Emphysema is a phenotype of chronic obstructive pulmonary disease (COPD) that causes air trapping and lung hyperinflation and, consequently, dyspnea, reduced exercise tolerance, and poor health-related quality of life. Several randomized controlled clinical trials have shown that bronchoscopic lung volume reduction (BLVR) with endobronchial valves (EBV) achieves clinically relevant improvements in dyspnea, pulmonary function, exercise capacity and quality of life 12 months after valve implantation in patients with heterogeneous emphysema without collateral ventilation. The goal of our meta-analysis is to examine the efficacy and safety of BLRV in patients with COPD.
Material and methodsA literature search was performed with PubMed, Embase and Cochrane to identify randomized controlled trials on BLVR with endobronchial valves published from 2005 onwards.
ResultsNine studies with a total of 1352 patients were included; 827 received EBV therapy and 525 standard of care (SOC) medications. The first group showed statistically significant improvements in forced expiratory volume in 1 second (FEV1), Saint George Respiratory Questionnaire (SGRQ) score, modified medical research council (mMRC) dyspnea scale, and 6-minute walk distance (6MWD), and a statistically significant reduction in residual volume (RV). The incidence of pneumothorax and exacerbations in the EBV arm increase significantly, and there were no significant difference between mortality rates.
ConclusionsPatients with heterogeneous emphysema and no collateral ventilation showed significant improvements in lung function, exercise capacity, dyspnea score, and health-related quality of life after BLVR-EBV, although the risk of exacerbations and pneumothorax in the first 6 months increased compared with the group receiving standard care.
El enfisema, rasgo tratable de la enfermedad pulmonar obstructiva crónica (EPOC), provoca atrapamiento aéreo e hiperinsuflación y, en consecuencia, disnea, reducción de tolerancia al ejercicio y peor calidad de vida. En pacientes con enfisema heterogéneo sin ventilación colateral ensayos clínicos controlados aleatorizados han demostrado con la reducción broncoscópica de volumen pulmonar (RVBP) mediante la colocación de válvulas endobronquiales (VEB) mejoría en la disnea, función pulmonar, capacidad de ejercicio y calidad de vida 12 meses después de su implantación. El objetivo del metaanálisis fue examinar la eficacia y seguridad de la RVBP con VEB en pacientes con EPOC.
Material y métodosSe realizó una búsqueda bibliográfica en PubMed, Embase y Cochrane, incluyendo ensayos controlados aleatorizados de RVBP con VEB desde 2005.
ResultadosSe incluyeron 9 estudios con un total de 1352 pacientes; 827 recibieron tratamiento con VEB y 525 tratamiento estándar (SOC). El primer grupo mostró de manera estadísticamente significativa una mejoría en el volumen espiratorio forzado en el primer segundo (FEV1), cuestionario de St. George (SGRQ), escala de disnea (mMRC) y distancia caminada en 6 minutos (6MWD), una reducción en el volumen residual (RV), y un aumento de la incidencia de neumotórax y exacerbaciones sin diferencia entre las tasas de mortalidad.
ConclusionesLa RVBP-VEB en pacientes con enfisema heterogéneo sin ventilación colateral mostró mejoras significativas en función pulmonar, capacidad de ejercicio, disnea y calidad de vida, pero también un aumento del riesgo de exacerbaciones y neumotórax en los primeros 6 meses en comparación con el grupo de tratamiento estándar.
Chronic obstructive pulmonary disease (COPD) is currently one of the top 3 causes of death worldwide, and 90% of these deaths occur in low and middle-income countries.1 COPD is a major cause of chronic morbidity and mortality worldwide, and is a major public health challenge that is both preventable and treatable.2 Emphysema – a phenotype of COPD – is a progressive, debilitating disease characterized by irreversible destruction of alveolar tissue.3,4 The pathophysiological effects of emphysema are loss of lung elastic recoil, air trapping, and lung hyperinflation. The resulting progressive hyperinflation and gas trapping with impaired respiratory mechanics causes chronic dyspnea, reduced exercise tolerance, and poor health-related quality of life. In selected patients with heterogeneous or homogeneous emphysema and significant hyperinflation refractory to optimized medical care, surgical or bronchoscopic modes of lung volume reduction (e.g., endobronchial one-way valves, lung coils or thermal ablation) may be considered.2,5
The risk of surgery-related morbidity and mortality has led clinicians to evaluate different non-surgical endoscopic interventions to reduce lung volume known as bronchoscopic lung volume reduction (BLRV).6 Randomized clinical trials have shown that patients receiving unidirectional endobronchial valves (EBV) show statistically significant improvements in FEV1 and the 6-minute walk test (6MWT) at 6 and 12 months post-surgery compared with controls. These one-way valves are placed bronchoscopically to block inspiratory flow in a lobar bronchus and cause lobar atelectasis. This in turn improves vital capacity by relieving dead space ventilation and hyperinflation.7 The greatest benefit has been seen in patients with heterogeneous emphysema and in those with no evidence of collateral ventilation. In the latter group, a randomized controlled clinical trial reported clinically relevant improvements in dyspnea, pulmonary function, exercise capacity and quality of life 12 months after valve implantation.8
Studies have shown that surgical volume reduction can reduce the rate of exacerbations and mortality; however, the effect of endoscopic volume reduction on exacerbations and mortality is unknown. The most frequent side effects of this procedure are pneumothorax and valve repositioning. The FDA has so far approved 2 one-way valve systems (Zephyr and Spiration) that reduce hyperinflation in patients with severe COPD.
The aim of our systematic review and meta-analysis is to examine the efficacy and safety of BLVR with EBV.
Material and methodsStudy designThis is a systematic review and metanalysis of randomized clinical trials comparing BLVR-EBV vs standard of care (SoC) performed in accordance with the PRISMA statement.9 The protocol was registered at PROSPERO under identification number CRD42024618560.
Study populationThe study population included RCTs recruiting adult (over 40 years of age) patients with COPD (defined as postbronchodilator FEV1/FVC<0.7), former or current smokers of more than 10 pack-years. The intervention was BLVR-EBV compared to SoC.
Search strategyA comprehensive literature search was conducted in 3 major databases – PubMed, Embase, and Cochrane Library – to identify relevant studies published between 2005 and February 2024. Search terms included combinations of keywords and MeSH terms such as “bronchoscopic lung volume reduction”, “BLVR”, “endoscopic lung volume reduction”, “lung volume reduction valves”, “endobronchial valves,” “COPD,” and “emphysema.” Boolean operators (“AND,” “OR”) were used to maximize the scope of the search strategy. Terms were related to the intervention and modified according to the index terms used in each database, such as Medical Subject Heading (MeSH) and EMTREE. Filters were applied to restrict the search results to randomized controlled trials (RCTs) in humans, published in English. Gray literature and conference abstracts were not included. The search results were exported to a reference management program (e.g., EndNote) for de-duplication.
Inclusion and exclusion criteriaAll published human bronchoscopic lung volume reduction with endobronchial valves trials in English from 2005 to 19/02/24 were considered for inclusion. Only randomized controlled trials were included. We included adult patients with COPD according to current guidelines with FEV1 ≤45% and with severe heterogenous or homogeneous emphysema and hyperinflation with TLC >100% based on high-resolution chest CT (HRCT) imaging findings. Since multiple valve trials have already shown disappointing results in patients without intact interlobar fissures, and current clinical practice emphasizes the need for fissure completeness, we prioritized studies or subgroups including patients with intact fissures. When trials included mixed populations, we included them only if the study provided subgroup analyses relevant to our target population.
Animal studies or preclinical studies and nonoriginal articles such as reviews, editorials, letters, opinions, and comments were excluded. Articles not published in English, case reports, studies with less than 6 months follow-up were also excluded. If two publications reported on the same study cohort, the article with the smallest sample size or the shortest follow-up was excluded to avoid duplication. We also excluded studies that did not report outcomes of interest, studies with no placebo or SoC control group, those in which BLVR was not performed with EBV, and studies where BLVR modalities were used for diseases other than COPD (e.g., valves for giant emphysematous bullae and persistent air leak).
Two reviewers (JDM and MJG) independently checked the relevance of the RCTs identified from the literature and databases. RCTs were selected using the aforementioned criteria, and any difference in opinion about eligibility was resolved by a third independent reviewer (BAN).
The review protocol was registered on PROSPERO.
Quality assessmentThe methodological quality of included studies was assessed using the Cochrane Risk of Bias (RoB 2) tool,10 which assesses selection bias, performance bias, detection bias, attrition bias, and reporting bias. Studies were grouped as low, moderate, or high risk of bias.
Outcome measuresThe primary outcome of this systematic review and metanalysis is the efficacy of BLRV-EBV expressed as improvements in FEV1, RV, 6MWT, quality of life (assessed on the Saint George Respiratory Questionnaire – SGRQ) and symptoms assessed with the modified Medical Research Council (mMRC) dyspnea scale. The secondary outcomes included the proportion of moderate and severe exacerbations (defined as changes in COPD symptoms that led to systemic corticosteroids and/or antibiotics treatment and hospital admissions due to COPD), all-cause mortality, and pneumothorax.
Statistical analysisThe statistical analysis was performed using random-effects models to account for variability both within and between the included studies. Continuous variables were reported as weighted mean differences (WMDs) with 95% confidence intervals (95% CIs). Dichotomous outcomes were expressed as odds ratios (ORs) with 95% CIs. A random-effects model was used to account for between-study variability.
Heterogeneity among the studies was evaluated using the Q statistic and the I2 statistic. An I2 score greater than 50% was considered indicative of substantial heterogeneity. Model fit was assessed using the log-likelihood, Akaike Information Criterion (AIC), and Bayesian Information Criterion (BIC). Publication bias was assessed using the Rosenthal Fail-safe N calculation, which estimates the number of additional studies needed to nullify the observed effect, and funnel plot asymmetry tests, including Kendall's Tau and the regression test for funnel plot asymmetry. The statistical significance of the results was determined using Z-tests for the log odds ratios, with p-values less than 0.05 considered statistically significant.
We also calculated 95% CIs to provide a range of values within which the true effect size is expected to lie. All statistical analyses were performed using Jamovi software (version 1.6) with the ‘metafor’ package from R software, which is specifically designed for meta-analyses in R.11
ResultsIncluded studiesThe study flow diagram is shown in Fig. 1. Initially, a comprehensive search identified 492 records from various databases. After removing 205 duplicates and excluding 68 records using automation tools and manual checks for eligibility, 219 records were assessed for eligibility. From these, 210 records were excluded based on predefined criteria, 75 reports were excluded for reasons such as non-BLVR-EBV arm, lack of outcome data (86), non-COPD related studies (18), less than 6 months of follow-up (5), and non-RCT studies (26). Ultimately, 9 studies were included in the final review and analysis. A summary of the studies included can be found in Table 1. The total number of patients included in this meta-analysis is 1352. The RoB assessment of included studies is shown in Table 2.
Characteristics of the studies included in the systematic review.
| Trial | BeLieVeR-HIFi21 | LIBERATE19,24,25 | IMPACT26,27 | TRANSFORM30 | STELVIO29 | REACH22 | VENT17,28 | IBV20 | EMPROVE23 |
|---|---|---|---|---|---|---|---|---|---|
| First author | Davey | Criner | Valipour | Kemp | Klooster | Li | Sciurba | Wood | Criner |
| Year | 2015 | 2018 | 2016 | 2017 | 2016 | 2018 | 2010 | 2014 | 2019 |
| Design | Single-center, double blind RCT | Multicenter RCT | Multicenter RCT | Multicenter RCT | Single-center prospective RCT | Multicenter unblinded RCT | Multicenter RCT | Multicenter RCT | Multicenter RCT |
| Valve | Zephyr PulmonX | Zephyr PulmonX | Zephyr PulmonX | Zephyr PulmonX | Zephyr PulmonX | Spiration Olympus | Zephyr PulmonX | Spiration Olympus | Spiration Olympus |
| Duration (weeks) | 12 | 52 | 24 | 24 | 24 | 24 | 24 | 24 | 24 |
| Sample (n) | 25 EBV25 SoC | 128 EBV62 SoC | 34 EBV44 SoC | 65 EBV32 SoC | 34 EBV34 SoC | 66 EBV33 SoC | 220 EBV101 SoC | 142 EBV135 SoC | 113 EBV59 SoC |
| Emphysema | HE/CV− | HE/CV− | HO/CV− | HE/CV− | HO/HE/CV− | HE | HE | HE | HE |
| Δ FEV1, % | 5.89 | 18.40 | 13.02 | 29.30 | 17.80 | 15.01 | 6.80 | 2.15 | 12.36 |
| Δ RV (mL) | −180 | −520 | −400 | −670 | −831 | −370 (NS) | −94 | −380 | −360 |
| Δ SGRQ | −0.83 | −7.05 | −7.47 | −6.50 | −14.62 | −10.50 | −3.40 | −3.56 | −12.90 |
| Δ mMRC | 0 | −0.35 | −0.41 | −0.56 | −0.22 (NS) | −0.37 | −0.30 | −0.10 (NS) | −0.80 |
| Δ 6MWD (m) | 22 | 39.28 | 28.40 | 78.70 | 74.00 | 36.40 | 20.00 | 20.62 | 6.90 (NS) |
| Severe exacerbations | 0.61 (NS) | −0.30 (NS) | 1.22 | 0.15 (NS) | 0.76 (NS) | 0.84 (NS) | 2.13 | 1.24 (NS) | 0.58 (NS) |
| Pneumotorax | 0.74 (NS) | 4.12 | 3.90 | 2.99 | 2.76 (NS) | 1.57 (NS) | 2.21 (NS) | 1.92 (NS) | 2.85 |
| Mortality | 1.69 (NS) | 0.91 (NS) | −1.40 (NS) | 0.41 (NS) | 1.13 (NS) | −1.81 (NS) | 0.84 (NS) | 1.78 (NS) | −0.65 (NS) |
Δ: improvement compared to SoC arm; CV: collateral ventilation; EBV: endobronchial valve; HE: heterogeneous emphysema distribution; HO: homogeneous emphysema distribution; m: meters; mL: milliliters; NS: not significant; RCT: randomized control trial; SoC: standard of care.
When multiple publications reported outcomes from the same trial, only one source (typically the version with the largest sample size or longest follow-up with a valid control group) was used for the meta-analysis. Additional publications remained in the table for reference but were not included in the quantitative synthesis.
Risk of bias (RoB 2) assessment of included studies.
| Author name, year | Sequence generation | Allocation concealment | Blinding of participants and personnel | Blinding of assessment | Missing data | Selective reporting | Other bias* |
|---|---|---|---|---|---|---|---|
| BeLieVeR-HIFi (Davey, 2015) | |||||||
| LIBERATE (Criner, 2018) | ////////////// | ////////////// | |||||
| IMPACT (Valipour, 2016) | ////////////// | ||||||
| TRANSFORM (Kemp, 2017) | ////////////// | ||||||
| STELVIO (Klooster, 2016) | ////////////// | ||||||
| REACH (Li, 2018) | ////////////// | ||||||
| VENT (Sciurba, 2010) | ////////////// | ||||||
| IBV (Wood, 2014) | ////////////// | ////////////// | |||||
| EMPROVE (Criner, 2019) | ////////////// |
* Other bias refers to bias due to problems not covered elsewhere in the table (e.g. the study had a potential source of bias related to the specific study design used, or there is insufficient information to assess whether an important risk of bias exists, or insufficient rationale or evidence that an identified problem will introduce bias).
White: low risk of bias; pattern ///////: unclear risk of bias; black: high risk of bias.
Our metanalysis revealed that patients who received EBV showed an increase in baseline FEV1 (WMD=12.73%, 95% CI: 4.73–18, p<0.001; I2=98.29%, p<0.001) compared to the standard of care (SOC) group (Fig. 2a).
According to the Q-test, the true outcomes appear to be heterogeneous (Q(8)=131.8395, p<0.0001, tau2=56.3952, I2=98.2905%). A 95% prediction interval of −2.8964 to 28.3662 was found for the true outcomes; hence, although the average outcome is estimated to be positive, in some studies the true outcome may in fact be negative. An examination of the studentized residuals revealed that none of the studies had a value greater than ±2.7729, so there was no indication of outliers in the context of this model. The Cook's distances showed that none of the studies could be considered to be overly influential. The regression test indicated funnel plot asymmetry (p=0.0014) but not the rank correlation test (p=0.4767).
Changes in VR (mL)RV showed a statistically significant reduction following EBV therapy, with a WMD of −413.35mL (95% CI: −591.59 to −235.11; p<0.001). This indicates effective deflation of hyperinflated lungs, a core therapeutic target in emphysema management. Heterogeneity remained high (I2=96.91%), highlighting the differences in patient selection and procedural approaches across studies (Fig. 2b).
Changes in the SGRQ (score)Quality of life, assessed by the SGRQ, improved significantly with EBV therapy. The WMD was −7.16 points (95% CI: −10.39 to −3.93; p<0.001). Heterogeneity (I2=97.24%) suggested variations in subjective outcomes across populations (Fig. 2c).
Changes in 6MWTEBV therapy significantly improves exercise capacity, as measured by the 6MWT. The WMD was 35.37m (95% CI: 19.12–51.63; p<0.001), reflecting enhanced physical endurance and mobility in treated patients. Heterogeneity was substantial (I2=96.83%), and was potentially linked to baseline differences in exercise tolerance and comorbidities (Fig. 2d).
Changes in the mMRC dyspnea scaleDyspnea severity improved significantly in the EBV group compared to SOC, with a WMD of −0.35 grades (95% CI: −0.52 to −0.17; p<0.001). This outcome reflects better symptom control in treated patients, supporting the efficacy of EBV in relieving breathlessness. Heterogeneity (I2=75.96%) was moderate compared to other endpoints (Fig. 2e).
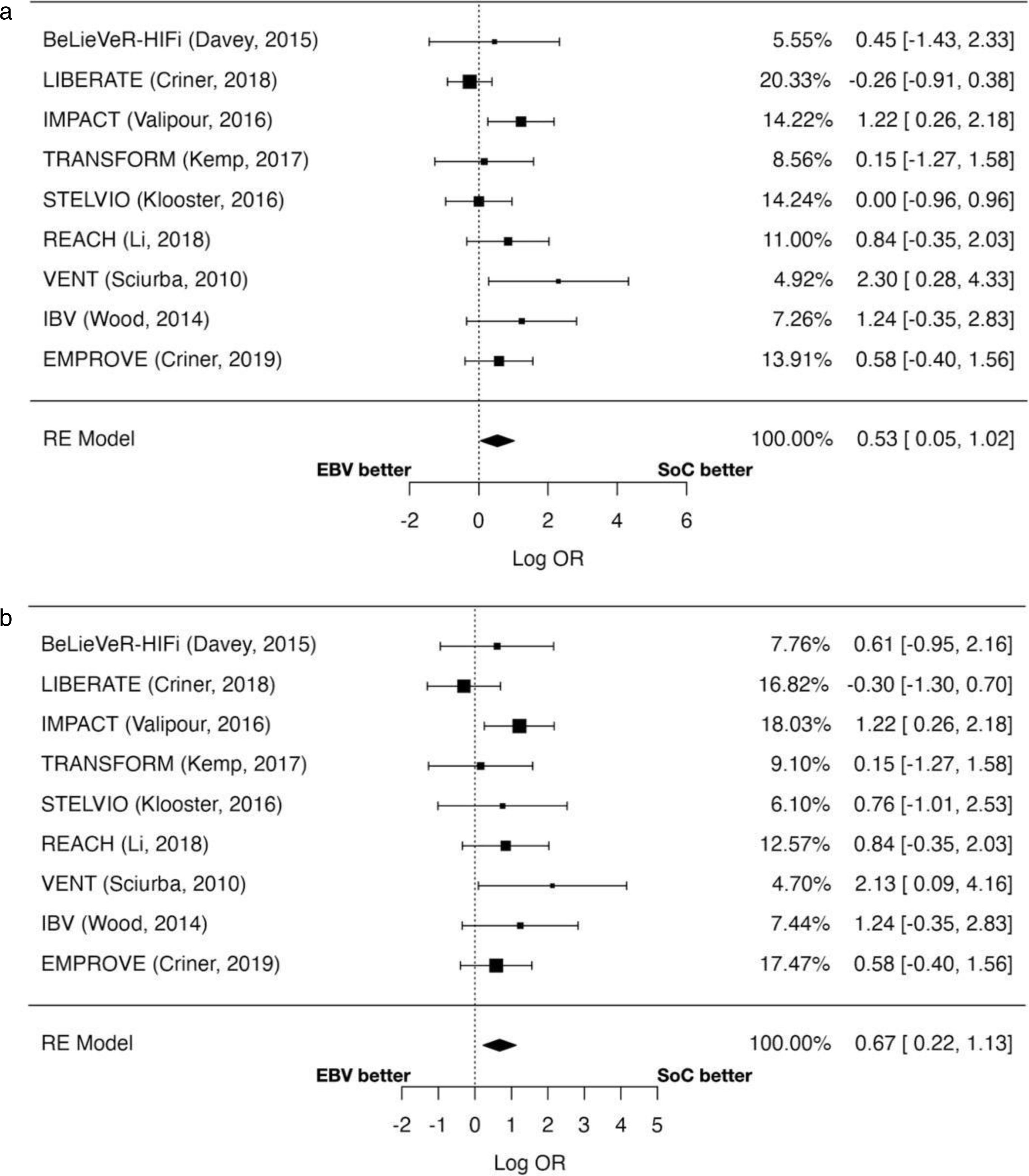
Moderate and severe exacerbationsThe odds of experiencing moderate-to-severe exacerbations were significantly higher in the EBV group (OR=1.71; 95% CI: 1.05–2.78; p=0.032) (Fig. 3a). The risk of severe exacerbations was also increased risk (OR=1.96; 95% CI: 1.25–3.08; p=0.003) (Fig. 3a). Heterogeneity was moderate for moderate-to-severe exacerbations (I2=37.93%) and low for severe exacerbations (I2=11.48%).
All-cause mortalityMortality rates did not differ significantly between the EBV and SOC groups (OR=1.78; 95% CI: 0.69–4.61; p=0.236). Heterogeneity was minimal (I2=0%), indicating consistent findings across studies (Fig. 3c).
Risk of pneumothoraxThe incidence of pneumothorax was significantly elevated in patients receiving EBV (OR=12.31; 95% CI: 4.81–31.58; p<0.001). This highlights the need for vigilant post-procedural monitoring, as pneumothorax is a known complication linked to lobar collapse. No significant heterogeneity was observed (I2=0%) (Fig. 3d).
DiscussionThe results of this meta-analysis provide substantial evidence supporting the efficacy and safety profile of BLVR-EBV therapy in patients with severe emphysema, particularly those with heterogeneous emphysema and intact interlobar fissures. The findings align with prior studies and metanalyses,12–17 and show that EBV is a valid minimally invasive alternative to surgical lung volume reduction. However, several critical points warrant discussion, particularly regarding the interpretation of outcomes, variability in findings, and implications for clinical practice. Our findings are consistent with previous meta-analyses, which also reported improvements in lung function and adverse events following EBV treatment. However, our study adds value by incorporating the latest RCTs published up to 2024 and by providing a more detailed safety analysis that includes exacerbations, pneumothorax, and mortality in a unified model. Importantly, previously published meta-analyses have included overall exacerbations but did not differentiate between total and severe exacerbations. This distinction is a novel contribution of our study, and gives a more detailed understanding of the safety profile of EBV therapy. This comprehensive approach allows for a broader assessment of both benefits and risks in a real-world clinical context.18
The significant improvement in FEV1 (weighted mean difference [WMD]=12.73%, 95% CI: 7.47–17.99, p<0.001) shows the physiological benefits of EBV therapy. Enhanced lung deflation, as indicated by reduced residual volume (RV; WMD=−413.35mL, 95% CI: −591.59 to −235.11, p<0.001), further corroborates the efficacy of this intervention in mitigating hyperinflation. These outcomes are clinically meaningful, given that reduced hyperinflation directly improves respiratory mechanics, leading to enhanced gas exchange and reduced dyspnea. The improvement in the 6-minute walk distance (6MWD; WMD=35.37m, 95% CI: 19.12–51.63, p<0.001) shows that functional capacity was improved. Patient-reported outcomes, including the SGRQ and mMRC dyspnea scale, reflect significant symptomatic relief and quality-of-life improvements. These findings are consistent with the mechanism of EBV therapy, which targets hyperinflation to alleviate the ventilatory load, which in tun reduces breathlessness and improves activity levels. Although the observed increase may not meet the minimal clinically important difference (MCID) threshold in all patients, the cumulative impact of improvements across multiple domains (e.g., dyspnea, quality of life) likely contributes to a significant overall benefit. This is particularly important in a population characterized by severe functional limitations. While the benefits of EBV therapy are evident, the increased risk of pneumothorax and exacerbations must be taken into consideration. Pneumothorax, a well-documented complication of lobar collapse, shows the importance of post-procedural monitoring and patient selection. Strategies such as limiting volume reduction to high-risk lobes and optimizing procedural techniques may mitigate this risk. The increased incidence of moderate-to-severe exacerbations warrants further investigation. It remains unclear whether these exacerbations are directly attributable to the intervention or reflect a natural progression of disease in a high-risk cohort.
The high heterogeneity (I2>90%) observed in primary outcomes such as FEV1 and RV suggests variability in study populations, procedural protocols, and follow-up durations. Differences in patient characteristics – including baseline hyperinflation severity, distribution of emphysema, and presence of comorbidities – may partially account for these findings. Additionally, procedural factors such as the type of valve used (Zephyr vs Spiration) and operator experience likely influenced outcomes. Sensitivity analyses excluding single-center studies or those with short follow-up durations confirmed the robustness of the pooled estimates, suggesting that the observed benefits are generalizable across diverse settings.
Our meta-analysis should be interpreted together with the evidence from similar studies. It is particularly important because it includes all available RCTs up to 2024,17–30 and therefore provides the most comprehensive evaluation to date of the efficacy and safety of EBV therapy. Unlike previous reviews, it is not only focuses on improvements in lung function, exercise capacity, and quality of life, but also systematically analyzes safety outcomes, including pneumothorax, exacerbations, and mortality. However, certain limitations should be acknowledged. High heterogeneity in primary outcomes reduces the precision of pooled estimates, and the exclusion of non-English studies may introduce language bias. Additionally, the reliance on short- to medium-term follow-up data limits the generalizability of long-term conclusions. A key limitation is the inability to determine the timing of adverse events (early post-intervention or during longer-term follow-up), which limits our ability to fully understand causality and the natural progression of disease in high-risk patients. Standardized reporting of adverse event timing in future trials would improve the interpretability of safety outcomes.
ConclusionThis meta-analysis highlights the efficacy and safety of BLVR-EBV therapy as a transformative intervention for selected patients with severe emphysema. While the benefits in lung function, exercise capacity, and quality of life are compelling, the associated risks show the need for meticulous patient selection and procedural execution. Future research should aim to refine patient selection criteria, enhance procedural safety, and elucidate long-term outcomes to maximize the therapeutic potential of this innovative approach.
Ethics committeeDue to the characteristics of the study no ethics committee approval was required.
Compliance with ethical standards ethical approvalThis research involves data extracted from already published literature and hence no human or animal subjects were directly involved.
Declaration of generative AI and AI-assisted technologiesNot used in the preparation of the manuscript.
Informed consentNo informed consent was deemed necessary.
FundingThis research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Authors’ contributionsJD-M and BA-N: conception and design of the study, writing the core content of the study, analysis and interpretation of data, drafting the article and revising it critically for important intellectual content. JD-M, MJ-G and EC-M: proposed the search strategy for the articles in the different selected databases; retrieved the selected articles, eliminated repeats, and performed the initial screening. Any difference in opinion about eligibility was resolved by a third independent reviewer BA-N. JD-M: statistical analysis and interpretation of data, preparation and critical review of the manuscript. AR-L, JIDG-OAC-V, PRJ-P: critical review of the manuscript. All authors approved the current version of the manuscript.
Conflicts of interestJDM has nothing to disclosure. ARL has nothing to disclosure. MJG has nothing to disclosure. ECM has nothing to disclosure. JIDGO has received honoraria for lecturing, scientific advice, participation in clinical studies or writing for publications from the following (alphabetical order): Aflofarm, Adamed, AstraZeneca, Boehringer, Chiesi, Esteve, Faes, Gebro, Menarini, and Pfizer. ACV has received honoraria for lecturing from GSK, PulmonX. PJRP reports honoraria or attendance fees from GSK, Novartis, Boehringer Ingelheim, Chiesi, Menarini and Esteve. BAN reports fees for lectures and advisory boards and grants from AstraZeneca, and Boehringer.