Pleuroscopy is a minimally invasive and highly effective procedure used for diagnosing pleural diseases. Despite its utility, pain during and after the procedure can be significant. Traditional analgesic approaches, including systemic opioids and local anaesthetics albeit potent, may provide incomplete pain relief and can be associated with side effects. Cryoneurolysis has emerged as a feasible analgesic technique in general surgery. However, an equivalent treatment modality has yet to be established for pleuroscopy.
ObjectivesTo investigate the safety and feasibility of cryoneurolysis in pleuroscopy performed using a 1.7mm cryoprobe via a semi-rigid pleuroscope.
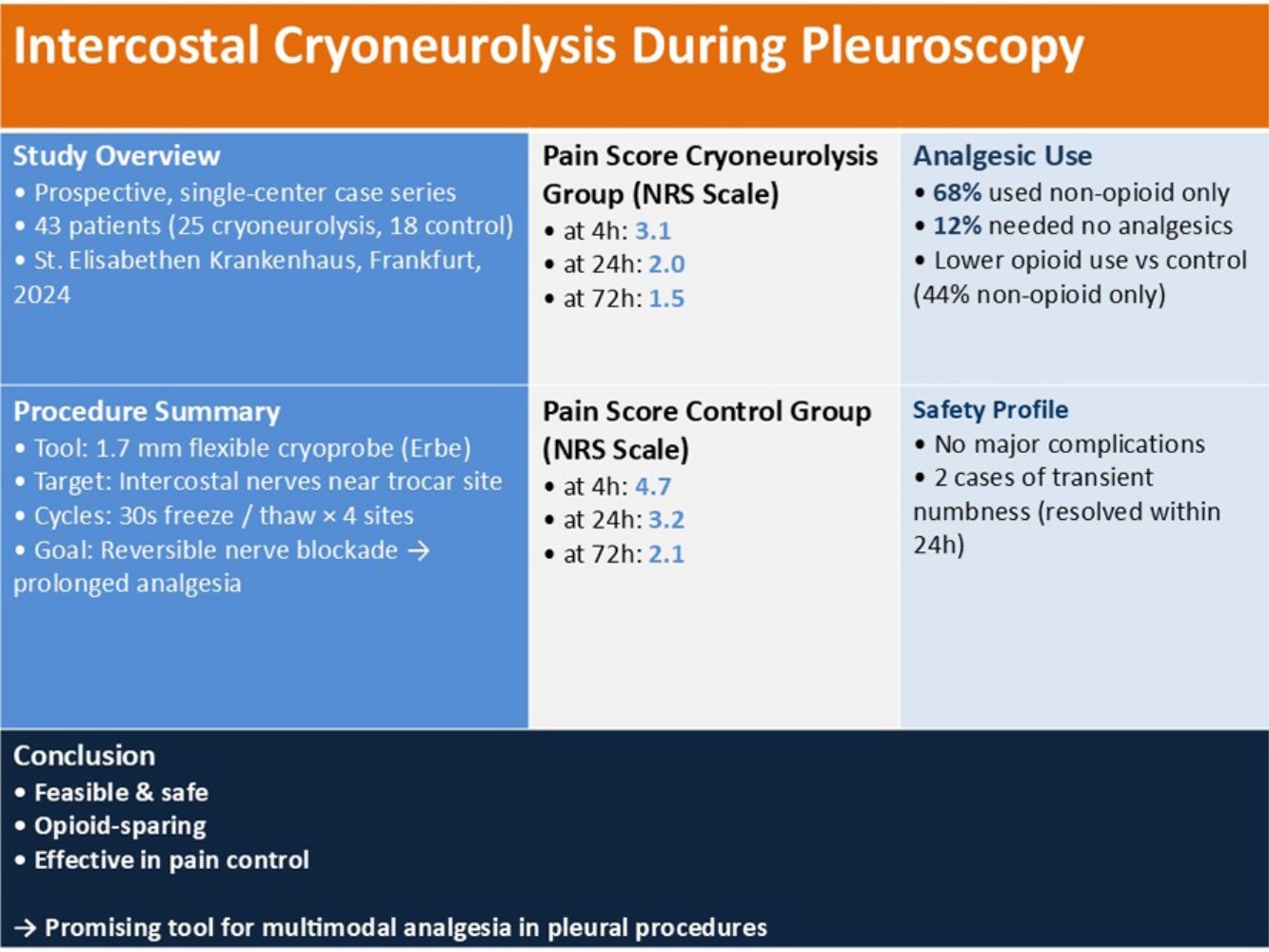
Materials and methodsWe conducted a prospective, single-centre observational study on 43 patients who underwent pleuroscopy at our institution between January 2024 and December 2024, of which 25 received cryoneurolysis. Pain levels were assessed using a Numeric Rating Scale (NRS) immediately post-procedure and at 24 and 72h. Complications and long-term adverse events were documented.
ResultsNRS scores after cryoneurolysis showed successful pain management both immediately post-procedure (mean NRS: 3.1) and at subsequent time points (24h: 2.0; 72h: 1.5). No major complications, such as major haemorrhage or nerve injury leading to persistent deficits, were observed. Minor complications occurred in 2 patients, who experienced a transient numbness immediately post-procedure. Analgesic requirements were lower than the control group, with 68% of patients requiring only mild oral analgesics post-procedurally.
ConclusionThe use of cryoneurolysis during pleuroscopy appears to be a feasible, safe, and effective technique for pain management. However, further studies with larger cohorts are warranted to validate the findings.
La pleuroscopia es una técnica mínimamente invasiva y muy eficaz para el diagnóstico de las enfermedades pleurales. A pesar de su utilidad, no obstante, se asocia con dolor significativo durante y después del procedimiento. Las técnicas de analgesia tradicionales, como los opiáceos sistémicos y los anestésicos locales, aun siendo potentes, no siempre comportan un alivio completo del dolor y pueden producir efectos secundarios. La crioneurólisis ya es una técnica analgésica viable en cirugía general, pero todavía es preciso fijar una modalidad terapéutica equivalente para la pleuroscopia.
ObjetivosEstudiar la seguridad y la viabilidad de la crioneurólisis en la pleuroscopia, realizada con una criosonda de 1,7mm por medio de un pleuroscopio semirrígido.
Materiales y métodosRealizamos un estudio observacional prospectivo y unicéntrico con 43 pacientes sometidos a pleuroscopia en nuestro hospital, entre enero y diciembre de 2024, a 25 de los cuales se les practicó la crioneurólisis. Se evaluaron los niveles de dolor con una escala numérica, inmediatamente después de la intervención y a las 24 y 72 horas. Se documentaron las complicaciones y los acontecimientos adversos a largo plazo.
ResultadosLas puntuaciones de la escala numérica indican que el control del dolor fue efectivo tanto inmediatamente después de la crioneurólisis (puntuación media: 3,1) como en los momentos posteriores (24 horas: 2,0; y 72 horas: 1,5). No se observaron complicaciones importantes, como hemorragias graves o lesiones nerviosas asociadas a déficits persistentes. Tuvieron complicaciones leves dos pacientes, quienes refirieron un entumecimiento pasajero inmediatamente después de la intervención. Los requisitos de analgesia fueron más bajos que en el grupo de control: después de la intervención, el 68% de los pacientes solo necesitaron analgesia oral ligera.
ConclusiónLa crioneurólisis representa una técnica factible, segura y eficaz para controlar el dolor durante la pleuroscopia. Con todo, habrá que validar estos resultados en estudios con cohortes más grandes.
Pleuroscopy, also known as medical thoracoscopy, is a minimally invasive and highly effective procedure used for diagnosing pleural diseases. Despite its utility, pain during and after the procedure can be significant, particularly in cases requiring extensive manipulation of the pleural space. The pain is primarily attributed to pleural irritation and trauma to the thoracic wall, commonly resulting from trocar insertion, instrument manipulation within the pleural space, pleural biopsies, and chemical pleurodesis.
Traditional analgesic approaches, such as systemic opioids and local anaesthetics, while potent, may provide incomplete pain relief and can be associated with side effects. Intercostal neurolysis using cryotherapy (cryoneurolysis) has emerged as a promising analgesic modality in surgical practice.1–5 The technique involves the application of controlled freezing to induce a reversible conduction blockade of intercostal nerves, thereby interrupting the transmission of pain signals from the pleura and adjacent thoracic structures. This effect occurs because the extreme cold induces Wallerian degeneration in the targeted nerve fibres. Rapid cycles of freezing and thawing disrupt the axons while preserving the structural integrity of the endoneurium, perineurium, and epineurium. As a result, nerve function is temporarily lost, effectively blocking pain signals until axonal regeneration restores normal conduction.
Cryoneurolysis has demonstrated effective analgesia in patients undergoing thoracic surgery, with studies reporting significant pain reduction and a favourable safety profile.6–12
Although cryotechnology has been explored in pleuroscopic diagnostics,13,14 its role as a therapeutic analgesic modality remains unstudied.
This study investigates the utility of cryotherapy as an analgesic method targeting intercostal nerves to enhance patient comfort during pleuroscopy.
Materials and methodsA non-comparative prospective case series was conducted on patients undergoing pleuroscopy between January and December 2024 at our pulmonology institution. A total of 43 medical thoracoscopies were performed, of which 25 included cryoneurolysis. Inclusion criteria were patients of legal age with suspected pleural disease requiring thoracoscopy. Exclusion criteria included coagulopathy, active infection, and prior thoracic surgeries. Written informed consent was obtained from each patient.
Allocation to the cryoneurolysis or control group was based on procedural availability and operator preference during the study period. No randomisation was employed, and group assignment was not influenced by clinical severity or collaboration status.
The patients underwent a medical thoracoscopy using a semi-rigid pleuroscope (LTF-H190, Olympus Medical Systems, Japan) under conscious sedation using solely propofol. Local anaesthesia was achieved with 1% Mepivacaine and the interventions were done under spontaneous breathing.
Patients were placed in lateral decubitus position with the affected side up. Ultrasonography was performed to determine the entry point. After disinfection with povidone-iodine and local anaesthesia with Mepivacaine, an approximately 1cm skin incision was made in the mid-axillary line between the 4th and 6th intercostal spaces of the chest wall. The subcutaneous tissue and intercostal muscles were bluntly dissected to access the pleural cavity, after which a disposable 8mm flexible trocar was inserted to serve as a conduit for the pleuroscope.
Systematic exploration of the pleural space was performed, and biopsies were obtained from various intrapleural sites. Subsequently, cryoneurolysis was carried out at the intercostal space adjacent to the trocar insertion site. A 1.7mm flexible cryoprobe (Erbecryo 20402-410, Erbe Elektromedizin, Tübingen, Germany) was first introduced through the working channel of the semi-rigid pleuroscope. The intercostal nerves were targeted under direct visualization and red dichromatic imaging (RDI) was employed to exclude the presence of large intercostal vessels. Cryotherapy using CO2 gas was then administered in repeated cycles of 30s of freezing followed by thawing, achieving temperatures as low as −70°C, for a total duration of 2min at each site. Cryoneurolysis was performed at a total of four sites along the selected intercostal spaces (Video 1). This process allowed for a disruption axonal transmission without causing permanent nerve damage, thus leading to pain reduction.
At the end of the procedure, a 24 Fr chest tube with underwater seal was placed via the thoracoscope insertion site after removal of the flexible trocar. All patients received a postprocedural chest radiograph to rule out complications and underwent a 72h observation.
Post-procedurally, patients received an analgesic regimen consisting either of a monotherapy with non-opioid analgesics (Metamizole) or a combination of scheduled non-opioid analgesics administered every 6h and on-demand opioid analgesics (Piritramide). Pain intensity was assessed at 4-, 24-, and 72-h post-procedure using the Numeric Rating Scale (NRS; 0=no pain, 10=worst pain imaginable), and analgesic requirements were closely monitored following the procedure.
The patients were discharged on the third post-interventional day after removal of the chest drain and verifying that no complications had occurred.
Long-term follow-up was conducted at our outpatient medical service centre, where patients attended two scheduled appointments. The first follow-up occurred one week after the pleuroscopy to review the diagnostic results, while the second was conducted six weeks post-intervention to assess the patients’ condition.
ResultsThe median age of the patients was 63 years (range: 52–84), with 29 male patients (67%) and 14 female patients (33%). Smokers and ex-smokers made up 79% of the cohort, with 24 patients being current smokers and 10 patients being ex-smokers. Patient characteristics are summarized in Table 1.
Indications for the pleuroscopy included exudative pleural effusion (n=20), pleural thickening (n=6), and suspected malignancy (n=17). Histological analysis revealed neoplastic findings in 35 of the 43 cases. In the cryoneurolysis group, 21 patients had malignant diagnoses (including 4 with mesothelioma), 2 had infectious etiologies, and 2 were non-diagnostic. In the control group, 14 had malignancy (including 2 with mesothelioma), none had infections, and 4 were non-diagnostic. A diagnostic yield of 87% was reported. A summary of the histological findings is presented in Fig. 1.
Pain scores were recorded post-procedurally and at subsequent time points at 24h and at 72h. Lower pain scores were reported in the cryoneurolysis cohort with a median NRS score of 3.1 at 4h, 2.0 at 24h and 1.5 at 72h. Their median pain scores are shown in Table 2.
In the control cohort, median NRS scores were 4.7 at 4h, 3.2 at 24h, and 2.1 at 72h.
Seventeen of 25 patients (68%) in the cryoneurolysis group required only Metamizole as therapy to achieve pain relief, while 3 of 25 (12%) required no additional analgesia post procedure. Five of 25 patients (20%) required the standard of care which was the combination of on-demand Piritramide as well as repeated doses Metamizole. A summary of the treatment regimens is shown in Table 3.
In the control group, 8 of 18 patients (44%) required only Metamizole, while the remaining 10 patients (56%) achieved pain relief with combination therapy consisting of on-demand Piritramide alongside Metamizole. All patients received some form of analgesia during the postprocedural phase.
No major adverse events, such as bleeding, persistent pneumothorax or nerve injury leading to permanent deficits, were reported in the postprocedural checks or in the long-term follow-ups.
Minor adverse events included transient localized numbness (n=2), that spontaneously resolved within 24h.
DiscussionThis study demonstrates that intercostal cryoneurolysis is a safe and effective adjunct to standard analgesic regimens in patients undergoing medical thoracoscopy. Patients in the cryoneurolysis cohort reported lower pain scores at all measured time points compared to the control group.
The majority of patients in the cryoneurolysis group (17 out of 25 patients) achieved adequate pain control with non-opioid analgesia alone, and 3 out of 25 required no postprocedural analgesia at all. In contrast, a significantly larger proportion of the control group (10 out of 28 patients) required opioid-based combination therapy to achieve adequate pain relief. These findings highlight the potential of cryoneurolysis to reduce opioid requirements, which is particularly relevant in the context of current efforts to minimize opioid exposure and associated side effects in clinical practice.
Importantly, the intervention was well-tolerated, with no major complications observed during postprocedural assessments or follow-up. Only two cases of transient localized numbness were reported, both resolving spontaneously within 24h, indicating a favourable safety profile. Additionally, its ease of application and rapid onset make it a practical addition to thoracoscopy procedures.
Our findings are consistent with prior studies demonstrating the efficacy of cryoneurolysis in thoracic procedures, such as video-assisted thoracoscopic surgery (VATS), and extend its potential application to minimally invasive diagnostic procedures like pleuroscopy. The ability to achieve significant analgesia without systemic opioids may also improve patient comfort and facilitate earlier recovery.
Despite the promising results, the study is limited due to its relatively small sample size and lack of randomization. Additionally, pain perception is subjective and may be influenced by individual patient factors. Future randomized controlled trials with larger sample sizes are warranted to confirm the findings and to further explore the long-term benefits and cost-effectiveness of incorporating cryoneurolysis into routine pleuroscopy protocols, as well as rule out delayed complications.
ConclusionsIntercostal cryoneurolysis using a 1.7mm cryoprobe appears to be a feasible and safe method for reducing pain in pleuroscopy. By freezing intercostal nerves, it provides prolonged analgesia, minimizes opioid reliance, and improves patient recovery. It represents a promising tool in the multimodal management of procedural pain. Further larger, controlled trials are needed to confirm efficacy and establish standardised guidelines.
Ethical approvalAll procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. The study protocol was reviewed and approved by the ethics committee of the St. Elisabethen Krankenhaus (A-01/2025).
Declaration of generative AI and AI-assisted technologies in the writing processThe authors declare that the study was done without the help of any artificial intelligence software or tool.
Informed consentInformed consent was obtained from all individual participants included in the study.
FundingThe authors declare that no funds, grants, or other support was received during the preparation of this manuscript.
Authors’ contributionsAll the authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by Sammy Onyancha, Isabelle Dettmer, Njuxhersa Maloku and Gernot Rohde. The first draft of the manuscript was written by Sammy Onyancha, and all the authors commented on previous versions of the manuscript. All the authors have read and approved the final manuscript.
Conflicts of interestSammy Onyancha has received honoraria as a consultant and as part of educational programs from Olympus, Medi-Globe, Erbe and Ambu.
Gernot Rohde has received honoraria as a consultant and as part of educational programs from AstraZeneca, Berlin Chemie, BMS, Boehringer Ingelheim, Chiesi, Essex Pharma, Grifols, GSK, Insmed, MSD, Roche, Solvay, Takeda, Novartis, Pfizer, and Vertex.
Data availabilityThe data that support the findings of this study are not publicly available as they contain information that could compromise the privacy of the research participants. Data may be made available from the corresponding author upon reasonable request.