Infections of the central nervous system caused by atypical bacteria are becoming more common. Borrelia burgdorferi and Rickettsia conorii are microorganisms transmitted by ticks; infection with these bacteria result in a wide spectrum of manifestations on imaging. In areas where these tick-borne microorganisms are endemic, including Spain, these infections must be included in the differential diagnosis of patients with a variety of systemic and neurologic symptoms. The clinical presentation of these infections is nonspecific, and CT is normally the initial imaging technique, although MRI is more sensitive to early changes. On MRI, these infections can manifest as small lesions in the deep supratentorial white matter that are hyperintense on T2-weighted/FLAIR sequences. It is fundamental to know the imaging characteristics of the different atypical bacterial infections and their differential diagnoses. Good history taking combined with complementary tests (blood tests and CSF analysis) and the neuroimaging findings can help reach the right diagnosis and enable appropriate treatment, thereby preventing possible neurological sequelae.
Las infecciones del sistema nervioso central causadas por bacterias atípicas se encuentran en aumento. Borrelia burgdorferi y Rickettsia conorii son microorganismos transmitidos por garrapatas cuyas infecciones presentan un amplio abanico de manifestaciones por imagen. Deben considerarse entre los diagnósticos diferenciales ante la presencia de síntomas sistémicos y neurológicos variados en un medio endémico como es España. La presentación clínica de estas infecciones es inespecífica, siendo la tomografía computarizada la técnica de imagen inicial. Sin embargo, la resonancia magnética es más sensible evaluando cambios precoces. Estas infecciones pueden presentarse en la resonancia magnética como pequeñas lesiones hiperintensas en secuencias T2/FLAIR en la sustancia blanca supratentorial profunda. Es fundamental conocer las características radiológicas de las distintas infecciones bacterianas atípicas y sus diagnósticos diferenciales. Una buena historia clínica, combinada con pruebas complementarias (análisis de líquido cefalorraquídeo y serología), y los hallazgos de la neuroimagen ayudan a llegar a un diagnóstico y tratamiento certeros, evitando posibles secuelas neurológicas.
Tick-borne infections are uncommon in Spain but their diagnosis is very important, because if they are not treated, symptoms may last months or even years. An added difficulty is that, in more than a few cases, the patient does not recall having been bitten by a tick. Therefore, a good medical history and a suitable understanding of context are necessary.
Central nervous system (CNS) signs are common but radiologically can be quite non-specific and varied, making their diagnosis even more difficult. For this reason, radiologists must be familiar with this disease and able to recognise it in order to diagnose these infections and start treatment as early as possible.
Borrelia burgdorferiLyme disease is caused by Borrelia burgdorferi, a Gram-negative spirochaete. It is transmitted to humans through the bites of ticks belonging to the genus Ixodes. The incidence of such bites reaches its peak in summertime. Its animal reservoirs are usually small mammals such as rats, mice and squirrels, although deer can also be affected. It is the most common tick-borne disease in the temperate zones of Europe and North America.1
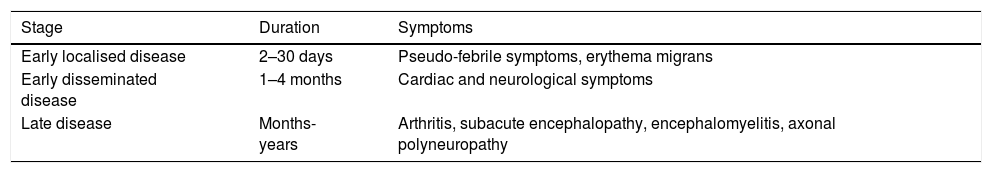
Symptoms generally begin with a characteristic expansive skin lesion, called erythema migrans, which ultimately resolves, even without treatment.2 Classically, Lyme disease is divided into three stages (Table 1).
Clinical stages of Lyme disease.
| Stage | Duration | Symptoms |
|---|---|---|
| Early localised disease | 2–30 days | Pseudo-febrile symptoms, erythema migrans |
| Early disseminated disease | 1–4 months | Cardiac and neurological symptoms |
| Late disease | Months-years | Arthritis, subacute encephalopathy, encephalomyelitis, axonal polyneuropathy |
Neurological signs occur in approximately 1 in 10 patients.3 The characteristic triad of neurological symptoms includes meningitis, neuritis and radiculoneuritis.
The diagnostic method of choice consists of indirectly demonstrating infection using serology techniques. However, examination of clinical presentation, laboratory test results and imaging findings usually constitute the initial approach to this type of patient.
For early-stage neuroborreliosis, the most common treatment is intravenous ceftriaxone at a dose of 2 g daily for two weeks; for late-stage neuroborreliosis, it is intravenous ceftriaxone at a dose of 2 g daily for three weeks. Oral doxycycline is as effective and safe as intravenous ceftriaxone.4 Antibiotic therapy accelerates the eradication of the pathogen, improves the healing process and prevents the development of late neuroborreliosis. Response to antibiotic therapy may be slow and incomplete, especially in late neuroborreliosis.1
Neuroimaging findings are rare, even in patients with known Lyme disease and neurological signs. Neuroborreliosis has a wide range of signs on imaging, including diffuse involvement; meningeal, parenchymal and nerve-root enhancement; vascular involvement with a presentation similar to a cerebrovascular accident; and bleeding. From a clinical and neuroimaging perspective, it can also simulate multiple sclerosis.5
Neuroborreliosis can affect both the peripheral and central nervous systems.
Peripheral nervous systemPeripheral nervous system involvement may be in the form of radiculoneuritis or cranial nerve involvement.
RadiculoneuritisThe most typical sign of neuroborreliosis or Lyme disease is Bannwarth syndrome with radiculoneuritis, which causes radicular pain and sometimes paresis of the limbs or abdominal wall.6
Moreover, plexus neuritis or mononeuritis multiplex is seen in 5%–10% of cases of neuroborreliosis.7 This can present as chronic asymmetric neuropathy, and these cases are generally not associated with meningitis or intrathecal antibody production.8 Neuritis due to neuroborreliosis can yield non-specific findings, such as a signal intensity increase in the nerves on long repetition time (RT) sequences, e.g. the short tau inversion recovery (STIR) sequence, sensitive to the detection of oedema.5
Cranial nerve involvementApproximately 80% of cranial nerve involvement in neuroborreliosis is located in the facial nerves, and it is bilateral in 25% of cases.9,10 A study by Ogrinc et al. in 77 patients with early neuroborreliosis found that 36% had facial paralysis.11 In neuroborreliosis, facial paralysis is the cause of hospitalisation in 50% of cases.12 Therefore, diffuse cranial nerve enhancement is a common finding on magnetic resonance imaging (MRI) (Fig. 1), as is marked enhancement in the geniculate ganglion and the tympanic and mastoid facial nerve segments. Enhancement is not usually seen in the cisternal, intracanalicular, labyrinthine or parotid segments.13
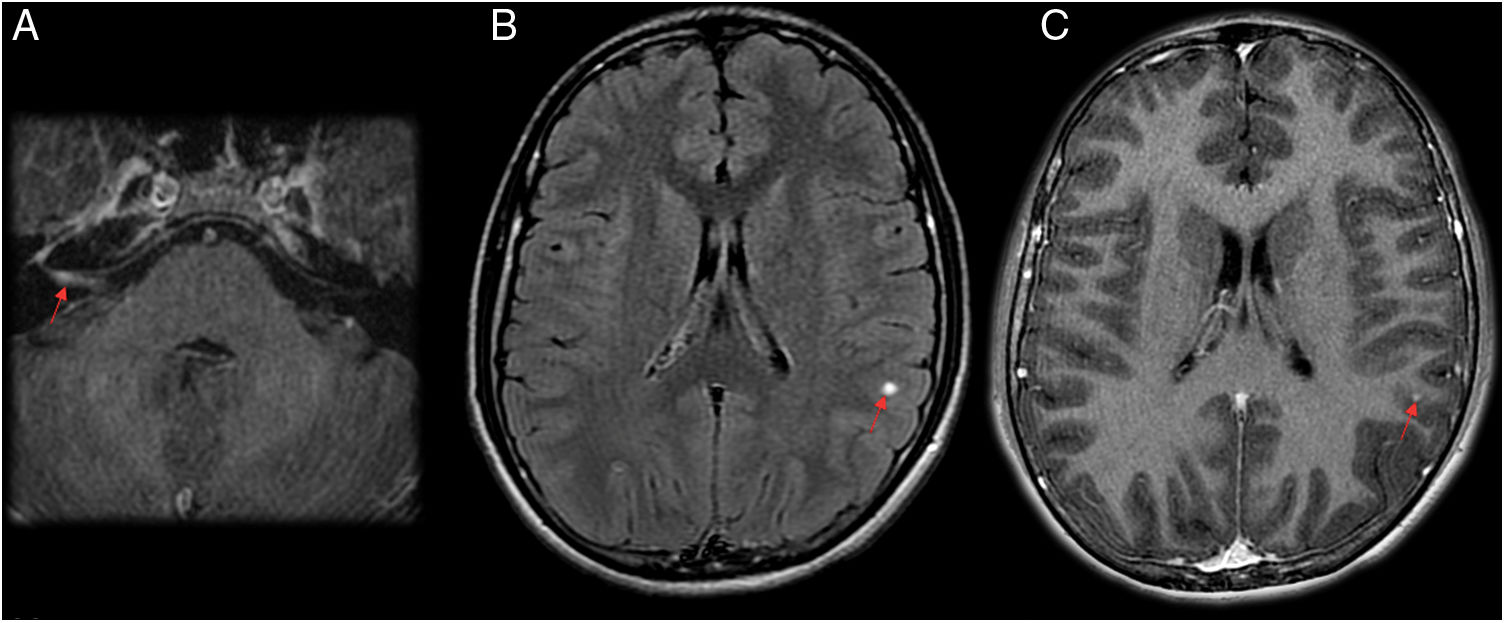
A 15-year-old patient who had fever for a month. Right peripheral facial paralysis, headache, dizziness and a sensation of stiffness in the neck and back subsequently developed. The patient was diagnosed with facial paralysis secondary to neuroborreliosis. (A and C) Axial T1-weighted imaging after intravenous contrast administration. (B) Axial imaging on a FLAIR sequence. Magnetic resonance imaging showed greater uptake by the right facial nerve in the internal auditory canal and intrapetrous course on a T1-weighted sequence with contrast (A). Round subcortical millimetric punctiform focus in the left parietal lobe, hyperintense on FLAIR (B) and exhibiting contrast uptake on T1 (C).
Lyme disease may present as meningitis, encephalomyelitis, encephalopathy, vasculitis or post-Lyme or chronic Lyme syndrome, or with evidence of intracranial hypertension.
MeningitisClinical signs of meningitis are seen in up to 29% of cases of neuroborreliosis.11,12 These cases may exhibit enhancement of the meninges, cranial nerves and spinal nerves. In a study by Agarwal et al., with a series of 63 patients, three showed leptomeningeal enhancement on MRI after intravenous contrast administration.14
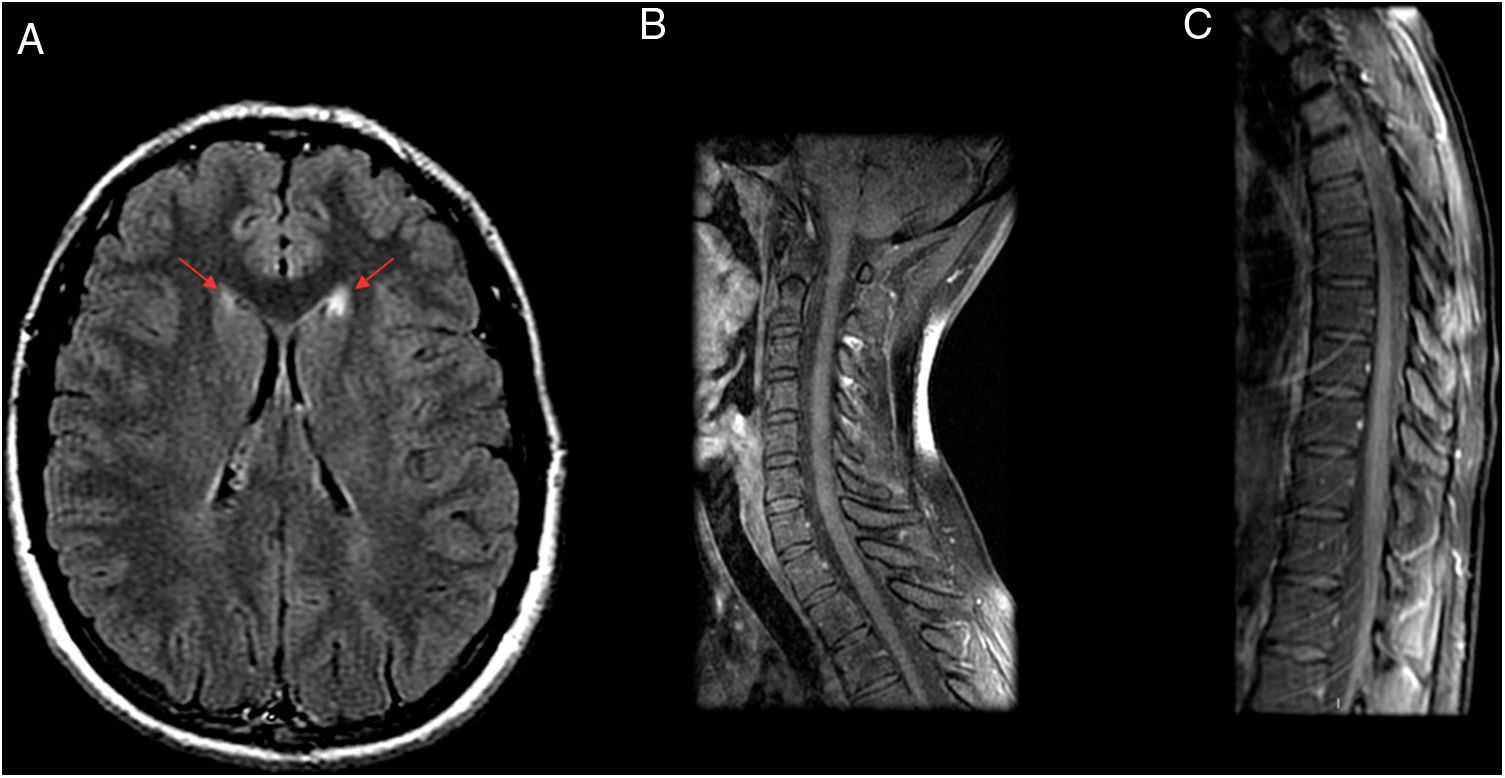
MyelitisMyelitis accounts for 7% of signs of neuroborreliosis leading to hospitalisation.12 A study by Lindland et al. reported 11 cases of myelitis due to neuroborreliosis on MRI in adults. The lesions affected the cervical spine and had a longitudinal and central distribution along the spinal cord (Fig. 2). After contrast administration, some did not enhance, while others showed nodular or diffuse enhancement.5 There are reports in the literature of lumbosacral myelitis with normal MRI findings.5,15
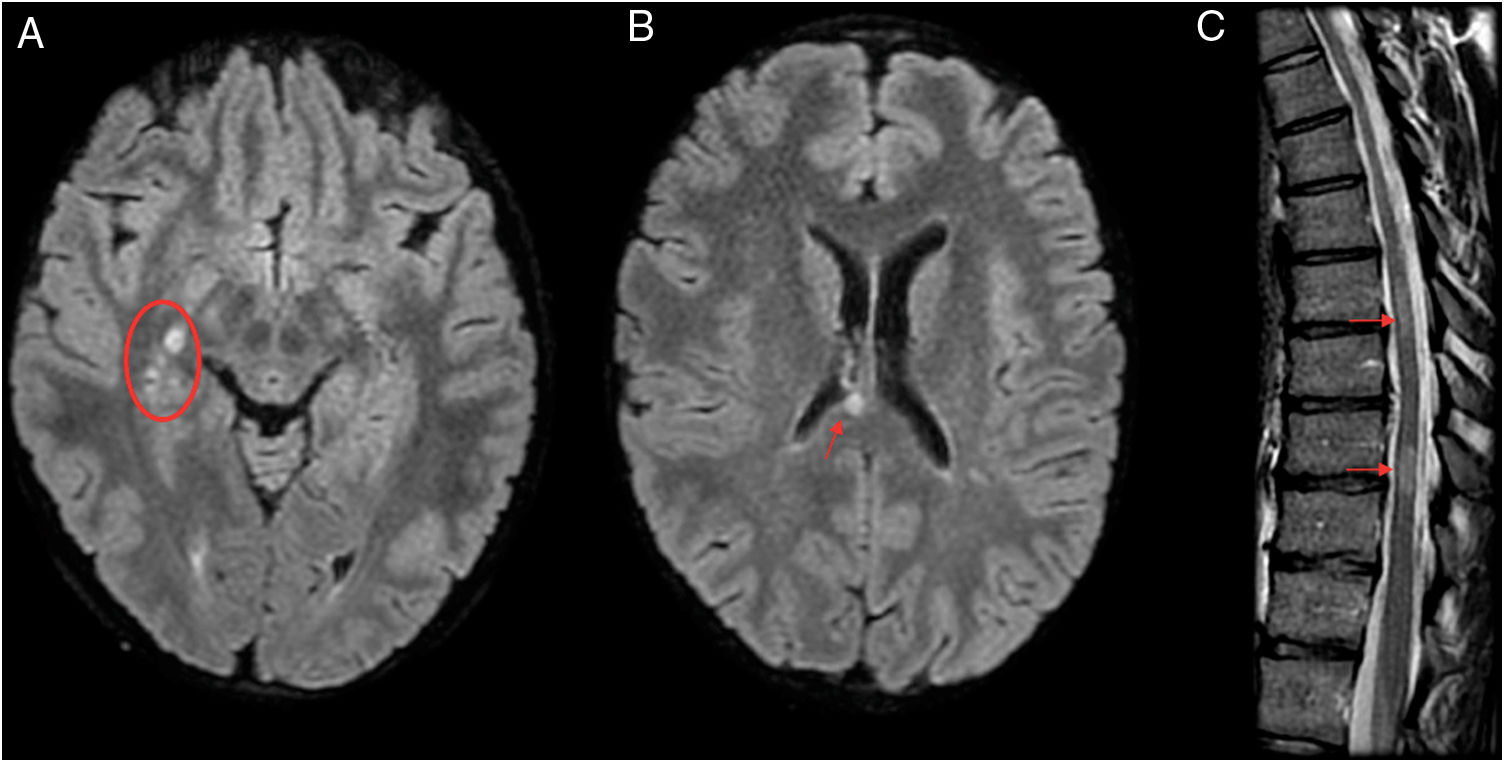
A 29-year-old patient admitted to neurology with hyperreflexia and lower limb paraesthesia was initially diagnosed with relapsing–remitting multiple sclerosis with partial response to treatment. However, laboratory testing revealed persistent positivity for IgM; hence, a decision was made to administer antibiotic therapy (doxycycline), after which the patient’s symptoms completely subsided in a month. Four years later, the patient has not experienced any recurrent symptoms or presented any new lesions on magnetic resonance imaging (MRI). MRI prior to antibiotic therapy. (A and B) Axial imaging on a FLAIR sequence. (C) T2-weighted sagittal imaging. MRI showed several hyperintense lesions on FLAIR sequences with an oval morphology in the right thalamic mesencephalic sulcus (A), corpus callosum (B), cervical spine (C3, C4 and C5) and thoracic spine (T7–T8 and T9–T10) (C).
No specific imaging findings have been reported in the few known cases of encephalitis due to neuroborreliosis.5 Schwenkenbecher et al. studied 68 patients with neuroborreliosis. Three were clinically classified as having acute encephalitis, and just one showed abnormal MRI findings, in the form of hyperintense lesions on T2-weighted imaging in the basal ganglia and left parietal lobe.12
The only reported case in the literature with involvement of the thalamus due to neuroborreliosis is that of Haene et al.16
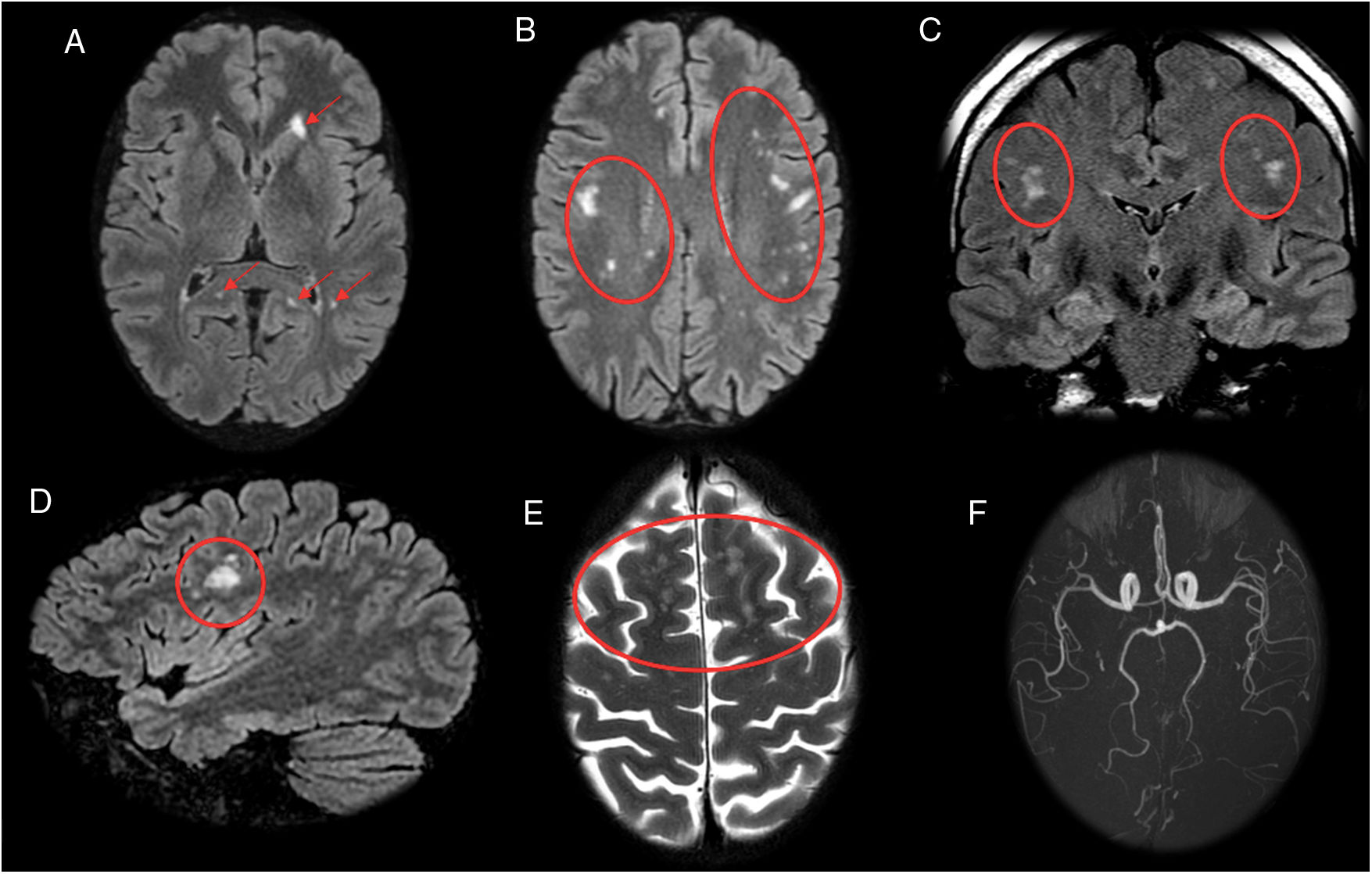
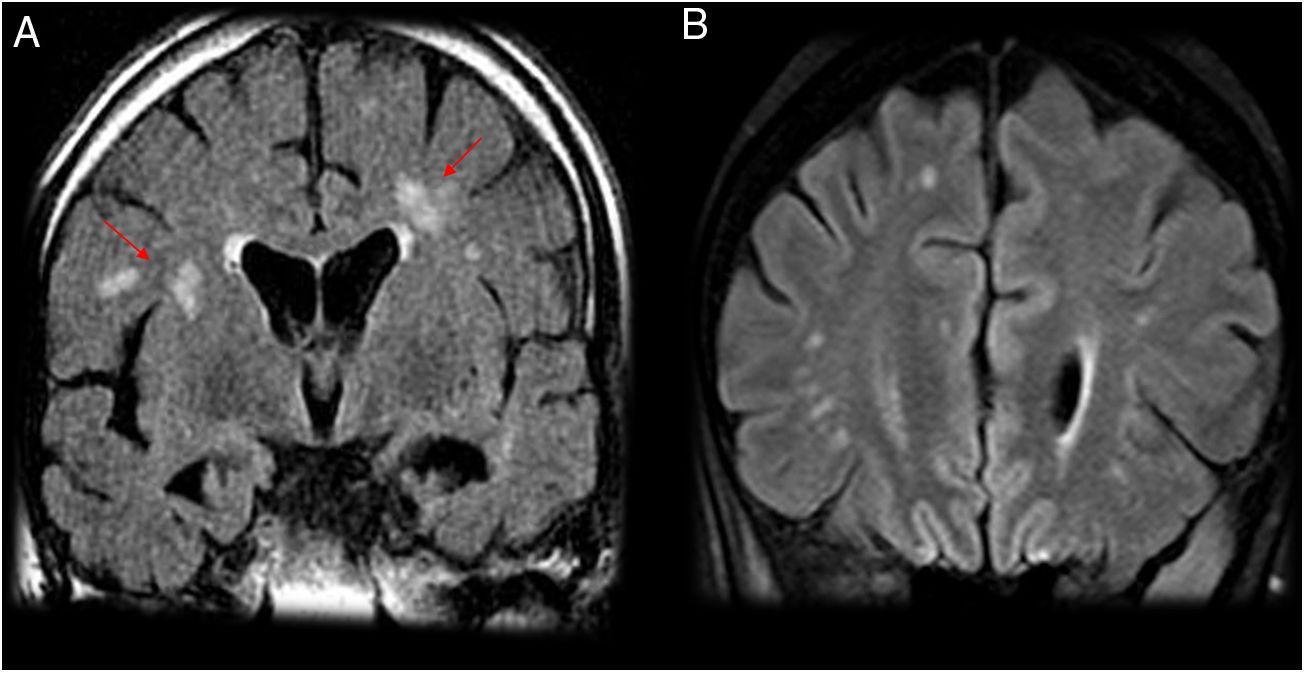
Cerebral vasculitisVasculitis secondary to neuroborreliosis is due to the secondary inflammatory response to spirochaetal infection.17 The reported frequency in the medical literature of vasculitis secondary to neuroborreliosis is 0.3%,18 warranting consideration of this condition in the differential diagnosis in patients with exposure to ticks in whom infarctions in the posterior region are observed.5 These vascular lesions secondary to Borrelia manifest radiologically in the form of small lesions in the deep supratentorial white matter, hyperintense on T2/fluid-attenuated inversion recovery (FLAIR) sequences (Figs. 3 and 4). Diffusion-weighted imaging (DWI) can identify acute and subacute ischaemic lesions, while angiography can show vessel lumen irregularities with occlusion, stenosis or segmental dilation.
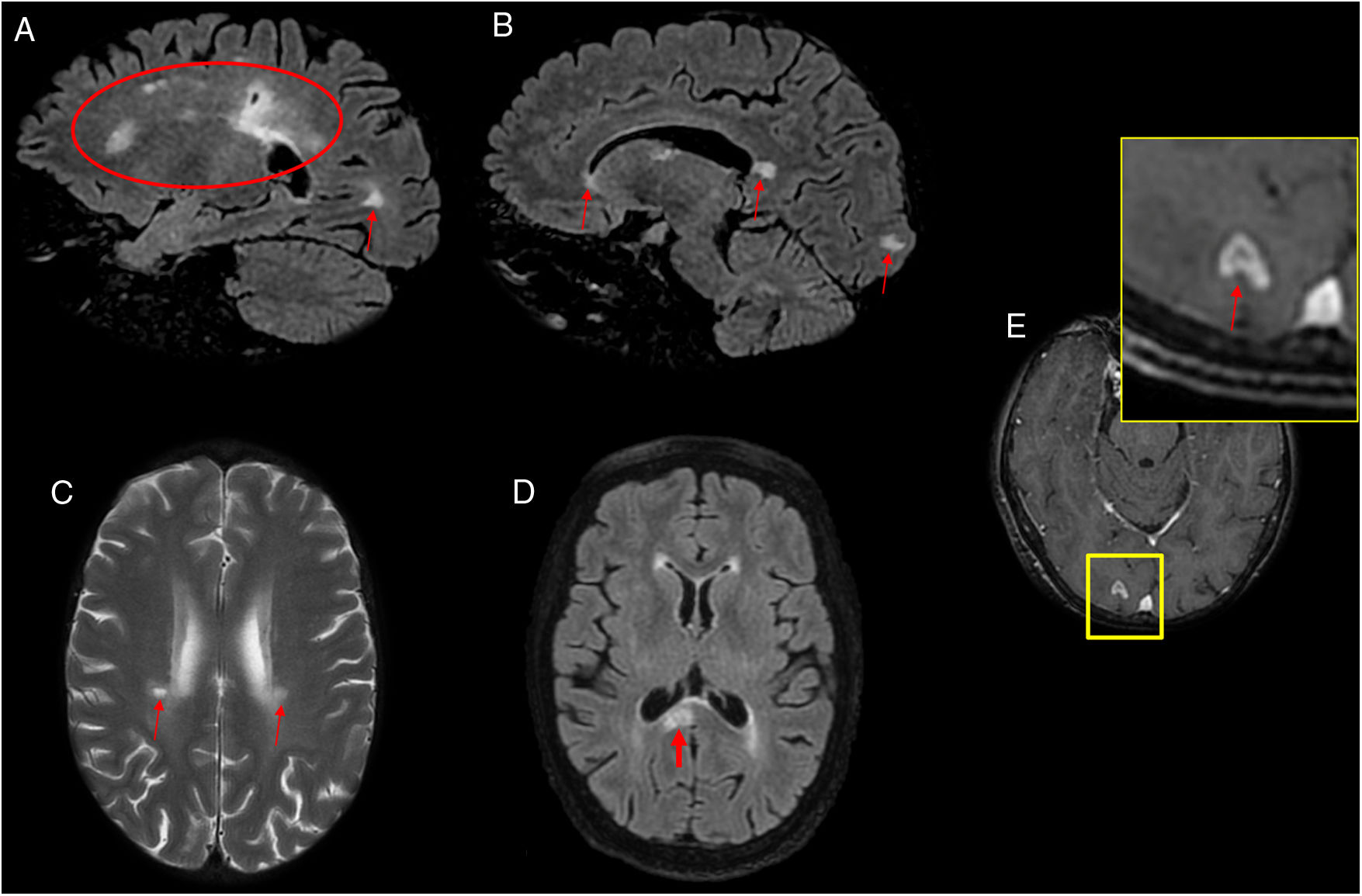
A 53-year-old patient with hemiplegia, headache and instability with positive serology for Borrelia. (A, B and D) Sagittal and axial imaging on a FLAIR sequence. (C) Axial T2-weighted imaging. (E) Axial T1-weighted imaging after intravenous contrast administration. Magnetic resonance imaging showed hyperintense lesions on T2/FLAIR in the periventricular white matter, coronae radiatae and splenium of the corpus callosum with no enhancement (A–D). A ring-enhancing lesion hyperintense on T2/FLAIR in a right occipital juxtacortical location was seen (E). The cerebrospinal fluid tested negative for oligoclonal bands, and the patient experienced improvement after antibiotic therapy.
A 21-year-old patient diagnosed with relapsing–remitting multiple sclerosis with poor treatment response and positive serology for Borrelia. Coronal (A and B) and sagittal (C) imaging on a FLAIR sequence. Magnetic resonance imaging revealed multiple white matter lesions hyperintense on FLAIR with a random distribution (A–C), without showing the juxtacortical or periventricular lesion distribution typical in multiple sclerosis and without affecting the brainstem.
Mediterranean spotted fever (MSF) or Boutonneuse fever (BF) is a form of spotted fever caused by R. conorii, an obligate intracellular bacterium belonging to the family Rickettsiaceae. The disease develops when humans are bitten by the brown dog tick Rhipicephalus sanguineus, which acts as a vector. Its peak incidence is recorded in summertime. MSF is endemic throughout the Mediterranean, including Spain, as well as the regions of the Black Sea and sub-Saharan African countries. Sporadic cases have been reported in non-endemic areas, either by travellers or by people in contact with infected dogs in endemic areas.19
MSF is usually a benign, self-limiting disease. It presents in the form of sudden headache, fever and maculopapular rash (97%–99% of cases) one to three weeks after the bite. In 70% of cases, a lesion with a necrotic black eschar (“tâche noire”) is seen at the inoculation site.20
The complications of the disease include acute kidney failure, thrombocytopenia, myocarditis, pneumonitis, gastric bleeding, shock and multiple organ failure. The development of systemic vasculitis is the primary pathogenic factor in the origin of systemic complications of MSF.
In MSF, neurological complications are rare, in contrast to rocky mountain spotted fever (caused by Rickettsia rickettsii, less common in Spain). However, MSF can affect both the CNS and the peripheral nervous system. CNS complications include meningitis, encephalitis and myelitis.
The diagnosis of MSF is based on clinical signs, epidemiological data and laboratory tests confirming recent exposure to the pathogen (culture techniques and serology tests). Indirect immunofluorescence is the most commonly used confirmation test.19
The most common drug treatment is oral or intravenous doxycycline at a dose of 200 mg daily for 7–14 days, depending on the patient’s clinical course. The majority of patients improve within 24 h of starting treatment.21
There is scarce literature on neuroimaging findings corresponding to R. conorii.
Ezpeleta et al. reported a case of R. conorii that presented with meningoencephalomyelitis. MRI showed small lesions hypointense on T1-weighted imaging and hyperintense on proton density-weighted (PD-weighted) and T2-weighted imaging. The lesions were located in both middle cerebellar peduncles, the splenium of the corpus callosum and the left frontal subcortical white matter, and all showed paramagnetic contrast uptake. The meninges showed neither lesions nor contrast uptake. These lesions were interpreted as demyelinating inflammatory areas, since they were clinically asymptomatic despite their size and strategic location; an ischaemic vascular origin was considered less likely, given the enhancement seen.22 However, a case published by Boulahri et al. reported an ischaemic stroke in a patient admitted for rickettsiosis with no cardiovascular risk factors.23
Another study by Aliaga et al. reported seven cases of encephalitis that illustrated the greater impact of R. conorii infection on the CNS. Five patients underwent a computed tomography scan of the brain, which showed abnormalities in two cases. The abnormalities appeared in the form of hypodensities in the subcortical white matter and both internal capsules. Two of the patients also underwent an MRI of the brain, revealing in both of them diffuse lesions in the subcortical white matter of the cerebral lobes, cerebellar peduncles and corpus callosum.21
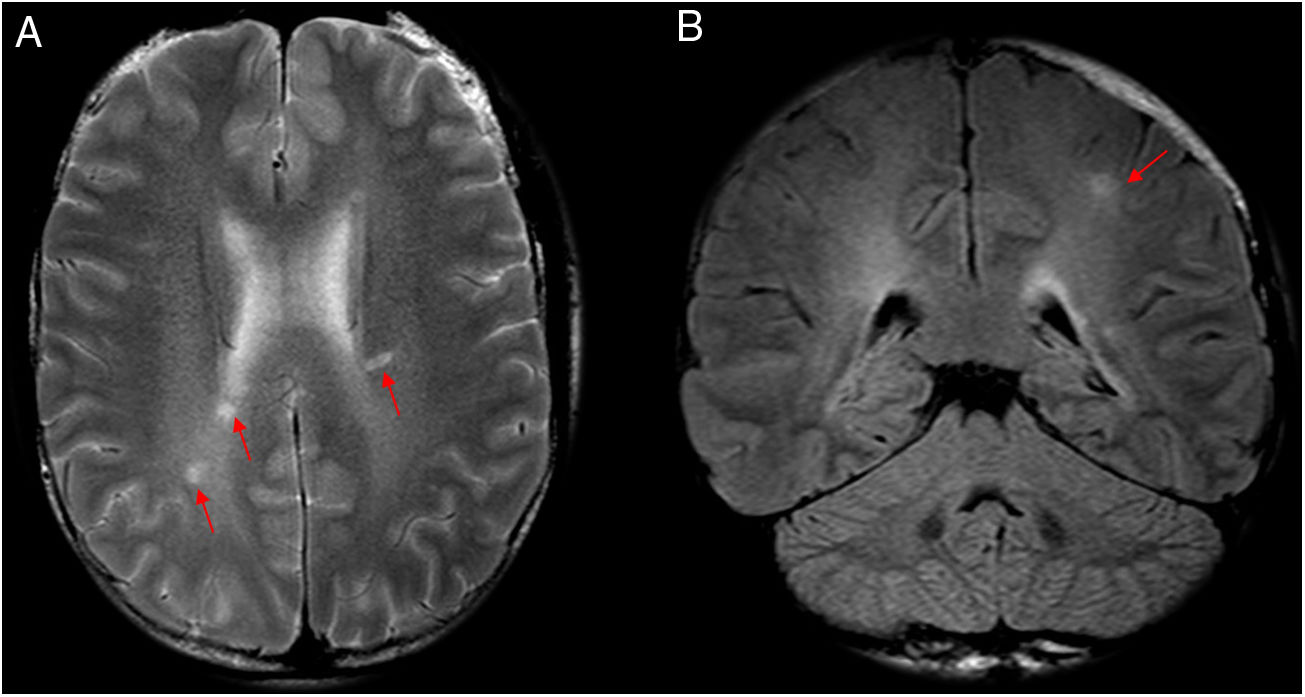
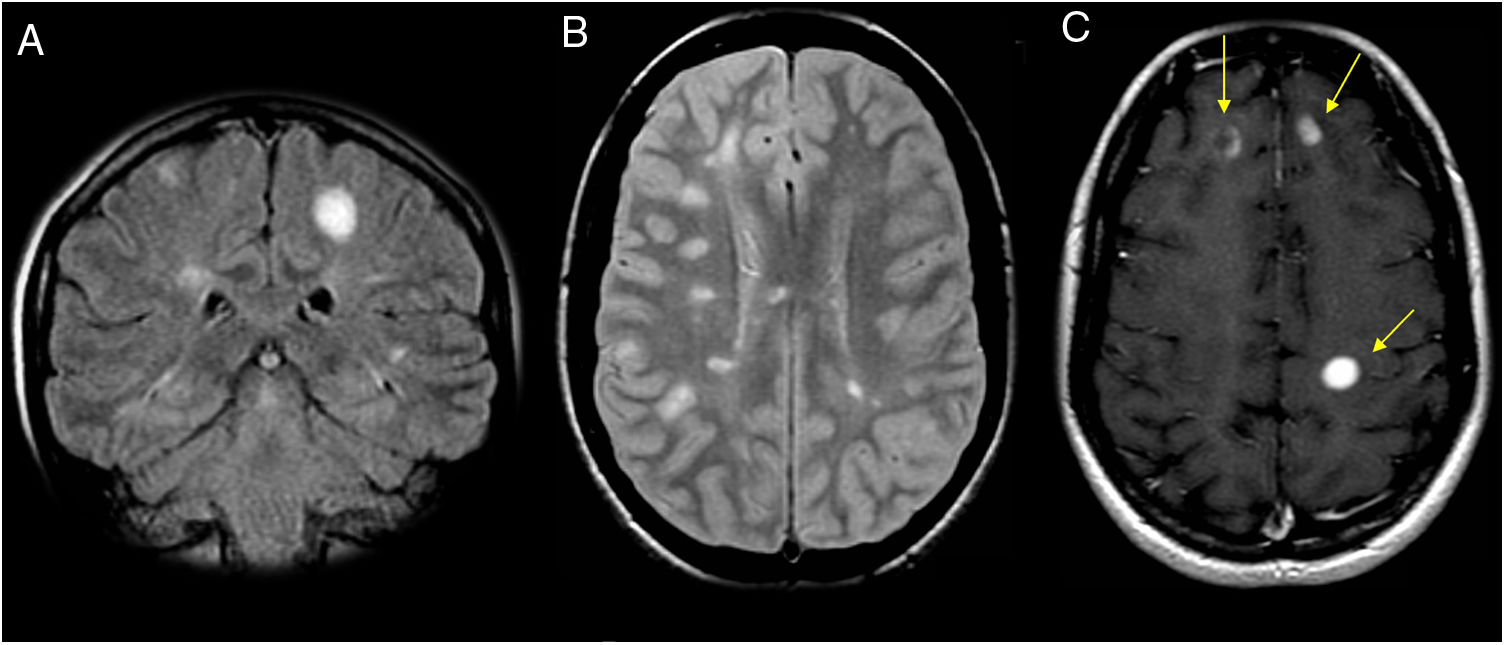
Among cases seen at our centre, the most common neuroimaging findings consisted of well-defined lesions hyperintense on T2/FLAIR sequences, in the supratentorial and periventricular white matter and the white matter adjacent to the head of the caudate nuclei (Figs. 5–7). However, in contrast to that reported by Ezpeleta et al., these lesions did not enhance after intravenous contrast administration. We did not find any cases with radiological evidence of spinal involvement.
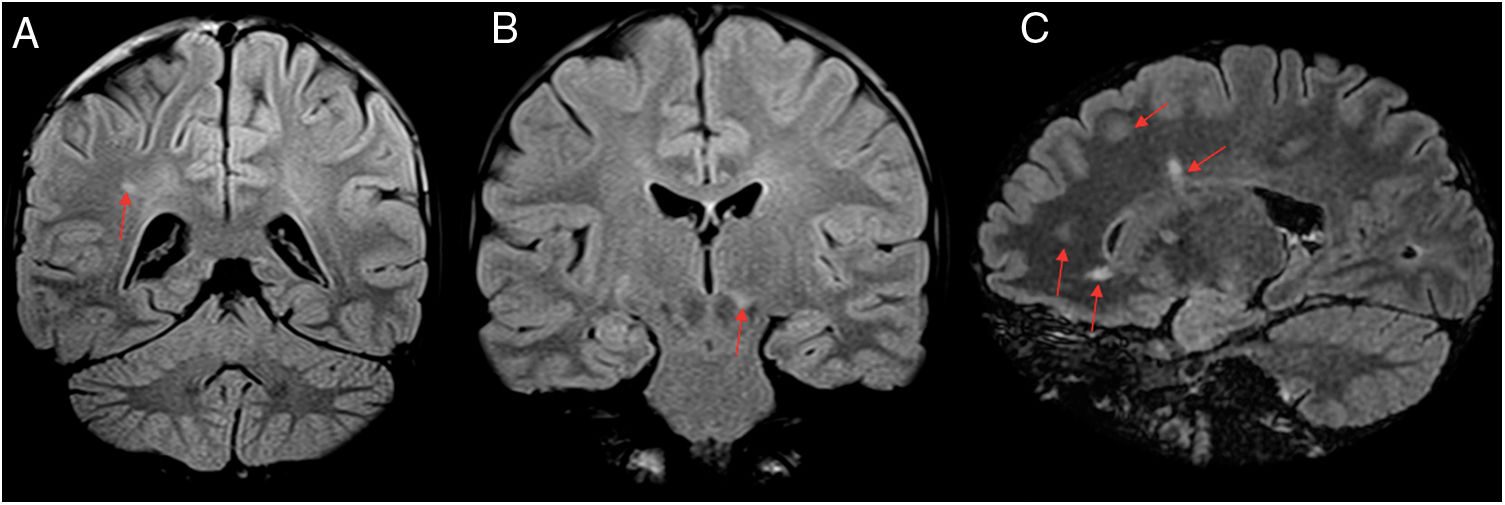
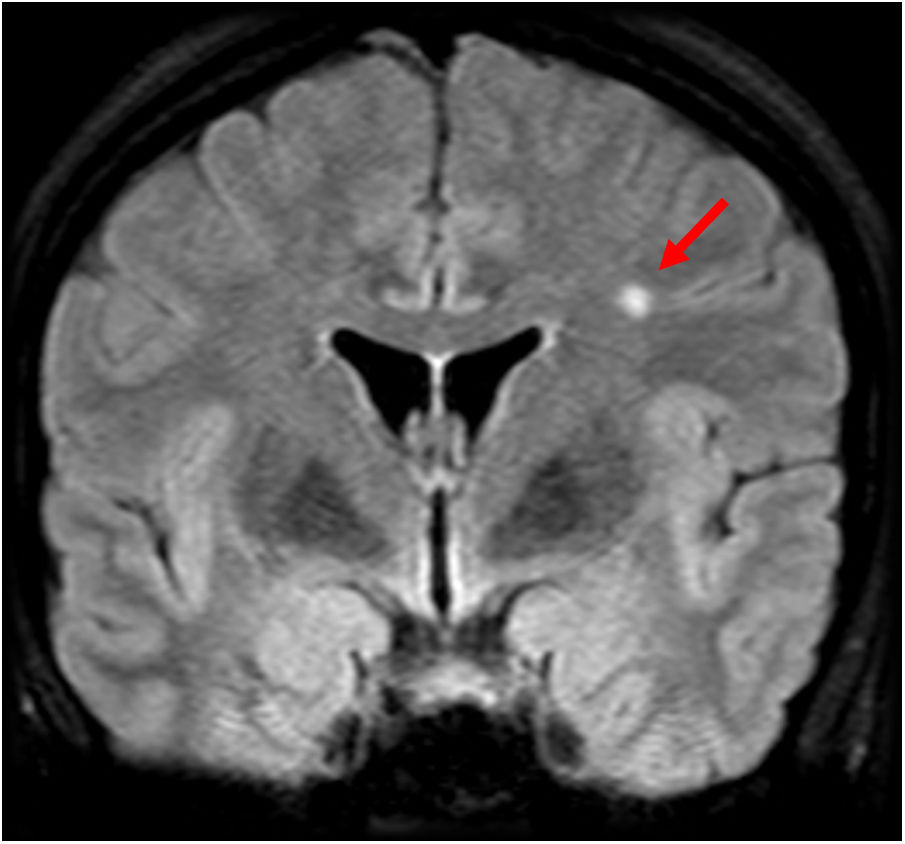
A 46-year-old patient who had psychomotor retardation for a month and developed sudden instability and migraine with aura. In view of the patient’s positive serology for Rickettsia, treatment was started with doxycycline, with subsequent clinical improvement. Axial (A and B), coronal (C) and sagittal (D) imaging on a FLAIR sequence. Axial T2-weighted imaging (E). Magnetic resonance angiography (F). This revealed punctiform foci, with a patchy distribution, in the juxtacortical white matter with a frontal predominance (A–E) and both coronae radiatae, without showing irregularities in the walls of the arteries suggestive of vasculitis at present. The patient had no cardiovascular risk factors, so Rickettsia infection was proposed as an alternative cause of microvascular involvement.
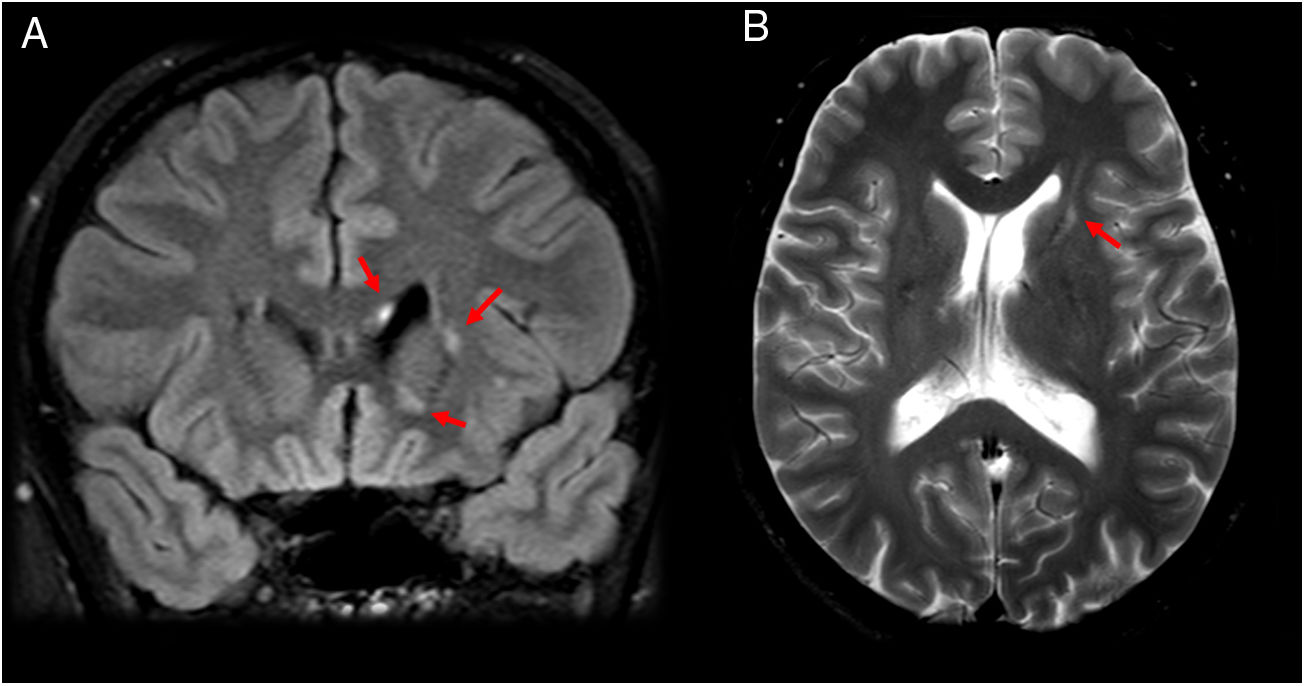
An 18-year-old patient with episodes of paralysis and sudden weakness in both legs, lasting for minutes, with no sensory abnormalities. The patient’s serology for Rickettsia was positive. (A) Axial imaging on a FLAIR sequence. (B and C) Sagittal T1-weighted fat-saturated imaging after intravenous contrast administration. Magnetic resonance imaging showed two small lesions adjacent to the head of the two caudate nuclei hyperintense on T2-weighted and FLAIR sequences and hypointense on T1-weighted sequences, with no expansiveness (A). No abnormalities are seen in the spinal cord (B and C).
A 39-year-old patient with asthenia and non-specific generalised pain, diagnosed with relapsing–remitting multiple sclerosis and considered to have “loss of medication efficacy”. Subsequently, the patient was found to have positive serology for Rickettsia. Clinical improvement after antibiotic therapy with doxycycline supported the probable diagnosis of Rickettsia infection. (A) Axial T2-weighted imaging. (B) Coronal imaging on a FLAIR sequence. Magnetic resonance imaging showed lesions hyperintense on T2/FLAIR sequences in a periventricular location with some in the coronae radiatae (A). Another similar left parietal subcortical lesion was seen (B). They did not enhance after contrast administration (images not shown).
The essential radiological finding in Borrelia and Rickettsia infections in the CNS consists of small, patchy lesions hyperintense on T2/FLAIR in the supratentorial deep white matter. These non-specific findings lead to a broad differential diagnosis by imaging that includes multiple diseases, such as multiple sclerosis, systemic lupus erythematosus and other forms of vasculitis, migraine, sarcoidosis, viral infections, metabolic disease and toxicity.
Multiple sclerosisThis is a chronic demyelinating disease that affects the CNS. It is most common in women in their thirties. The most common form, relapsing–remitting, is characterised by multiple flare-ups throughout life. MRI findings include plaques of ovoid morphology in periventricular and juxtacortical locations. These lesions in their acute phase exhibit contrast uptake (Fig. 8). Although the diagnosis is always clinical and supported by cerebrospinal fluid (CSF) analysis,24 MRI plays a very important role, since the involvement of certain locations (juxtacortical, periventricular, brainstem and spine) supports the diagnosis of the disease, and a completely normal MRI makes it highly unlikely.
A 31-year-old patient who presented an episode of hypoaesthesia in the right arm. Cerebrospinal fluid analysis revealed oligoclonal bands; all things considered, the patient met the criteria for a diagnosis of multiple sclerosis. (A) Coronal imaging on a FLAIR sequence. (B) Axial imaging on a PD sequence. (C) Axial T1-weighted imaging after intravenous contrast administration. Magnetic resonance imaging showed numerous hyperintense ovoid white matter lesions (A–C). Some showed enhancement with punctiform morphology or open ring enhancement after intravenous contrast administration (C), thus demonstrating the temporal and spatial dissemination of the lesions.
Given its high prevalence in Spain (80–180 per 100,000 population25) and its similarity in terms of morphology and location of lesions, multiple sclerosis (MS) undoubtedly represents one of the main differential diagnoses.
Some imaging findings that support a diagnosis of MS are: hypointense lesion borders on susceptibility weighted imaging (SWI) due to the presence of iron, lesions on the inferior border of the corpus callosum, short spinal cord injury (affecting fewer than two segments) and the central vein sign.26,27 The initial study of all patients with suspected demyelinating disease in Spain should include the detection of possible Lyme disease or rickettsiosis. In relation to imaging, in our experience, one of these possible infectious causes should be considered, especially when none of the patient's lesions is prototypical callosomarginal demyelinating plaques.
Systemic lupus erythematosusSystemic lupus erythematosus (SLE) is a multisystem autoimmune disease that is up to 13 times more common in women. Some 80% of patients show neuropsychiatric signs, the most common of which is seizures.28
Clinical and radiological findings are many, varied and not specific to the disease. Radiological findings include the presence of multiple patchy lesions hyperintense on T2/FLAIR in the subcortical and deep white matter of multifactorial aetiology (Fig. 9). On the one hand, this disease presents clotting abnormalities, creating a predisposition to both arterial and venous infarctions secondary to sinus thrombosis. On the other hand, it causes demyelinating lesions of both the brain and spinal code very similar to those in MS; in addition, aseptic meningitis may occur. There are a number of neurological signs with no clear representation on imaging techniques, such as seizures, headache and movement abnormalities.29
A 35-year-old patient with hypertension who had an acute psychotic episode secondary to systemic lupus erythematosus. (A and B) Coronal imaging on a FLAIR sequence. Several hyperintense foci are seen on FLAIR in the deep subcortical white matter (A) and the frontal lobes (B). Radiological signs of lupus vary widely and sometimes are highly non-specific, as in this case, and very difficult to distinguish from Lyme disease in the absence of a suitable clinical context.
Migraine is a disease with a high prevalence that is sometimes difficult to manage. In some cases, it is accompanied by warning symptoms that can end up causing neurological deficits. At present, its pathophysiology is still not fully understood.30 It is associated with an increased risk of lesions in the frontal deep white matter and the semioval centres (centrum semiovale) (Fig. 10),31 subclinical infarctions in the region of the posterior circulation and brain iron accumulation.32 In cases of hemiplegic migraine, venous dilation may be seen in the cerebral hemisphere contralateral to the hemiplegic side on SWI; perfusion abnormalities may be observed in the same way.33 In many cases, it is difficult to distinguish from rickettsiosis in terms of signs and symptoms as well as imaging; to a large extent they coexist.
SarcoidosisThis is a multisystem granulomatous disease. Neurological involvement (neurosarcoidosis) accounts for 5%–25% of cases.34 It most often affects black women in their thirties. The most common neurological involvement includes the brain parenchyma in the form of hyperintense lesions on T2 with a periventricular distribution (Fig. 11), often indistinguishable from MS and small vessel disease. In addition, there may be homogeneous pachymeningeal thickening and enhancement, or focal or generalised enhancement of the leptomeninges, cranial nerves35 and pituitary stalk (infundibulum).35
A 36-year-old patient with sarcoidosis who experienced an episode of hearing loss, facial paralysis and right hypoaesthesia. (A) Axial T2-weighted imaging. (B) Coronal imaging on a FLAIR sequence. Small foci hyperintense on T2/FLAIR are seen in the corpus callosum (A) and internal capsule (A and B). This case showed no pachymeningeal enhancement, leptomeningeal enhancement or thickening of the pituitary stalk (infundibulum), with MRI findings being highly non-specific.
It must not be overlooked that non-specific lesions in deep white matter are found in up to 5.3% of the healthy population 16–65 years of age, which may further complicate the differential diagnosis.36
ConclusionsIt is increasingly common to find patients with white matter lesions and positive serology for Borrelia and Rickettsia. It has been demonstrated that, in Lyme disease, enhancement of the meninges and nerves after contrast administration is almost as common as white matter lesions, often not detected due to the lack of contrast in the studies done. Lyme disease should be suspected in patients with lesions similar to multiple sclerosis with rash and flu-like symptoms.
At present, there is very little literature on neuroimaging findings in patients with positive serology for R. conorii, despite its high prevalence. Borrelia and Rickettsia most commonly present in the form of small lesions in the deep supratentorial white matter, hyperintense on T2/FLAIR sequences. It is important to obtain a full history of the patient’s profession, as well as any trips that he or she might have taken, to aid in confirming the diagnosis of these tick-borne diseases. In any case, most patients remember neither being bitten nor the presence of the initial typical lesion.
Unfortunately, neuroimaging findings are non-specific, complicating the differential diagnosis.
The primary function of imaging consists of ruling out other, more common causes that could account for patients’ symptoms. The radiologist plays an essential role, given the non-specific nature of the clinical signs, in helping the clinician arrive at the correct diagnosis, which is key to starting targeted treatment and preventing future neurological sequelae.
Authorship- 1.
Responsible for study integrity: JAS
- 2.
Study conception: JAS, DHG
- 3.
Study design: JAS, DHG
- 4.
Data collection: JAS, DHG, AMAG, CASV, EML
- 5.
Data analysis and interpretation: JAS, DHG, EML
- 6.
Statistical processing: JAS, DHG, AMAG
- 7.
Literature search: JAS, DHG, EML
- 8.
Drafting of the article: JAS, DHG
- 9.
Critical review of the manuscript with intellectually significant contributions: JAS, DHG, EML
- 10.
Approval of the final version: JAS, DHG.
The authors declare that they have no conflicts of interest.
Please cite this article as: Azcona Sáenz J, Herrán de la Gala D, Arnáiz García AM, Salas Venero CA, Marco de Lucas E. Infecciones bacterianas atípicas del sistema nervioso central transmitidas por garrapatas: una amenaza desconocida. Radiología. 2021. https://doi.org/10.1016/j.rx.2021.07.003