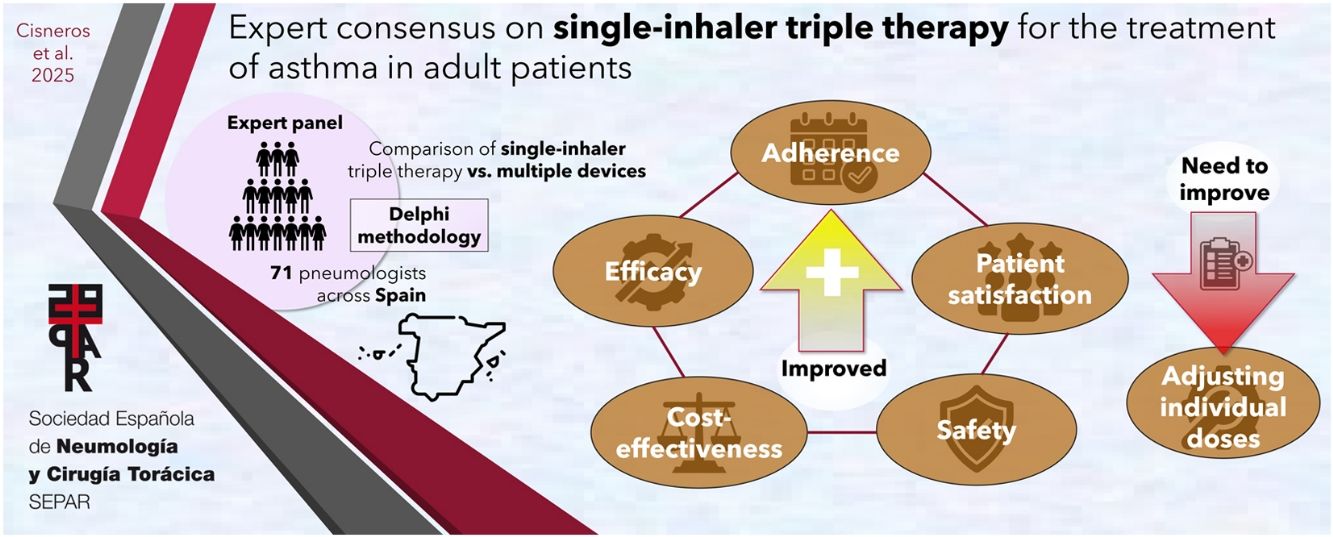
Despite existing guidelines, many patients with asthma do not achieve adequate control of their disease. This is largely due to low adherence to inhaled therapy. Single-inhaler administration may improve this and other aspects of asthma therapy, such as cost-effectiveness. The aim of this study is to gather the opinions of a panel of experts on single-inhaler triple therapy (SITT) for the treatment of asthma in adult patients.
Material and methodsA recommendation task force reviewed the available evidence and formulated 45 statements divided into 5 sections: efficacy, cost-effectiveness, therapeutic adherence, safety, and patient satisfaction. A validation task force of 71 experts evaluated these recommendations using the 2-round Delphi technique. Panellists rated their agreement with each statement on a 9-point scale.
ResultsConsensus was achieved in 42 of the 45 statements. Panellists broadly agreed that SITT improves lung function, reduces exacerbations, is cost-effective, and promotes therapeutic adherence. The safety of TT was considered favourable, even in patients with cardiovascular comorbidities. The panellists also agreed on the importance of evaluating patient satisfaction with the inhaler. However, no consensus was reached regarding the suitability of TT as a first-line treatment, nor on whether TT is more beneficial than up-dosing ICS in patients with a low inflammatory profile currently receiving dual therapy. Additionally, panellists did not agree on whether sick leave due to respiratory causes was associated with greater adherence to TT.
ConclusionsThe consensus indicates that SITT is considered an effective, safe option for the treatment of asthma that improves therapeutic adherence and patient satisfaction. Further real-world studies are needed to evaluate its implementation in different clinical contexts.
A pesar de las directrices existentes, muchos pacientes con asma no logran un control adecuado de la enfermedad. Esto se debe en gran parte a la baja adhesión a la terapia inhalada. La administración en dispositivo único puede mejorar este y otros aspectos, como coste-efectividad del tratamiento. Este estudio tiene como objetivo desarrollar un consenso de expertos sobre la triple terapia (TT) en un solo inhalador para el tratamiento del asma en pacientes adultos.
Material y métodosUn grupo elaborador de recomendaciones revisó la evidencia disponible y formuló 45 enunciados divididos en cinco bloques: eficacia, coste-efectividad, adhesión, seguridad y satisfacción del paciente. Un grupo validador de 71 expertos evaluó estas recomendaciones mediante el método Delphi en dos rondas. Los panelistas calificaron cada enunciado en una escala de 9 puntos para determinar su grado de acuerdo.
ResultadosSe alcanzó consenso en 42 de los 45 enunciados. Los panelistas mostraron amplio acuerdo en la eficacia de la TT en dispositivo único para mejorar la función pulmonar y reducir exacerbaciones, así como en su buen perfil de coste-efectividad y de adhesión al tratamiento. La seguridad de la TT fue considerada favorable, incluso en pacientes con comorbilidades cardiovasculares. Los panelistas también estuvieron de acuerdo en la importancia de la satisfacción del paciente con el dispositivo inhalador. Sin embargo, no se alcanzó consenso sobre la idoneidad de la TT como tratamiento de primera línea, ni sobre si la TT es más beneficiosa que el aumento de dosis de CSI en pacientes con un perfil inflamatorio bajo que ya reciben doble terapia. Además, los panelistas no estuvieron de acuerdo en que la baja laboral por causas respiratorias estuviera asociada con una mayor adhesión a la TT en dispositivo único.
ConclusionesEl consenso indica que la TT en un solo dispositivo se considera una opción efectiva y segura para el tratamiento del asma, mejorando la adherencia y la satisfacción del paciente. Se destaca la necesidad de más estudios en vida real para evaluar su implementación en diferentes contextos clínicos.
Asthma is a chronic condition characterised by inflammation of the respiratory tract.1 Its main pathophysiological features are bronchial hyperreactivity, inflammation, and fluctuating airflow obstruction. The disease affects more than 260 million people worldwide,2 including approximately 2 million adults in Spain (more than 5% of the population).3 Asthma treatment involves controlling symptoms and reducing the risk of death and serious complications, such as lung function decline, exacerbations, and the adverse effects (AEs) of treatment.4 Both national and international guidelines recommend a stepwise approach to treatment to achieve these goals.4,5
Nevertheless, despite these guidelines and the availability of effective therapies, a considerable proportion of patients still do not achieve disease control.6,7 One of the main factors behind this suboptimal control is low adherence to inhaled therapy, which is associated with an increase in asthma-related morbidity and mortality, and a greater use of healthcare services.8 For this reason, continuous monitoring is essential to maximise the benefits of treatment. Asthma should be managed from a more personalised perspective based on the identification and evaluation of treatable traits, such as eosinophilic airway inflammation, airflow limitation, and tendency towards exacerbations, together with other parameters such as adherence and inhalation technique.9,10
First-line therapy for long-term asthma control recommended by the guidelines consists of a combination of long-acting β-2 agonist (LABA) bronchodilators and inhaled corticosteroids (ICS),4,5 known as dual therapy (DT).11 In adult patients with severe uncontrolled asthma despite DT with high-dose LABA + ICS, guidelines recommend adding a long-acting muscarinic antagonist (LAMA), since well-designed studies have shown that this triple therapy (TT) reduces exacerbations and improves lung function.4,5,12,13 The safety of inhaled TT has been evaluated in several studies,14–17 and in recent years, combinations of these 3 drugs have appeared in a single inhaler for use once or twice daily.18,19
Simplifying treatment in a single inhaler versus open TT may improve adherence,20 and the availability of cost-effective treatments for asthma is beneficial for both patients and healthcare systems.21 However, evidence on these key issues is still scarce, and the clinical positioning of open or closed TT is not well established. Research into TT for the management of asthma and chronic obstructive pulmonary disease (COPD) has provided consistent evidence on patient preference for and satisfaction with different inhalation devices.22,23 Studies show that patient satisfaction with the inhaler plays a crucial role in therapeutic adherence and effectiveness.24 The choice of inhaler, the patient's ability to use it correctly, and overall satisfaction with the device are critical aspects to consider when prescribing inhaled treatments.25
Although these studies suggest that simplifying the method of treatment delivery in a single inhaler could improve adherence and asthma control, there is no clear expert consensus on this issue. Recommendations based on expert consensus are useful in situations where evidence is scarce or insufficiently clear to establish guidelines. In the current context of TT, we believe that an expert opinion study can play an important role in guiding decision-making in clinical practice and the generation of more evidence for future recommendations. Therefore, the aim of this project was to develop a Delphi consensus statement on single-inhaler TT (SITT) for the treatment of asthma. Key aspects such as efficacy, cost-effectiveness, adherence, and patient satisfaction were evaluated comparing SITT with multi-inhaler therapy.
Material and methodsA recommendation task force (RTF) consisting of 10 experts from the Spanish Society of Pulmonology and Thoracic Surgery (SEPAR, in its Spanish acronym) was formed for the purpose of reviewing and synthesising the evidence available up to the end of 2023 and formulating conclusions and recommendations. After discussing and reviewing these in a virtual meeting, the task force drafted a questionnaire containing 45 statements divided into 5 sections: 1, efficacy (15 statements); 2, cost-effectiveness (5 statements); 3, adherence (10 statements); 4, safety (6 statements); and 5, patient satisfaction (9 statements). A validation task force (VTF) of 71 experts in asthma from different autonomous communities was recruited as panellists by the RTF to validate the conclusions and recommendations formulated (Table 1 and Supplementary Table 1). Experts were selected based on their recognised expertise in asthma management, as demonstrated by their scientific contributions (e.g., publications, participation in research projects, and involvement in national and international guidelines) and their clinical experience in treating asthma patients. The RTF sought to ensure a balanced representation of specialists from different regions to obtain a representative panel of the Spanish population.
Sociodemographic characteristics of the expert panel.
| Autonomous community, n (%) | |
| Andalucía | 16 (22.5%) |
| Aragón | 2 (2.8%) |
| Asturias | 3 (4.2%) |
| Baleares | 2 (2.8%) |
| Canarias | 3 (4.2%) |
| Cantabria | 1 (1.4%) |
| Castilla La Mancha | 1 (1.4%) |
| Castilla y León | 3 (4.2%) |
| Cataluña | 10 (14.1%) |
| Comunidad Valenciana | 8 (11.3%) |
| Extremadura | 2 (2.8%) |
| Galicia | 5 (7.0%) |
| La Rioja | 0 (0.0%) |
| Madrid | 10 (14.1%) |
| Murcia | 3 (4.2%) |
| Navarra | 0 (0.0%) |
| País Vasco | 2 (2.8%) |
| Total | 71 |
| Age, mean±SD | 52.61±10.11 |
| Male/female, n (%) | 38/33 (53.5%/46.5%) |
| Years of specialty, mean±SD | 23.7±9.87 |
SD: standard deviation.
RAND/UCLA methodology was used for the consensus process and analysis.26 The VTF evaluated the findings and recommendations remotely using an interactive 2-round Delphi process. During each round, panel members were asked to rate their degree of agreement or disagreement with each statement on a 9-point scale, where 1=“totally disagree” and 9=“totally agree”.27 Between rounds, members of both the VTF and RTF met in a virtual consensus conference to assess the results of the first round, hear the panellists’ comments, and discuss the non-consensus statements in light of these comments and the opinion of the experts in the RTF. At this meeting, the RTF considered the possibility of changing the wording of some statements. Only the statements that did not achieve consensus in the first round were re-evaluated in the second round.
Following the RAND/UCLA manual, recommendations were classified as inappropriate, uncertain, or appropriate, depending on whether they achieved a mean score of 1–3, 4–6, or 7–9, respectively. To reach consensus on the “appropriateness” or “inappropriateness” of a recommendation, at least two-thirds of the panellists had to have rated the statement within the range that includes the median. A statement was considered controversial when more than one third of the scores fell in the range opposite to that containing the median, and neutral if it did not meet either of the foregoing criteria. The data were analysed using the statistical package R version 4.3.2 (R Core Team, 2024).28
ResultsSeventy-five of the 93 experts invited to join the panel accepted the invitation, and 71 (94.7%) completed both rounds of the Delphi questionnaire. The sociodemographic characteristics of the panel are described in Table 1. Forty-two of the 45 statements (93.3%) were validated by the panel of experts. The results of the consensus are shown in Table 2.
Results of the Delphi questionnaire. Statements with no agreement are highlighted in grey.
| Statement | Median | Appropriateness | Agreement | Round |
|---|---|---|---|---|
| Section 1: Efficacy | ||||
| 1. Triple therapy (LABA/LAMA/ICS) improves lung function in patients with severe uncontrolled asthma (SUA) on ICS/LABA | 8 | Appropriate | Agreement | 1 |
| 2. Triple therapy (LABA/LAMA/ICS) reduces the rate of exacerbations in patients with SUA on ICS/LABA | 8 | Appropriate | Agreement | 1 |
| 3. Triple therapy (LABA/LAMA/ICS) reduces the rate of exacerbations in patients with SUA on ICS/LABA regardless of their T2 phenotype | 7 | Appropriate | Agreement | 1 |
| 4. Both single-inhaler triple therapy (LABA/LAMA/ICS) and dual therapy (ICS/LABA) are suitable as first-line treatment to achieve asthma control in previously untreated (naive) patients receiving step 4 care or higher | 7 | Uncertain | Disagreement | 2 |
| 5. Triple therapy (LABA/LAMA/ICS) may be especially recommended in patients with asthma not controlled with medium- or high-dose ICS and a history of smoking | 8 | Appropriate | Agreement | 1 |
| 6. Triple therapy (LABA/LAMA/ICS) may be especially recommended in patients with asthma not controlled with medium- or high-dose ICS/LABA and with persistent airflow limitation (PAL) | 8 | Appropriate | Agreement | 1 |
| 7. The addition of LAMA to medium-dose ICS/LABA could be considered an alternative to increasing the ICS dose in adherent patients with uncontrolled asthma and without increased blood eosinophils | 8 | Appropriate | Agreement | 1 |
| 8. Before starting biological therapy in patients with uncontrolled asthma, it is advisable to treat with triple therapy (LABA/LAMA/ICS) with high-dose ICS | 9 | Appropriate | Agreement | 1 |
| 9. When asthma is controlled with high-dose triple therapy, de-escalation to medium-dose triple therapy may be considered rather than switching to high-dose ICS/LABA | 8 | Appropriate | Agreement | 1 |
| 10. In adherent patients with non-T2 asthma that is poorly controlled despite medium-dose ICS/LABA, we should consider starting single-inhaler triple therapy (LABA/LAMA/ICS) with medium-dose ICS to avoid exacerbations | 8 | Appropriate | Agreement | 1 |
| 11. In adherent patients with poorly controlled asthma despite high-dose ICS+LABA, we could consider starting high-dose single-inhaler triple therapy (LABA/LAMA/ICS) before starting oral corticosteroids or biological agents, particularly in patients with PAL and/or severe exacerbations. | 9 | Appropriate | Agreement | 1 |
| 12. In patients with good asthma control on high-dose ICS/LABA or on single-inhaler triple therapy (LABA/LAMA/ICS) with high-dose ICS but at risk of ICS-related adverse effects, consider switching to single-inhaler triple therapy with medium-dose ICS. | 8 | Appropriate | Agreement | 1 |
| 13. Adding a LAMA in patients with uncontrolled asthma, airflow limitation, and low inflammatory profile on medium-dose ICS/LABA is more beneficial than increasing the ICS dose | 7 | Appropriate | Neutral | 2 |
| 14. Triple therapy with indacaterol/glycopyrronium/MF improves lung function and reduces asthma exacerbations compared with dual therapy (indacaterol/MF) regardless of baseline blood eosinophil levels | 8 | Appropriate | Agreement | 1 |
| 15. Fixed dose indacaterol/glycopyrronium/MF once daily is effective in patients with persistent asthma with and without chronic airflow limitation | 8 | Appropriate | Agreement | 1 |
| Section 2: Cost-effectiveness | ||||
| 16. In adults with asthma previously uncontrolled with LABA + medium-dose ICS, single-inhaler triple therapy with medium-dose ICS (beclomethasone/formoterol/glycopyrronium) has a more favourable cost-effectiveness profile than beclomethasone/formoterol in a single inhaler | 7 | Appropriate | Agreement | 2 |
| 17. In adults with asthma previously uncontrolled with high-dose LABA/ICS, single-inhaler triple therapy (beclomethasone/formoterol/glycopyrronium) with high-dose ICS is more cost-effective than beclomethasone/formoterol in a single inhaler | 7 | Appropriate | Agreement | 1 |
| 18. In adults with asthma, single-inhaler triple therapy (beclomethasone/formoterol/glycopyrronium) with high-dose ICS is more cost-effective than beclomethasone/formoterol in a single inhaler/tiotropium in a second inhaler | 8 | Appropriate | Agreement | 1 |
| 19. Cost-effectiveness studies are needed to promote the sustainability of the healthcare system | 9 | Appropriate | Agreement | 1 |
| 20. There is a need for independent cost-effectiveness studies of single-inhaler triple therapy in asthma patients that measure the real situation in each country and assess real-world data | 9 | Appropriate | Agreement | 1 |
| Section 3: Therapeutic adherence | ||||
| 21. Both adherence and persistence must be accurately assessed in the follow-up of patients with asthma, and the use of electronic devices is recommended | 8 | Appropriate | Agreement | 2 |
| 22. Adherence to inhaled therapy should be considered a treatable trait in asthma and requires individualised treatment adaptation | 9 | Appropriate | Agreement | 1 |
| 23. Adding a LAMA to the LABA/ICS combination using multiple inhalers may contribute to suboptimal Adherence to inhaled therapy should be considered a treatable trait in asthma and requires individualised treatment adaptation | 8 | Appropriate | Agreement | 1 |
| 24. Adherence and persistence with inhaled therapy is greater when using single-inhaler triple therapy (LABA/LAMA/ICS) | 8 | Appropriate | Agreement | 1 |
| 25. Once-daily dosing of single-inhaler triple therapy (LABA/LAMA/ICS) promotes therapeutic adherence | 8 | Appropriate | Agreement | 1 |
| 26. Administering LABA/ICS and a LAMA in a single inhaler promotes adherence and persistence with asthma treatment | 9 | Appropriate | Agreement | 1 |
| 27. Single-inhaler triple therapy (LABA/LAMA/ICS) reduces the risk of asthma exacerbations because the combination of lower doses in a single inhaler reduces critical errors | 8 | Appropriate | Agreement | 1 |
| 28. Non-adherence is a treatable trait of asthma patients that can be identified to optimise the use of single-inhaler triple therapy (LABA/LAMA/ICS) | 8 | Appropriate | Agreement | 1 |
| 29. Poor adherence associated with certain asthma comorbidities such as anxiety/depression could be improved with single-inhaler triple therapy (LABA/LAMA/ICS) | 8 | Appropriate | Agreement | 2 |
| 30. Sick leave due to respiratory illness is associated with greater adherence to single-inhaler triple therapy (LABA/LAMA/ICS) | 5 | Uncertain | Neutral | 2 |
| Section 4: Safety | ||||
| 31. Single-inhaler triple therapy (LABA/LAMA/ICS) is safe, irrespective of the age of the asthma patient | 8 | Appropriate | Agreement | 2 |
| 32. Single-inhaler triple therapy (LABA/LAMA/ICS) may make it difficult to adjust the dose of the different components | 7 | Appropriate | Agreement | 1 |
| 33. Single-inhaler triple therapy (LABA/LAMA/ICS) has a safety profile that is at least as favourable as open triple therapy (with multiple inhalers) in the adult population | 8 | Appropriate | Agreement | 1 |
| 34. Single-inhaler triple therapy (LABA/LAMA/ICS) should not be interrupted during pregnancy in asthma patients | 8 | Appropriate | Agreement | 1 |
| 35. Single-inhaler triple therapy (LABA/LAMA/ICS) should not be interrupted during breastfeeding in patients with asthma | 8 | Appropriate | Agreement | 1 |
| 36. Single-inhaler triple therapy (LABA/LAMA/ICS) can be indicated in patients with asthma and cardiovascular comorbidity | 8 | Appropriate | Agreement | 1 |
| Section 5: Patient satisfaction | ||||
| 37. Satisfaction with the inhalation device among asthma patients is not adequately evaluated in clinical studies or in routine clinical practice | 8 | Appropriate | Agreement | 1 |
| 38. Several validated questionnaires are available to measure patient satisfaction with inhalation devices (e.g. FSI-10 or PASAPQ), but they are not implemented | 8 | Appropriate | Agreement | 1 |
| 39. Studies measuring asthma patient satisfaction with their inhaler show very heterogeneous results | 8 | Appropriate | Agreement | 1 |
| 40. One of the factors that can influence patient satisfaction with the inhalation device is the molecule it contains | 7 | Appropriate | Agreement | 2 |
| 41. One of the factors that can influence patient satisfaction with the inhalation device is the prescribed dose | 8 | Appropriate | Agreement | 1 |
| 42. To date, no well-designed studies have measured the degree of satisfaction of asthma patients with the various single-inhaler triple therapy options available on the market | 8 | Appropriate | Agreement | 1 |
| 43. It is advisable to adapt to the patient's needs and preferences and explore which treatable traits can be addressed with the different treatment combinations available on the market (dose, regimen, molecules, device, and particle size) | 9 | Appropriate | Agreement | 1 |
| 44. Single-inhaler therapy should be prioritised in patients with low adherence | 9 | Appropriate | Agreement | 1 |
| 45. One of the factors that can influence patient satisfaction with single-inhaler triple therapy is the speed with which the disease is controlled | 8 | Appropriate | Agreement | 1 |
Panellists were in broad agreement with statements regarding the efficacy of TT, specifically with regard to improved lung function and reduced exacerbations compared with DT (regardless of T2 phenotype), especially in patients with a history of smoking, persistent airflow limitation (PAL), and no increased blood eosinophils (Table 2). However, although experts did not doubt the effectiveness of these combinations, there was no consensus on the suitability of TT as first-line treatment, or on the statement that TT is more beneficial than up-dosing ICS in patients with a low inflammatory profile currently receiving DT.
Section 2: Cost-effectivenessThe panellists agreed with all statements about the cost-effectiveness of TT (Table 2). Specifically, there was agreement that SITT (beclomethasone/formoterol/glycopyrronium) with medium/high doses of ICS has a more favourable cost-effectiveness profile than single-inhaler DT (beclomethasone/formoterol). The experts consider it important to perform further independent cost-effectiveness studies that use real-world data to evaluate SITT in asthmatic patients in the context of each country.
Section 3: AdherenceThere was also broad agreement on statements about the benefits of TT in improving therapeutic adherence (Table 2). The panellists consider it appropriate to approach adherence as a treatable trait of asthma and to monitor it accurately, preferably using electronic devices. However, panellists did not agree that sick leave due to respiratory causes was associated with greater adherence to SITT.
Section 4: SafetyThe panellists reached consensus on all statements about the safety of TT (Table 2). They agreed that its safety profile is at least as favourable as DT, regardless of the patient's age, and that it should not be discontinued during pregnancy, breastfeeding, or in patients with cardiovascular (CV) comorbidities. However, experts also agreed that it may be difficult to adjust the dosage of the different TT components when using a single inhaler.
Section 5: Patient satisfactionIn this section there was also total agreement among the panellists (Table 2). Clinicians are advised to adapt to the patient's needs and preferences and explore which treatable traits can be addressed with the different treatment combinations available on the market, prioritising single-inhaler treatment in patients with low therapeutic adherence. The panellists also agreed that there are few studies that analyse asthma patient satisfaction with the different inhalation devices on the market, since this factor is not properly evaluated in either clinical studies or routine clinical practice.
DiscussionEfficacyInstead of dose escalation and starting biologics, some studies have evaluated the addition of a LAMA as a viable alternative to increasing the dose of ICS in patients who do not achieve adequate asthma control. The IRIDIUM, ARGON and CAPTAIN studies showed that the addition of a LAMA to the ICS/LABA combination significantly improves several parameters compared to conventional dual therapies (salmeterol+fluticasone propionate), including forced expiratory volume in one second (FEV1).29–31 Although TT may be particularly recommendable in patients with PAL and uncontrolled asthma despite medium- or high-dose ICS/LABA, a subanalysis of the IRIDIUM study showed that patients without PAL also benefited from TT.32 In addition to improving lung function, some studies have shown that TT substantially reduces the rate of exacerbations. In the TRIMARAN and TRIGGER studies, the combination of beclomethasone dipropionate/formoterol fumarate/glycopyrronium bromide (BDP/FF/GB) significantly reduced exacerbations in patients with uncontrolled asthma compared with DT with BDP/FF.33
Compared with standard DT, TT has been shown to produce these effects irrespective of initial eosinophil levels,31,34 extending the time to the first severe exacerbation and providing more effective and long-lasting asthma control.35,36 This means that TT may waive the need for more intensive treatments in some cases, depending on the profile of each patient and their treatable traits. If good asthma control is achieved with this strategy, clinicians can consider down-dosing ICS in patients with a low T2 profile, thereby reducing the AEs of high-dose corticosteroids.37 However, there was no consensus on the statement that in patients with PAL, a low T2 profile, and uncontrolled asthma despite a combination of ICS/LABA with medium doses of ICS, adding a LAMA is more beneficial than increasing the dose of ICS, since there is insufficient evidence to support this strategy.
It is important to note that the experts did not consider TT an appropriate first-line treatment in a naive patient (or even in patients on step 4 or higher); instead, treatment should start with ICS/LABA and a LAMA added depending on the response obtained. Although SITT is emerging as a robust, effective option for asthma management, it would be important to promote the development of inhalers containing different dose combinations of the 3 components, so that clinicians can adjust the intensity of the treatment in a personalised manner according to the response of each patient.
Cost-effectivenessStudies evaluating the cost of health interventions provide information that can help shape evidence-based healthcare policies. Although there are few cost-effectiveness analyses in asthma, there is evidence from one study in England supporting that SITT is more cost-effective than multiple inhalers. In this study, Orlovic et al. 38 analysed the cost-effectiveness of extrafine beclomethasone dipropionate/formoterol fumarate/glycopyrronium in patients with uncontrolled asthma based on the cost per exacerbation in England. The study included patients with uncontrolled asthma under treatment with LABA+ICS (at medium and high doses) who received either a second inhaler with a LAMA or SITT with a LAMA. Both medium- and high-dose BDP/FF/G were found to have an improved cost-effectiveness ratio than BDP/FF, and high-dose BDP/FF/G had a better cost-effectiveness ratio than BDP/FF+tiotropium in a second inhaler. However, in the Spanish healthcare model, these results may differ due to various factors, such as drug prices, different service costs, or prescribing habits, among others.
Since the evidence in asthma is scarce, studies of cost-effectiveness comparing DD with TT in patients with COPD could help shed light on the topic. A systematic review analysed cost-effectiveness studies of SITT compared to 2 inhalers (ICS/LABA, LAMA/ICS+LABA, LAMA/LABA+ICS or LAMA+LABA) in patients with moderate or severe COPD.39 The authors concluded that SITT was more cost-effective (especially in patients with severe COPD), as it was associated with a reduced loss of productivity and less use of healthcare resources. SITT also achieved better results in health parameters, reducing exacerbations and improving life years gained and quality-adjusted life years. However, it is important to bear in mind that the studies included in the review analysed cost-effectiveness using theoretical models, so further independent, real-world studies would be necessary.
AdherenceIn asthma management, adherence should be considered a treatable trait that must be accurately assessed in order to personalise the patient's therapy. Smart electronic monitoring devices can track adherence in real time and provide audiovisual reminders and customised feedback, all of which are crucial to improve long-term adherence. However, the widespread implementation of these devices still needs to be validated in cost-effectiveness studies, since they are rarely available in clinical practice and there are other means of monitoring, such as pharmacy refill records and the test of adherence to inhalers.40,41
Using multiple inhalers to deliver different medications requires patients to learn different inhalation techniques. This can be complicated and, therefore, lead to significant errors and non-adherence. Escalation to multi-inhaler TT results in a high rate of discontinuation of critical treatment components such as ICS, with 44% of patients discontinuing therapy 6 months after starting treatment.42 Administration of LAMA, LABA and ICS in a single inhaler, particularly once daily, has been associated with improved adherence and persistence compared to therapies requiring multiple inhalations throughout the day.43–46 Real-world studies suggest that patients who start with a single inhaler are 31% more likely to remain adherent and 49% more likely to persist than those who use multiple devices.47,48
SafetyStudies indicate that the safety profile of SITT is comparable, if not superior, to that of multi-inhaler therapies. Simplifying the therapeutic regimen does not increase the risk of ICS-induced systemic AEs such as adrenal suppression and osteoporosis, since the corticosteroid dose is somewhat lower than that used in open triple therapy. In addition, SITT can also reduce the risk of administration errors.35,37 Existing evidence suggests that adding LAMA to standard ICS/LABA therapy has an excellent safety profile.15 Despite initial concerns about potential LABA- and LAMA-related CV events, studies in various TT combinations found no significant differences in the risk of serious AEs, showing them to be safe in all asthma patients including those with CV comorbidities.17,31,35
The safety profile does not vary significantly with patient age. Several studies analysing AEs in adults have observed no significant differences (p>0.05) in the risk of serious adverse events, pneumonia, and serious CV adverse events between the combinations investigated.16,17 Although TT was associated with a slight increase in mild AEs, such as dry mouth and dysphonia, the overall safety profile of TT confirms that it may be suitable for a wide age spectrum of asthma patients.
Most asthma medications are well tolerated during pregnancy, especially considering the maternal and foetal risks of uncontrolled asthma. However, although no individual components have shown significant AEs, caution is advisable due to the lack of specific data. Evidence on the safety of TT during pregnancy and breastfeeding remains limited and is based mainly on data from observational studies. The benefits of asthma control should be weighed up against the potential risks before making decisions about treatment during pregnancy and lactation.49,50
Patient satisfactionInstruments such as the Feeling of Satisfaction with Inhaler (FSI-10)51 and quantitative patient satisfaction and preference questionnaires (PASAPQ)52 can be used to explore the patient's preferences, proficiency with the inhaler, and opinion of the treatment. Several studies focusing on asthma patient satisfaction based on the type of inhaler have shown that the characteristics of the inhaler can significantly influence user preferences,53–55 and satisfaction with the device and its performance directly affect adherence to treatment. In fact, greater inhaler satisfaction is associated with improved health outcomes, including a reduction in exacerbations and hospital visits.56 There is also evidence that patients who participate in choosing their inhalers are more likely to use them correctly.52
Although few studies have analysed asthma patient satisfaction with different inhalation devices, several have explored the use of TT for the management of COPD, showing the importance of patient preference and satisfaction.57 For example, it was observed that the inhalation technique improved significantly after appropriate training among users of dry powder inhalers compared to those using metered-dose and capsule-based dry powder inhalers.58 These studies indicate that proper inhalation technique could be a determining factor in patient satisfaction, having a direct impact on treatment effectiveness and underscoring the importance of satisfaction as a potential indicator of positive health outcomes in asthma treatment.
ConclusionThis study shows that there is broad consensus in Spain on the significant advances made in asthma therapy for adults following the introduction of SITT. These devices not only have the potential to improve lung function and reduce exacerbations, but may also increase treatment adherence and patient satisfaction. Although experts do not yet recommend SITT as first-line treatment, there is evidence that it is safe and has a low risk for serious AEs, including CV and other systemic AEs. Recent studies support its use in a wide age range and in several different clinical contexts. These factors, combined with a favourable cost-effectiveness evaluation, make SITT a promising option for the management of poorly controlled asthma.
On the other hand, the use of TT in a single device makes it difficult to adjust the dosage of individual components. This problem could be solved by developing TT combinations with different dosages that allow both escalation and de-escalation of treatment according to clinical guidelines. Nevertheless, it is important to acknowledge the inherent limitations of expert consensus documents, including their dependence on current evidence and expert opinion, which may evolve as new data emerge. Further clinical trials and real-world studies are needed to document in detail the situations in which it is preferable to use single or multiple devices.
Artificial intelligence involvementThe authors declare that they have not used any type of generative artificial intelligence to produce this article.
FundingGebro Pharma funded services of Delphi methodological support and editorial assistance provided by Medical Science Consulting (MSC), but these organizations have had no input in the preparation of the project. The Scientific Committee worked at all times with complete independence of opinion and freedom to select the content of treatments and recommendations discussed.
Authors’ contributionsC.C.S., E.M.M., A.P.G., C.F.A., A.T.A., G.P.C, A.P.B., A.R.F., J.A.C.G., and J.G.S.C. contributed to the conception and design of the study, as well as the investigation and analysis performed. The first draft of the manuscript was written by C.C.S., E.M.M., A.P.G. and J.G.S.C. The Members of the Validation Task Force voted and commented on the recommendations during the Delphi process.
C.C.S., E.M.M., A.P.G., C.F.A., A.T.A., G.P.C, A.P.B., A.R.F., J.A.C.G., and J.G.S.C. critically reviewed, edited, and commented on the manuscript until the final version was obtained. The final version of the manuscript was read and approved by all authors.
Conflicts of interestsC.C.S. has received honoraria for speaking at sponsored meetings from AstraZeneca, Chiesi, Gebro Pharma, GSK and Sanofi; has received assistance for travel from AstraZeneca, Chiesi and Sanofi; and has acted as a consultant for AstraZeneca, Chiesi, GSK, CIPLA and Sanofi. E.M.M. has received honoraria for speaking at meetings sponsored by AstraZeneca, Chiesi, Menarini, Gebro Pharma, GSK and Sanofi; has received travel support from AstraZeneca, Chiesi, GSK, Bial and Sanofi; and has acted as a consultant for AstraZeneca, GSK, ALK, Gebro Pharma and Sanofi. A.P.G. has received honoraria for speaking at sponsored meetings from AstraZeneca, Alk-Abelló, Bial, Chiesi, Gebro Pharma, and Sanofi; has received assistance for travel from AstraZeneca, Chiesi and Sanofi; and has acted as a consultant for AstraZeneca, Chiesi, GSK and Sanofi. C.F.A. received honoraria for speaking at sponsored meetings from AstraZeneca, GSK, Chiesi, Menarini, and Gebro Pharma and received congress travel assistance from Sanofi, AstraZeneca, and GSK. A.T.A. has received honoraria for speaking at meetings sponsored by AstraZeneca, Chiesi, GSK and Sanofi; has received travel support from AstraZeneca, Chiesi and Sanofi; and has acted as a consultant for AstraZeneca, Chiesi, GSK and Sanofi. G.P.C. received honoraria for speaking at sponsored meetings from AstraZeneca, Boehringer Ingelheim, Chiesi, Faes, Gebro Pharma, GSK, MSD, Sanofi and Teva; received help assistance to meeting travel from AstraZeneca, Chiesi and Sanofi; and act as a consultant for AstraZeneca, GSK and Sanofi. A.P.B. received honoraria for speaking at sponsored meetings from GSK, Sanofi, Chiesi and AstraZeneca and received congress travel assistance from Gebro Pharma, Sanofi, Faes. A.R.F. received honoraria for speaking at sponsored meetings from Astrazeneca, Gebro Pharma, GSK, and Menarini; received assistance to meeting travel from Sanofi; and acted as a consultant for AstraZeneca, Sanofi, and GSK. J.A.C.G. received honoraria for speaking at sponsored meetings from AstraZeneca, Chiesi, FAES Pharma, GSK, Menarini and Sanofi. J.G.S.C. received honoraria for speaking at sponsored meetings from AstraZeneca, Gebro Pharma, GSK, Menarini and Sanofi; received assistance to meeting travel from AstraZeneca and Sanofi; and act as a consultant for AstraZeneca, Sanofi, and GSK.
Ánchel González Barriga and Blanca Piedrafita, from MSC, provided editorial support in the form of medical writing, table assembly according to the authors’ detailed instructions, collection of author comments, editing, data checking, and reference checking.