There is little control of cardiovascular (CV) risk factors in secondary prevention after an ischaemic stroke, in part due to a lack of adherence to treatment. The CV polypill may contribute to proper treatment adherence, which is necessary for CV disease prevention. This study aimed to establish how and in what cases the CV polypill should be administered.
MethodsA group of 8 neurologists drafted consensus recommendations using structured brainstorming and based on their experience and a literature review.
ResultsThese recommendations are based on the opinion of the participating experts. The use of the CV polypill is beneficial for patients, healthcare professionals, and the health system. Its use is most appropriate for atherothrombotic stroke, lacunar stroke, stroke associated with cognitive impairment, cryptogenic stroke with CV risk factors, and silent cerebrovascular disease. It is the preferred treatment in cases of suspected poor adherence, polymedicated patients, elderly people, patients with polyvascular disease or severe atherothrombosis, young patients in active work, and patients who express a preference for the CV polypill. Administration options include switching from individual drugs to the CV polypill, starting treatment with the CV polypill in the acute phase in particular cases, use in patients receiving another statin or an angiotensin ii receptor antagonist, or de novo use if there is suspicion of poor adherence. Nevertheless, use of the CV polypill requires follow-up on the achievement of the therapeutic objectives to make dose adjustments.
ConclusionsThis document is the first to establish recommendations for the use of the CV polypill in cerebrovascular disease, beyond its advantages in terms of treatment adherence.
El control de los factores de riesgo cardiovascular (CV) en la prevención secundaria tras un ictus isquémico es bajo, en parte debido a la falta de adherencia terapéutica. La polipíldora CV puede contribuir a la buena cumplimentación del adecuado tratamiento para la prevención cerebrovascular. El objetivo fue establecer cómo y en qué casos se debería administrar.
MétodosUn grupo de 8neurólogos redactaron recomendaciones consensuadas mediante una técnica de brainstorming estructurado, basándose en su experiencia y en una revisión bibliográfica.
ResultadosLos resultados atienden a la opinión de los expertos. El uso de la polipíldora CV tiene ventajas para pacientes, profesionales sanitarios y para el sistema de salud. Las situaciones clínicas más adecuadas para su uso son el ictus aterotrombótico, el lacunar, el asociado a deterioro cognitivo, el criptogénico con factores de riesgo CV y la enfermedad cerebrovascular silente. Su uso preferente incluye la sospecha de mal cumplimiento, a los pacientes polimedicados, ancianos, polivasculares o con alta carga aterotrombótica, jóvenes activos laboralmente y pacientes con preferencias por la polipíldora CV. Las opciones de administración incluyen el paso de fármacos individuales a la polipíldora CV, el inicio directo desde la fase aguda en casos particulares, a los pacientes con otra estatina o con un antagonista del receptor de la angiotensina ii, o de novo si hubiera sospecha de mala adherencia. No obstante, su uso implica realizar seguimiento del cumplimiento de los objetivos terapéuticos para ajustar la dosis.
ConclusionesEste documento es el primero en establecer recomendaciones de uso de la polipíldora CV en enfermedad cerebrovascular, aparte de sus ventajas sobre la adherencia.
Ischaemic stroke is one of the leading causes of death worldwide.1 According to the World Health Organization, 6.7 million people died due to ischaemic stroke in 2012.2 Stroke patients are at greater risk of recurrent cerebrovascular accidents. Recurrent ischaemic stroke is also associated with increased risk of vascular dementia.
Poor treatment adherence is regarded as one of the main obstacles to secondary prevention of vascular disease.3 Factors involved in poor treatment adherence include the chronic and sometimes scarcely symptomatic nature of cardiovascular disease, medication copayments, complex treatment approaches, and the lack of education programmes for healthcare professionals and patients.4 Poor treatment adherence increases the rate of severe cardiovascular complications, including stroke, leading to higher mortality rates, poorer quality of life in survivors, increased care burden, and greater healthcare costs associated with complications and hospital admissions.5
Measures including lower medication copayments, automatic reminders, mail-order pharmacies, professional healthcare advice, and fixed-dose combination therapy have helped improve treatment adherence. Simplifying treatment schedules with fixed-dose combination therapy is a complementary strategy used in a wide range of diseases to improve treatment adherence; furthermore, this approach is favourably viewed by patients. Fixed-dose combination therapy also reduces production and distribution costs, making treatment more affordable.4
The cardiovascular (CV) polypill, the first combined treatment to be approved in Europe for the secondary prevention of cardiovascular diseases, contains acetylsalicylic acid (ASA), atorvastatin, and ramipril; these 3 active ingredients have been shown to reduce mortality in patients with established vascular disease.6–9
Currently marketed polypills contain 100mg ASA, 20/40mg atorvastatin, and 2.5/5/10mg ramipril.
Our study analyses the impact of the CV polypill, establishes the most and least favourable clinical situations and patient profiles for this treatment, and makes some general recommendations about polypills for the secondary prevention of stroke.
Material and methodsA meeting was held between an expert panel of 8 neurologists to define the structure and content of the consensus recommendations. An external moderator participated in the structured brainstorming session, facilitating discussion, organising ideas, and promoting equal participation. It was agreed that the document should address the following topics: the implications of CV polypill use for different population groups, favourable and unfavourable clinical profiles, preferential indications, and use and management recommendations. Each neurologist drafted a different part of the consensus document based on the literature and their clinical experience. The final version was reviewed and approved by all neurologists via e-mail.
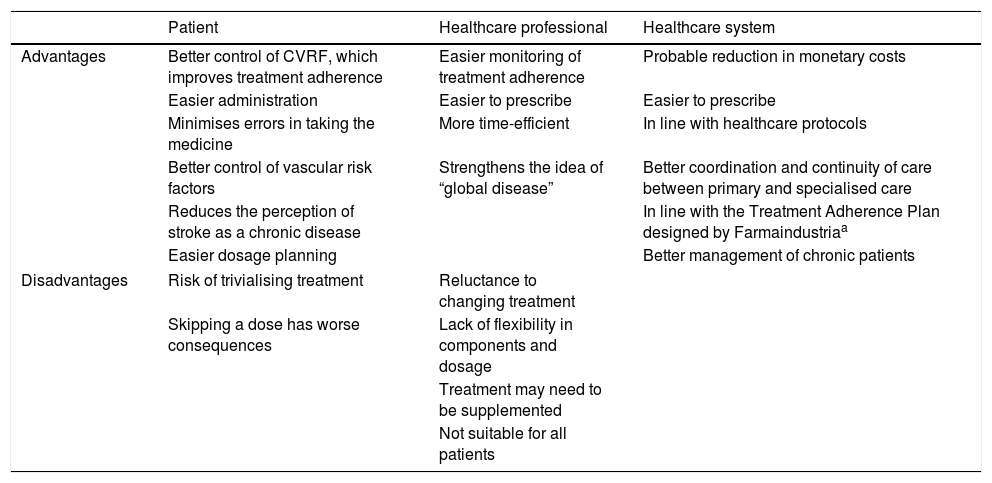
ResultsImplications of cardiovascular polypill administration for cerebrovascular disease preventionThe introduction of the CV polypill as a secondary prevention measure for cerebrovascular disease has considerable implications for patients, healthcare professionals, and the healthcare system (Table 1).
Implications of the use of cardiovascular polypills for cerebrovascular disease prevention.
| Patient | Healthcare professional | Healthcare system | |
|---|---|---|---|
| Advantages | Better control of CVRF, which improves treatment adherence | Easier monitoring of treatment adherence | Probable reduction in monetary costs |
| Easier administration | Easier to prescribe | Easier to prescribe | |
| Minimises errors in taking the medicine | More time-efficient | In line with healthcare protocols | |
| Better control of vascular risk factors | Strengthens the idea of “global disease” | Better coordination and continuity of care between primary and specialised care | |
| Reduces the perception of stroke as a chronic disease | In line with the Treatment Adherence Plan designed by Farmaindustriaa | ||
| Easier dosage planning | Better management of chronic patients | ||
| Disadvantages | Risk of trivialising treatment | Reluctance to changing treatment | |
| Skipping a dose has worse consequences | Lack of flexibility in components and dosage | ||
| Treatment may need to be supplemented | |||
| Not suitable for all patients | |||
CVRF: cardiovascular risk factors.
The main advantages for patients are simplicity of treatment for cardiovascular risk factors, and improved adherence. The complexity of a treatment regime is inversely correlated with the level of adherence.10 There is solid evidence of improved treatment adherence in patients receiving fixed-dose combination therapy as compared to those taking each drug separately.11–13 This may be explained by the lower risk of errors associated with taking multiple medications, and the simplicity of the treatment. Better adherence is associated with improved management of vascular risk factors. However, although polypill monocomponents alone have been shown to have an impact on risk factors, additional evidence is needed of their ability to reduce cerebrovascular events. CV polypills optimise drug prescription and treatment management, making it easier for healthcare professionals to control vascular risk factors and allowing them to allocate more time to promoting healthy habits. Despite occasions when it is necessary to supplement the CV polypill with other drugs,14 fixed-dose combination therapy is compatible with personalised secondary prevention treatment.
Fixed-dose combination therapy has also been shown to be cost-effective for primary and secondary prevention.15,16 Although further research into secondary prevention is needed, we may hypothesise that improved treatment adherence reduces healthcare costs when it results in better control of clinical progression and recurrence of non-cardioembolic cerebrovascular disease.
Favourable and unfavourable clinical situations for use of the cardiovascular polypill for cerebrovascular disease preventionTable 2 summarises the situations in which the CV polypill should and should not be used, although these consensus recommendations mainly focus on the clinical situations where patients benefit the most from this treatment.
Favourable and unfavourable clinical situations for use of cardiovascular polypills for cerebrovascular disease prevention.
| Recommended | Not recommended |
|---|---|
| Atherothrombotic stroke | Cardioembolic stroke |
| Lacunar stroke | Patients with stroke but not presenting arterial hypertension or dyslipidaemiaa |
| Cryptogenic stroke associated with CVRF | Intolerance to or contraindications for any of the polypill monocomponents |
CVRF: cardiovascular risk factors.
According to some guidelines, patients with history of atherothrombotic stroke should receive antiplatelets, statins, and antihypertensives.17–20 ASA dosed at 75-325mg/day is the antiplatelet agent of choice for both extracranial and intracranial stenoses. Patients with extracranial and intracranial stenoses (critical or otherwise), recurrent stroke, or progressive stroke may be treated with ASA (75-325mg/day), clopidogrel (75mg/day), or a combination of ASA (25mg)+extended-release dipyridamole (200mg) twice daily; this drug combination is to be preferred over ASA+clopidogrel.
Statins are recommended for asymptomatic patients and should be dosed to achieve a low-density lipoprotein (LDL) cholesterol level below 100mg/dL.21 In patients with atherothrombotic stroke, LDL cholesterol levels should be kept below 70mg/dL.17,21 Results from clinical trials of statins in patients with ischaemic heart disease, a meta-analysis of these trials, and the SPARCL trial of patients with atherothrombotic stroke reveal a significant reduction in stroke risk, especially in patients taking atorvastatin dosed at 80mg/day.22,23
Hypertensive treatment must be started in patients with history of hypertension or with blood pressure above 140/90mm Hg. Blood pressure should be kept within the normal range (< 140/90mm Hg); no recommendations have been made for specific drugs.19
Patients with atherothrombotic stroke require life-long polymedication to reduce the associated risk of recurrence; this may lower treatment adherence. Administering CV polypills to patients requiring all 3 drugs may improve adherence, always on the condition that treatment objectives be met.
Lacunar strokeArterial hypertension is the main risk factor for lacunar stroke. Correctly managing blood pressure in patients with ischaemic stroke may result in a 28% decrease in the risk of stroke recurrence.24 In the SPS3 trial, lower blood pressure achieved non-significant reductions in lacunar stroke recurrence. Furthermore, angiotensin-converting enzyme inhibitors prevent the progression of cerebral microangiopathy, according to MRI studies.25 Antihypertensive treatment should be started in patients with history of hypertension or with blood pressure above 140/90mm Hg. Blood pressure should be kept below 140/90mm Hg; no recommendations for specific drugs have been made.19
The SPARCL trial showed that statins achieve similar benefits in patients with lacunar and with other types of stroke,26 although patients with lacunar stroke rarely present large-vessel atheromatosis. Antiplatelet treatment with a single drug is recommended in patients with lacunar stroke. In the SPS3 trial, treatment with 325mg ASA plus 75mg clopidogrel did not significantly reduce the risk of stroke recurrence and increased the risk of haemorrhage and death, compared to treatment with 325mg ASA only.27 Antiplatelet treatment with ASA dosed at 75-325mg is recommended in these patients.28 The polypill may therefore be useful in patients with lacunar stroke requiring all 3 drugs.
Cryptogenic stroke associated with cardiovascular risk factorsAnticoagulants are occasionally used in patients with cryptogenic stroke, especially those meeting diagnostic criteria for embolic stroke of undetermined source. To date, however, anticoagulant treatment has not been found to be more effective for secondary prevention of cryptogenic stroke; ASA is therefore the antiplatelet treatment of choice.
Classic vascular risk factors such as arterial hypertension and dyslipidaemia must be controlled; these factors are associated with increased risk of recurrence, although the risk is lower than in other stroke subtypes. The CV polypill is therefore indicated for patients with cryptogenic stroke presenting arterial hypertension and hypercholesterolaemia.
Preferential indication of the cardiovascular polypill for cerebrovascular disease preventionIn addition to the previously described clinical situations, polypill treatment is more appropriate for certain patient profiles; the polypill should be preferentially indicated in these patients. Non-adherent patients constitute the most evident example. Different studies have shown that fixed-dose combination therapy achieves better treatment adherence than free drug combinations.12,29–34 No specific profile has been established for non-adherent patients, although several predictors of poor treatment adherence have been identified35–38: young age, old age, comorbidities, polymedication, unemployment or low income, rural setting, history of stroke, and care provided by less specialised centres. Improved adherence also results in economic savings.11,16
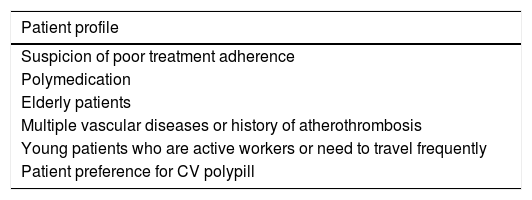
Management of patients with multiple vascular diseases or history of atherothrombosis is complex; the polypill is particularly beneficial in these cases. Copresence of atherothrombotic stroke and coronary or peripheral artery disease, whether symptomatic or not, is frequent in clinical practice and has a significant impact on prognosis.39–41 Furthermore, mortality due to ischaemic heart disease is elevated in patients with history of stroke, and stroke recurrence is more likely in patients with history of ischaemic heart disease. Controlling vascular risk factors and ensuring treatment adherence is therefore essential in these patients, who frequently receive multiple medications. Table 3 describes other patient profiles benefiting from treatment with the CV polypill.
Patients profiles with preferential indication for cardiovascular polypills for cerebrovascular disease prevention.
| Patient profile |
|---|
| Suspicion of poor treatment adherence |
| Polymedication |
| Elderly patients |
| Multiple vascular diseases or history of atherothrombosis |
| Young patients who are active workers or need to travel frequently |
| Patient preference for CV polypill |
The European guidelines on cardiovascular disease prevention include a section on the polypill, an essential component in the global strategy for cerebrovascular disease prevention.42 According to the above considerations, and the recommendations of published guidelines, our expert panel established a series of recommendations for polypill treatment initiation and follow-up.
There are several possible scenarios for starting treatment with the polypill:
Switching from personalised treatment to the polypill: ASA dose does not change over the course of patient follow-up, but ramipril and atorvastatin doses do require adjustment depending on blood pressure and LDL cholesterol levels, treatment adherence, and lifestyle changes. Doses should therefore be adjusted every 3-6 months to achieve objectives set for blood pressure and LDL cholesterol level, aiming to achieve good tolerability. In patients with non-cardioembolic stroke and LDL cholesterol levels above 100mg/dL, the recommended initial dose of atorvastatin is 80mg/24h. While this dose is poorly tolerated by some patients, others may achieve the target LDL cholesterol level (< 70mg/dL) with 40mg/24h.
Starting treatment with the polypill during the acute phase: polypill treatment may be started during hospitalisation if the physician anticipates poor treatment adherence or compliance, or difficulties accessing treatment.
Switching from a statin other than atorvastatin or an angiotensin II receptor antagonist to thepolypill35: the statin may be substituted for the polypill provided that the latter shows similar effectiveness in lowering LDL cholesterol levels.43 The angiotensin II receptor antagonist may be substituted for ramipril, except in patients with history of adverse reactions to angiotensin-converting enzyme inhibitors.44–48 It is essential to verify that the polypill achieves targets for blood pressure and LDL cholesterol.
Unmedicated patients: the polypill may be administered to patients who have not been treated with the 3 polypill monocomponents separately but are being considered for this treatment, especially when poor treatment adherence is anticipated or patients have difficulties accessing treatment, and in patients for whom treatment objectives are achieved with the polypill.
During follow-up, physicians should:
Manage blood pressure and LDL cholesterol levels, adjusting ramipril dose or adding/discontinuing other antihypertensive drugs, increasing the dose of atorvastatin or combining the drug with ezetimibe, or discontinuing the polypill and administering its monocomponents.
Evaluate tolerability: there is no evidence that the polypill causes more adverse reactions than the combination of its monocomponents, although a study comparing several polypills against placebo or one polypill monocomponent reported slightly lower tolerability for polypills.49 Any adverse reaction should be managed by adjusting the dose of the drug responsible.
DiscussionA wide range of polypills with different drug combinations have been approved for different diseases. Polypills are widely used to treat infectious diseases, and there is mounting evidence of their benefits for patients with cardiovascular diseases50,51; however, much work remains to be done in the field of cardiovascular prevention. This study makes recommendations for use of the CV polypill containing ASA, atorvastatin, and ramipril for secondary prevention of cerebrovascular disease. These recommendations were made by consensus by a panel of neurologists and focus on the most appropriate patient profiles, clinical situations, and administration approaches.
The benefits of the polypill involve the management of cardiovascular risk factors and improved treatment adherence. Therefore, the polypill is indicated for potentially non-adherent patients and those requiring strict follow-up of treatment objectives, such as elderly patients, polymedicated patients, patients with multiple vascular diseases, or individuals with history of atherothrombosis. The polypill is more beneficial in some clinical situations than in others; these recommendations are intended to provide an introduction to this topic.
Interest in fixed-dose combination therapy has increased in recent years, probably due to the associated advantages for patients, healthcare professionals, and the healthcare system. In fact, the European Medicines Agency's Committee for Medicinal Products for Human Use recently updated its guidelines on fixed combination medicinal products; the new version is expected to come into force in October 2017.
Some researchers have highlighted the potential of the CV polypill for improving secondary prevention of stroke.5,52,53 There are currently no recommendations establishing the patient profiles and clinical situations in which the polypill has the greatest benefits for cerebrovascular disease, although such recommendations do exist for CV polypill treatment for secondary prevention of cardiovascular disease.5 In this context, expert opinion is particularly important; this study provides a starting point for determining which patients may benefit the most from fixed-dose combination therapy for secondary prevention of cerebrovascular accidents.
This study has the limitation that it is based on expert opinion rather than on well-designed clinical trials; furthermore, no systematic review of the literature has been conducted to provide evidence for our recommendations. In any case, these recommendations were drafted based on the consensus of a panel of respected specialists with broad clinical experience in the management of these patients.
FundingThis study was funded by Grupo Ferrer Internacional, S.A.
Conflicts of interestAll authors received professional fees from Grupo Ferrer Internacional, S.A. for this study.
The authors wish to thank Grupo Ferrer Internacional for the financial support and GOC Networking for the methodological support.
Please cite this article as: Masjuan J, Gállego J, Aguilera JM, Arenillas JF, Castellanos M, Díaz F, et al. Uso de la polipíldora cardiovascular en la prevención secundaria de la enfermedad cerebrovascular. Neurología. 2021;36:1–8.