the epidemiological tracing of isolates, the cross-linked surveillance of isolates in human beings and food, the identification of sources of infections and the performance of outbreak controls.
Pulsed-field gel electrophoresis (PFGE) is considered the gold standard typing method in epidemiological studies of pathogenic organisms and, as such, it is used by the INEI-ANLIS. Ribotyping is a completely automated method based on restriction fragment length polymorphisms associated with ribosomal operons. Enterobacterial repetitive intergenic consensus polymerase chain reaction (ERIC-PCR) is an alternative typing method based on 124–127 base-long elements consisting of highly conserved central inverted repeat sequences found in the extragenic regions of the bacterial genome.
In this study, subtyping of L. monocytogenes by ERIC-PCR, automated ribotyping and PFGE was evaluated in 27 strains isolated from Argentinean meat and environmental samples belonging to the collection of the Institute of Veterinary Genetics, School of Veterinary Sciences, National University of La Plata.
For ERIC-PCR, a single colony of pure L. monocytogenes was inoculated into 5ml Brain Heart Broth (Biokar Diagnostics, France) and cultured at 37°C for 24h. Genomic DNA was extracted using the Wizard Genomic DNA Purification kit (Promega, WI, USA). Primers ERIC 1R (5′-ATGTAAGCTCCTGGGGATTCAC-3′) and ERIC 2 (5′-AAGTAAGTGACTGGGGTGAGCG-3′)14 were used. Amplification reactions were performed in 25μl of a solution containing 1 x Taq buffer with (NH4)2SO4 (Thermo Scientific, MA, USA), 1μM of each primer (Embiotec SRL, Argentina), 0.4mM of each deoxynucleoside triphosphate (PB-L, Argentina), 6mM MgCl2 (Thermo Scientific, MA, USA), 1.25U DNA polymerase (Thermo Scientific, MA, USA) and 2μl DNA extract. Amplifications were performed in a DNA thermal cycler (Life Express, Bioer, China) with the following temperature profiles: 1 cycle at 95°C for 5min; 35 cycles at 94°C for 30s, at 40°C for 3min, and at 72°C for 2min; and 1 cycle at 72°C for 7min. Finally, PCR products were separated by electrophoresis in 2% agarose (Genebiotech, South Korea) in 1× TBE running buffer at 80V for 3h. Molecular weight marker 1Kb Plus DNA Ladder (Thermo Scientific, MA, USA) was used as size standard.
EcoRI-Ribotyping was performed on L. monocytogenes isolates grown on Nutritive Agar (Biokar Diagnostics, France) for 24h at 37°C in an automated ribotyping system (RiboPrinter®, Qualicon DuPont™) following the manufacturer's instructions.
For PFGE analysis, the one-day standardized laboratory protocol for molecular subtyping of L. monocytogenes was employed4. Restriction digestion of DNA in agarose plugs was carried out with ApaI or AscI (Thermo Scientific, MA, USA) during 18h. MaestroGen slider imager (Maestrogen Inc., Nevada, USA) was used to obtain PFGE images of gels.
Tagged file format (TIFF) images obtained by ERIC-PCR, ribotyping and ApaI- and AscI-PFGE were analyzed with BioNumerics version 6.6 software package (Applied Maths, Sint-Martens-Latem, Belgium) using the Dice coefficient and the unweighted pair-group method with arithmetic mean (UPGMA) to generate dendrograms with 1.5% band matching tolerance. Two or more isolates with identical band pattern (100% similarity) were grouped into a cluster.
The three typing methods were compared using the Simpson's Diversity Index (DI) and the corresponding confidence intervals (CIs)11. Statistical differences among DI values were analyzed by calculating p-values according to the jackknife pseudo-values resampling method. The Comparing Partitions tool was used to calculate DI and for the statistical analysis (http://www.comparingpartitions.info/?link=Tool).
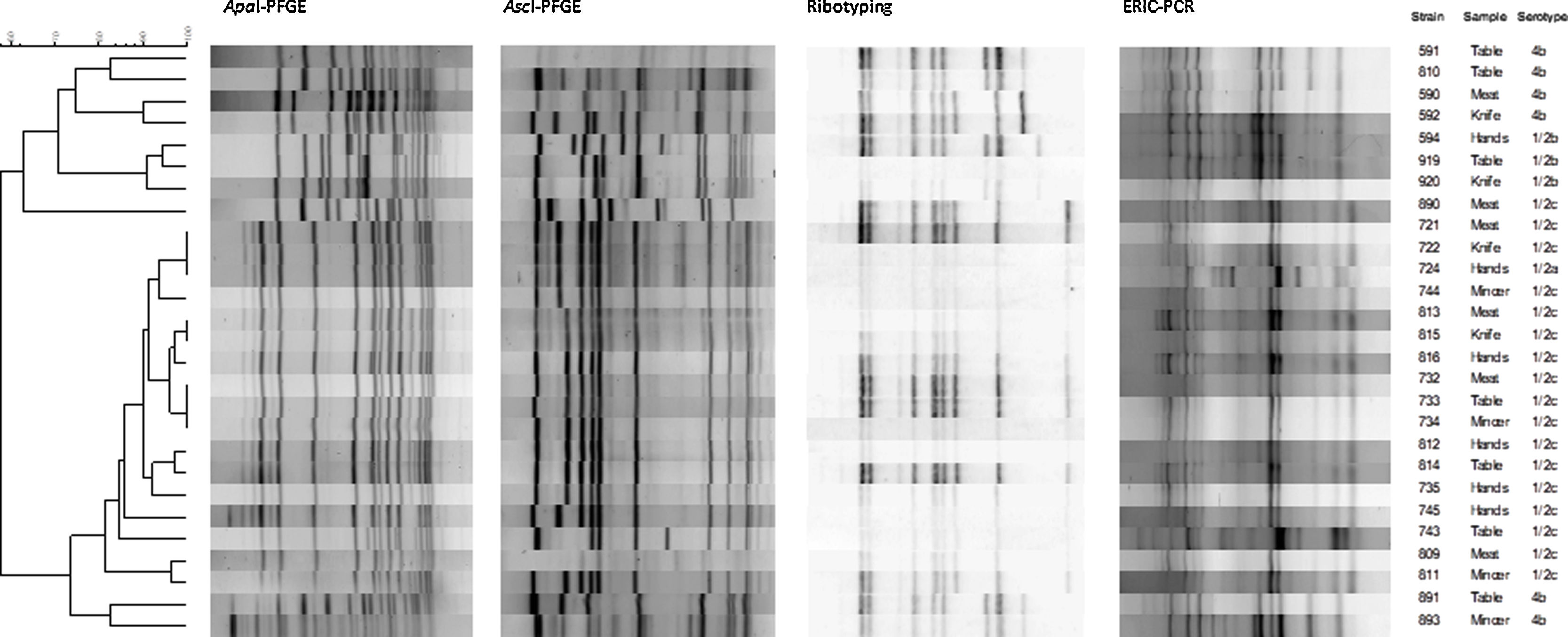
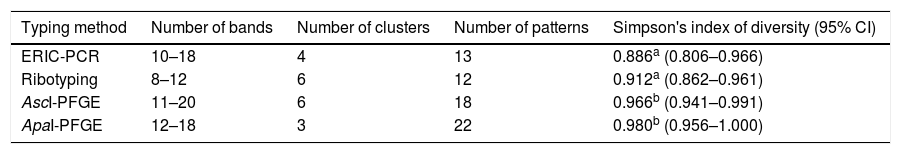
All L. monocytogenes strains analyzed (n=27) were typeable by ERIC-PCR, ribotyping and AscI/ApaI-PFGE (Fig. 1). Cluster analysis results are shown in Table 1. The number of bands was greater and the band pattern was more defined in the gels obtained by AscI- and ApaI-PFGE than in those obtained by ERIC-PCR and ribotyping. Accordingly, DI showed the following discriminatory power: ApaI-PFGE, AscI-PFGE, ribotyping and ERIC-PCR. DI values were neither significantly different between ApaI- and AscI-PFGE nor between ribotyping and ERIC-PCR (Table 1).
Results of cluster analysis for the different typing methods analyzed
| Typing method | Number of bands | Number of clusters | Number of patterns | Simpson's index of diversity (95% CI) |
|---|---|---|---|---|
| ERIC-PCR | 10–18 | 4 | 13 | 0.886a (0.806–0.966) |
| Ribotyping | 8–12 | 6 | 12 | 0.912a (0.862–0.961) |
| AscI-PFGE | 11–20 | 6 | 18 | 0.966b (0.941–0.991) |
| ApaI-PFGE | 12–18 | 3 | 22 | 0.980b (0.956–1.000) |
Numbers without common superscripts (a–b) differed significantly (p<0.05).
The number of unique profiles obtained with ApaI was greater than with AscI-PFGE, and only three clusters resulted from the cluster analysis. Strains with equal ApaI profile also presented the same profile with AscI, with the exception of strains 732, 733 and 734. Therefore, a greater discriminatory power was obtained by combining the results of AscI- and ApaI-PFGE. Clusters formed by strains 721, 722 and 724 and strains 813 and 815 were found to be clonal by the combined ApaI and AscI analysis. However, different profiles were obtained for these strains by ribotyping and ERIC-PCR, which could be explained by the presence of faint and diffuse bands in ERIC-PCR and ribotyping profiles, which interfered with complicated band assignment and result interpretation. ERIC-PCR patterns were the most difficult to interpret, as already reported by Harvey9.
In PFGE typing, DIs of AscI- and ApaI-PFGE (0.966 and 0.980, respectively) were similar to those described by Henri et al.10, who reported a DI of 0.992 in 687 strains isolated from meat and meat products and 0.987 in 209 strains isolated from the food processing environment.
Our results agree with those reported by Fugett et al.7, who found that the discriminatory power determined by the Simpson's index did not differ between ApaI and AscI (DI 0.992 each) in 495 L. monocytogenes isolates, while the combined analysis of both enzymes was more discriminatory than EcoRI ribotyping (0.995 vs. 0.950, respectively). Grif et al.8 also found a higher discriminatory power of PFGE than of automated ribotyping. However, some PFGE types were differentiated by ribotyping, suggesting that the combination of data obtained by both methods increased the likelihood of strain discrimination. Other authors found that DIs of automated ribotyping and ApaI-PFGE did not differ significantly5,12.
Since L. monocytogenes serotype 4b is responsible for most hospitalization rates and deaths3, its sensitive discrimination is particularly crucial for surveillance and monitoring of human listeriosis cases. Previous studies showed the highly clonal nature of L. monocytogenes 4b strains8. In the present study, the four serotype 4b isolates exhibited unique ApaI-PFGE patterns, although they presented similar profiles clustering in nearby branches by the other typing methods.
At present, the availability of molecular techniques for L. monocytogenes subtyping is essential to reinforce the development of epidemiological outbreak studies as well as to identify and trace strains in food, human and environmental samples isolated from different countries.
In this study, ERIC-PCR showed the lowest DI, suggesting the need to optimize the method to obtain accurate results. However, since it is a simple and economic technique that can be carried out in low-complexity laboratories, ERIC-PCR could be used as a first subtyping screening. On the other hand, ribotyping had higher DI than ERIC-PCR; despite being automatic, rapid and less laborious than PFGE, ribotyping is expensive and requires specific equipment. Finally, the high discriminatory power obtained by two-enzyme PFGE makes it a good technique for epidemiological outbreak studies. This method also produced stable and reproducible band patterns and showed high concordance with epidemiological data4. However, it is time-consuming, expensive, laborious and requires specific equipment4. In Argentina, its implementation is restricted to high-complexity research laboratories or centers. While new alternatives for foodborne disease surveillance such as whole genome sequencing (WGS) are gaining importance, the epidemiological surveillance of listeriosis should be reinforced in Argentina, since subtyping techniques alone are not sufficient and should be accompanied by effective food monitoring strategies and reliable clinical and epidemiological studies.
Conflict of interestThe authors declare that they have no conflicts of interest.
The authors thank A. Di Maggio for manuscript correction and editing.